Published online Apr 15, 2021. doi: 10.4239/wjd.v12.i4.453
Peer-review started: December 11, 2020
First decision: December 31, 2020
Revised: January 13, 2021
Accepted: March 7, 2021
Article in press: March 7, 2021
Published online: April 15, 2021
Processing time: 118 Days and 20.1 Hours
Sleeve gastrectomy (SG) can induce prominent remission of type 2 diabetes mellitus. However, the long-term remission rate of diabetes usually decreases over time. Oligofructose has been verified to modulate host metabolism. The aim of this study was to explore the protective effect of oligofructose on high-fat diet (HFD)-induced metabolic dysfunction after SG.
To study the effect and mechanism of oligofructose on diabetic remission in diabetic rats after SG.
SG and SHAM operation were performed on diabetes rats induced with an HFD, nicotinamide, and low-dose streptozotocin. Then the rats in the SHAM and SG groups were continuously provided with the HFD, and the rats in sleeve gastrectomy-oligofructose group were provided with a specific HFD containing 10% oligofructose. Body weight, calorie intake, oral glucose tolerance test, homeostasis model assessment of insulin resistance, lipid profile, serum insulin, glucagon-like peptide 1 (GLP-1), total bile acids, lipopolysaccharide (LPS), and colonic microbiota levels were determined and compared at the designated time points. All statistical analyses were performed using Statistic Package for Social Science version 19.0 (IBM, United States), and the statistically significant difference was considered at P < 0.05.
At 2 wk after surgery, rats that underwent SG exhibited improved indexes of glucose and lipid metabolism. Compared with the SG group, the rats from SG-oligofructose group exhibited better parameters of glucose and lipid metabolism, lower body weight (526.86 ± 21.51 vs 469.25 ± 21.84, P < 0.001), calorie intake (152.14 ± 9.48 vs 129.63 ± 8.99, P < 0.001), homeostasis model assessment of insulin resistance (4.32 ± 0.57 vs 3.46 ± 0.52, P < 0.05), and LPS levels (0.19 ± 0.01 vs 0.16 ± 0.01, P < 0.05), and higher levels of insulin (1.17 ± 0.17 vs 1.58 ± 0.16, P < 0.001) and GLP-1 (12.39 ± 1.67 vs 14.94 ± 1.86, P < 0.001), and relative abundances of Bifidobacterium (0.0034 ± 0.0014 vs 0.0343 ± 0.0064, P < 0.001), Lactobacillus (0.0161 ± 0.0037 vs 0.0357 ± 0.0047, P < 0.001), and Akkermansia muciniphila (0.0050 ± 0.0024 vs 0.0507 ± 0.0100, P < 0.001) at the end of the study. However, no difference in total bile acids levels was observed between the two groups.
Oligofructose partially prevents HFD-induced glucose and lipid metabolism damage after SG, which may be due to the changes of calorie intake, insulin, GLP-1, LPS, and the gut microbiota in rats.
Core Tip: Bariatric surgery is one of the important methods to treat obesity and type 2 diabetes mellitus, but the remission and recurrence of type 2 diabetes mellitus after surgery are still a hot issue. In this study, we demonstrated that oligofructose can partially resist the high-fat diet-induced glucose and lipid metabolism damage after sleeve gastrectomy in rats, and the influence of oligofructose on calorie intake, insulin, glucagon-like peptide-1, lipopolysaccharide, and gut microbiota may play an important role in the improving function.
- Citation: Zhong MW, Li Y, Cheng YG, Liu QR, Hu SY, Zhang GY. Effect of oligofructose on resistance to postoperative high-fat diet-induced damage of metabolism in diabetic rats after sleeve gastrectomy. World J Diabetes 2021; 12(4): 453-465
- URL: https://www.wjgnet.com/1948-9358/full/v12/i4/453.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i4.453
Bariatric surgery has been considered the most effective treatment for type 2 diabetes mellitus (T2DM)[1,2]. Over the past decade, the total number of bariatric surgeries worldwide is increasing, particularly sleeve gastrectomy (SG). Currently, SG is the most frequently used procedure in the United States/Canada and in Asian/Pacific regions[3]. Although bariatric surgery induces rapid and prominent remission of T2DM, the long-term remission rate usually decreases over time, and the recurrence of T2DM is observed in a part of patients with initial remission after bariatric surgery[4-7]. As a novel type of bariatric surgery, SG is characterized by lower complication rate, faster operation, fewer technical requirements, and fewer postoperative nutritional problems[8], so that a marked increase in the relative application rate of SG has been observed[3]. Many investigators have reported that SG and Roux-en-Y gastric bypass (RYGB) surgery have equal treatment efficacy for diabetes[9,10]. However, long-term randomized controlled comparison for the impact of SG coupled with RYGB as the gold standard in subjects with T2DM is surprisingly limited. The long-term effect of SG on diabetes is questionable[8]. What is more, SG has been reported to be an independent predictor of the relapse of diabetes at 48.7 mo of follow-up[5]. How to reduce or delay the recurrence of diabetes after surgery is a severe problem that the clinicians have to face.
Prebiotics are non-digestible oligosaccharides, such as oligofructose, galactoo-ligosaccharides, lactulose, and inulin. Prebiotics promote the loss of body weights and improve the metabolism of glucose and lipid in rodents and human[11-15]. The mechanisms underlying these benefits may be due to the reduction in energy intake, regulation of gut microbiota, improvements in low-grade inflammation, and increased levels of gut hormones, such as glucagon-like peptide-1 (GLP-1) and peptide YY[16-19], which prompted us to explore whether prebiotics reduce or delay the recurrence of diabetes after surgery.
A high-calorie diet appears to be one of primary factors contributing to obesity and diabetes[20], and our previous studies have confirmed that a high-fat diet (HFD) induces the deterioration of glucose tolerance after an initial improvement in diabetic rats subjected to duodenal-jejunal bypass[21]. In the present study, we conducted SG on nicotinamide-streptozotocin (STZ)-HFD-induced diabetic model rats with metabolic characteristics of human diabetes[22-24], and stimulated the recurrence of diabetes with postoperative HFD feeding. The glucose and lipid profiles, serum levels of insulin, GLP-1, total bile acids (TBA), and lipopolysaccharide (LPS), and changes in the composition of the gut microbiota were determined and compared.
Forty 8-wk-old male Wistar rats (Laboratory Animal Center of Shandong University, Jinan, China) were individually housed in independent ventilated cages at a constant temperature (24-26 °C), humidity (50%-60%), and a light-dark cycle (12 h light: 12 h dark). All animal procedures were approved by the Institutional Animal Care Committee of the First Affiliated Hospital of Shandong First Medical University. All rats were provided with an HFD (40% of calories as fat, Huafukang Biotech Company, Beijing, China) for 4 wk to induce insulin resistance, and then treated with a single intraperitoneal injection of nicotinamide (170 mg/kg, Sigma, St. Louis, MO, United States) followed by a single injection of STZ (65 mg/kg, Sigma, St. Louis, MO, United States) 15 min later after 12 h of fasting to induce a diabetic state. Two weeks after STZ injection, 29 rats were selected as diabetic rats with a fasting blood glucose level higher than 7.1 mmol/L or a blood glucose level after gavage for 2 h higher than 11.1 mmol/L during the oral glucose tolerance test (OGTT), and the rats with extreme hyperglycemia (blood glucose level > 16.7 mmol/L) were excluded from the study[22-24]. The diabetic rats were randomly allocated to a SHAM group (n = 9), SG group (n = 10), and SG-oligofructose (SG-OF) group (n = 10). The rats in the SHAM and SG groups were continuously provided with the HFD after the operation, and the rats in the SG-OF group were provided with a specific HFD supplemented with 10% oligofructose (Huafukang Biotech Company, Beijing, China) since 2 wk after surgery. Body weight and calorie intake were measured at baseline, and 2, 12, and 24 wk after surgery.
The diabetic rats were fed a low-residue diet from 48 h before the operation to 72 h after the operation. Then, they were allowed for ad libitum access to the HFD and water. Rats were anesthetized with a 10% chloral hydrate solution during surgery. The surgical processes were performed as described in our previous study and a published report[25,26].
SG surgery: The surgical procedure is as follows: (1) A 4 cm midline abdominal incision was made from the xiphoid process; (2) The gastric omentum was dissected to disclose the gastric cardium; (3) Short gastric vessels, corresponding gastroepiploic vessels, and the branches of left gastric vessels in the greater curvature were ligated and transected using a 7-0 silk suture (Ningbo Medical Needle, Ningbo, Zhejiang, China); (4) A vena caval clamp was placed to occlude the stomach wall through the cardia to pylorus for the prevention of bleeding; (5) The gastric fundus and a large portion of gastric body were removed (70% of the total stomach); and (6) The residual stomach was closed using a 7-0 silk suture (Ningbo Medical Needle, Ningbo, Zhejiang Province, China).
SHAM surgery: A similar abdominal incision was made as described for the SG procedure, and a similar process was conducted, but the gastric fundus and the gastric body were not removed. The operation time of the SHAM surgery was prolonged to the same time as SG surgery to acquire similar surgical and anesthetic stress.
OGTT was performed at baseline and 2, 12, and 24 wk after surgery. After 8 h of fasting, all rats were subjected to gavage with glucose at a dose of 1 g/kg. Blood glucose levels were measured in conscious rats at baseline and 10, 30, 60, 90, and 120 min after gavage.
During the OGTT conducted at baseline and 2, 12, and 24 wk after surgery, blood samples were collected from the retrobulbar venous plexus at baseline and 10, 30, 60, and 120 min after glucose gavage. Blood samples were centrifuged (1000 × g) at 4 °C for 15 min, and serum was immediately extracted and stored at -80 °C. Fasting serum TBA levels and serum lipid profiles were measured using an automatic biochemical analyzer (Hitachi, Tokyo, Japan). Serum insulin and fasting serum GLP-1 and LPS levels were measured using enzyme-linked immuno-sorbent assay kits (insulin: Millipore, MA, United States; GLP-1: Uscn Life Science incorporated, Wuhan, Hubei, China; LPS: Bio-Swamp, Wuhan, Hubei Province, China).
At baseline and 2, 12, and 24 wk after the operation, homeostasis model assessment of insulin resistance (HOMA-IR) was calculated to evaluate insulin resistance using the following formula: HOMA-IR = fasting insulin (mIU/L) × fasting glucose (mmol/L)/22.5[27].
The change in the gut microbiota may alter host metabolism partially due to the biological activity of the colonic microbiota. Thus, the colonic contents were collected at 24 wk after surgery, and stored at -80 °C immediately. Genomic DNA was extracted from the colonic contents using standard methods[28]. Amplicons of the 16S rRNA gene V4 region were sequenced using the Illumina MiSeq platform (BGI technology, Shenzhen, Guangdong Province, China).
All data are presented as the mean ± SD. Areas under the curves for OGTT (AUCOGTT) were calculated using the trapezoidal integration. The data in each group were analyzed by Shapiro-Wilk test for normality. Intergroup comparisons were evaluated using one-way analysis of variance followed by Bonferroni post hoc comparison. The concentrations of insulin and total GLP-1 after glucose gavage were analyzed using a mixed model analysis of variance followed by Bonferroni post hoc comparison. All statistical analyses were performed using Statistic Package for Social Science version 19.0 (IBM, United States), and the statistically significant difference was considered at P < 0.05.
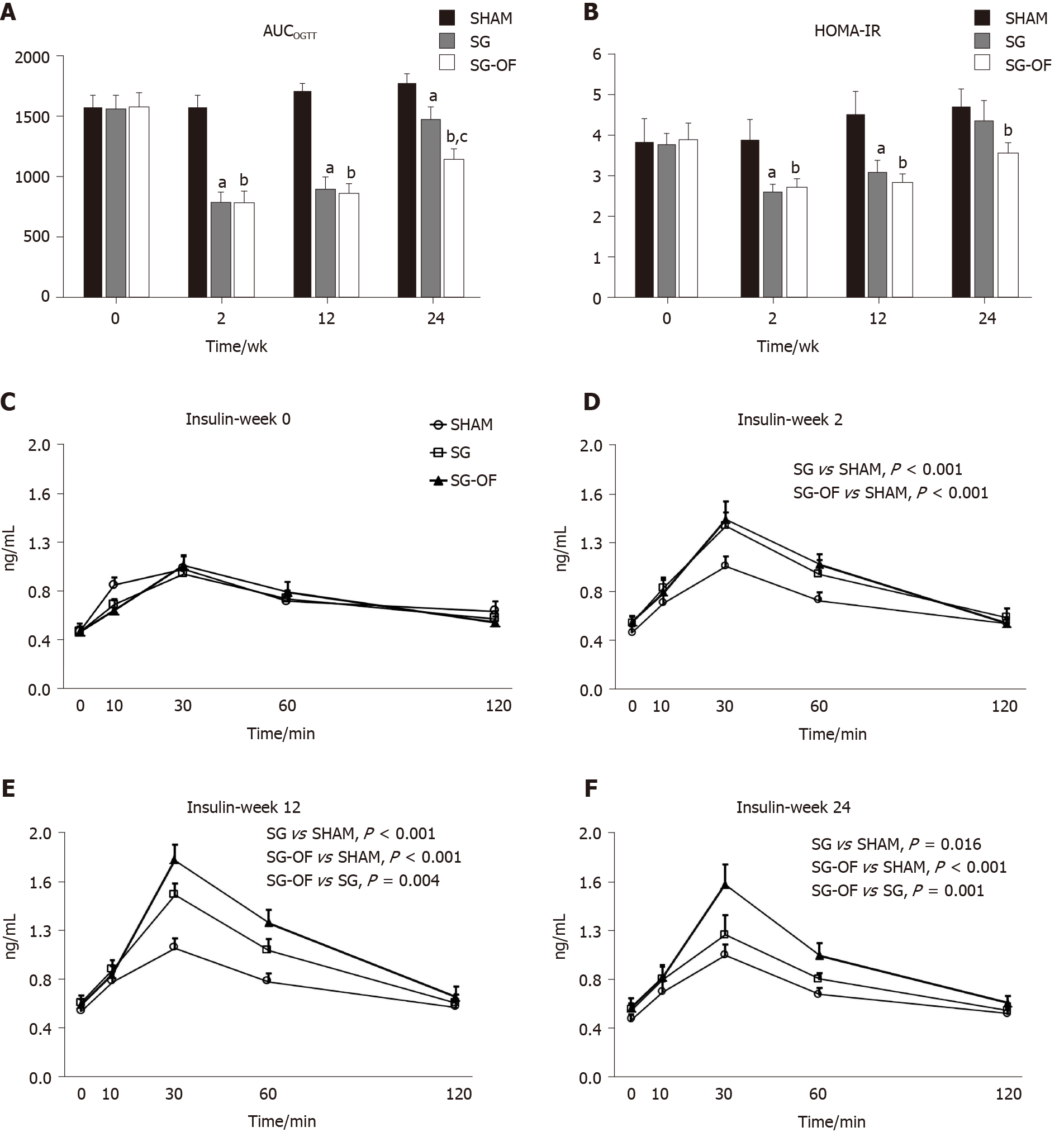
At the end of this experiment, 9, 7, and 8 rats were alive in the SHAM, SG, and SG-OF groups, respectively. Five rats died of residual stomach leakage after SG. As shown in Figure 1A, the AUCOGTT of the three groups were not significantly different at baseline assessment. At 2, 12, and 24 wk after surgery, the AUCOGTT in the SG and SG-OF groups were both lower than those in the SHAM group. The difference of AUCOGTT between the SG group and SG-OF group was observed since 24 wk after surgery. At 24 wk after surgery, rats from the SG-OF group showed significantly lower AUCOGTT than rats from the SG group.
HOMA-IR showed a similar trend to the AUCOGTT, as shown in Figure 1B. No significant differences of HOMA-IR among the three groups were observed at baseline assessment. At 2 and 12 wk after surgery, the rats that underwent SG showed significantly lower HOMA-IR than that from the SHAM group. At 12 wk after surgery, the HOMA-IR of the SG group appeared to be higher than that in the SG-OF group, but there was no statistic difference between the two groups. At 24 wk after surgery, the HOMA-IRs in the SHAM and SG groups were comparable, and higher than that in the SG-OF group.
The curves of serum insulin levels measured during OGTT at baseline and 2, 12, and 24 wk after surgery are shown in Figure 1C-F, respectively. No significant difference of serum insulin levels was observed among the three groups at baseline assessment. A greater amount of insulin was secreted into the serum in the SG and SG-OF groups during the OGTT than that in the SHAM group at 2, 12, and 24 wk after surgery. A difference in the secretion of insulin into the serum was observed between the SG and SG-OF groups, and detected earlier than changes in the AUCOGTT and HOMA-IR. A lower amount of insulin was secreted into the serum of the SG group than the SG-OF group beginning at 12 wk after surgery.
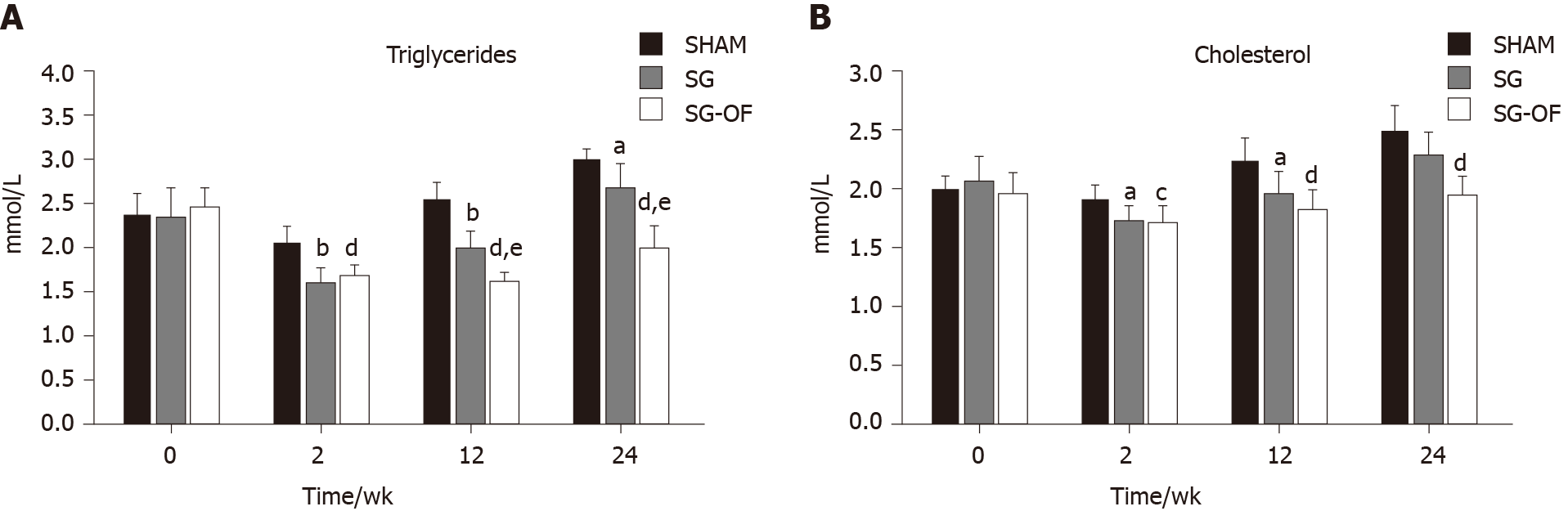
Fasting serum triglyceride and cholesterol levels are shown at Figure 2. There was no significant difference in lipid profiles among the three groups at baseline assessment. And triglyceride and cholesterol in the SG and SG-OF groups were significantly lower than those in the SHAM group at 2 and 12 wk after surgery. At 12 wk after surgery, the SG-OF group showed a lower triglyceride level than the SG group and a comparable cholesterol level to the SG group. At 24 wk after surgery, the SHAM group showed the highest triglyceride level among the three groups and the triglyceride level in the SG group was higher than that in the SG-OF group. Cholesterol levels in the SG group were comparable to the levels in the SHAM group, but higher than those in the SG-OF group.
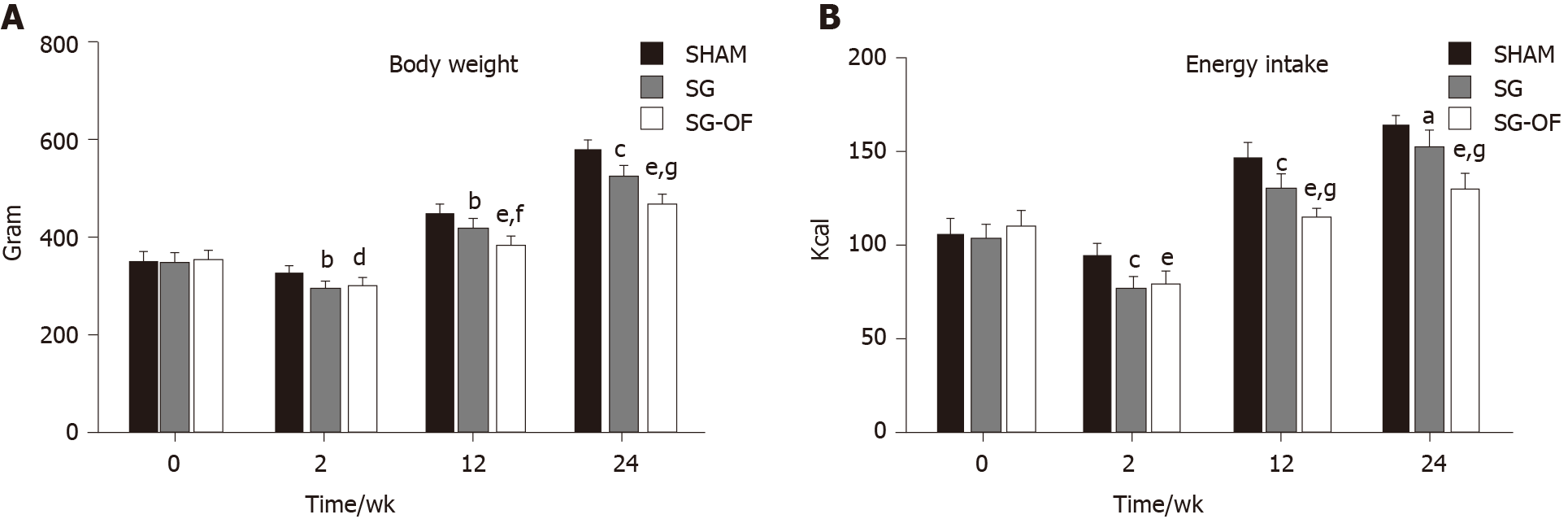
As shown in Figure 3, body weight and calorie intake exhibited a similar trend during this experiment. No significant differences in body weight or calorie intake were observed among the three groups at baseline, and all rats subjected to SG showed a lower body weight and calorie intake than the rats from the SHAM group at 2, 12, and 24 wk after surgery. The body weight and calorie intake in the SG-OF group were lower than those in the SG group beginning at 12 wk after surgery.
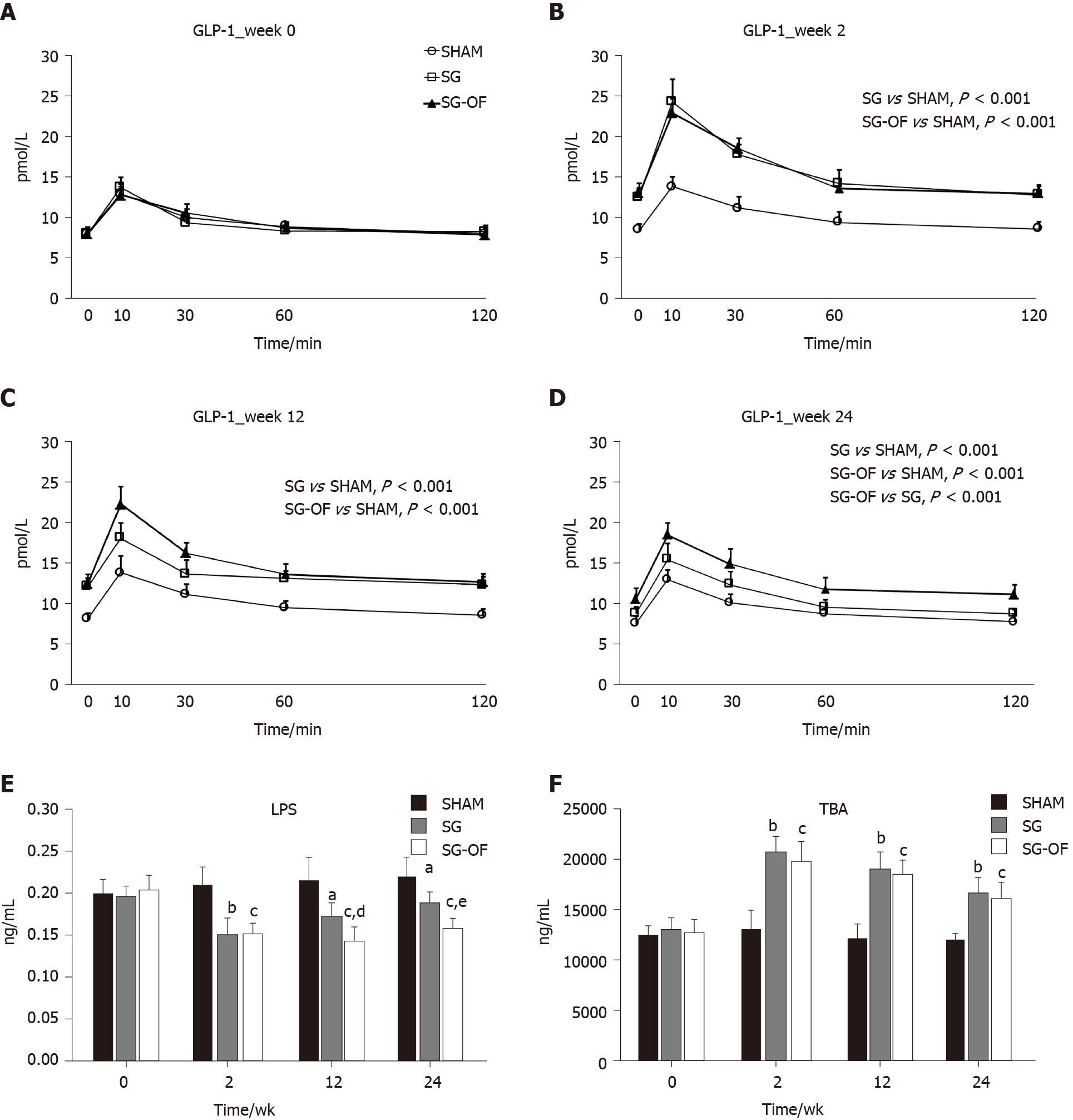
The fasting serum GLP-1 levels at baseline and 2, 12, and 24 wk after surgery are shown in Figure 4A-D, respectively. All the three groups showed similar baseline levels of GLP-1, but the SG and SG-OF groups showed higher GLP-1 levels at all postoperative time points. Compared with the SG group, the SG-OF group exhibited higher GLP-1 levels beginning at 12 wk after surgery.
As shown in Figure 4E, there was no significant difference in LPS levels between groups at baseline assessment. In contrast to the GLP-1 levels, the SG and SG-OF groups showed lower LPS levels at all postoperative time points than the SHAM group, and the LPS level in the SG-OF group was lower than that in the SG group beginning at 12 wk after surgery.
The fasting serum TBA levels are shown in Figure 4F. At all postoperative time points, the TBA levels in the SG and SG-OF groups were significantly higher than those in the SHAM group, but no significant difference was observed between the SG group and SG-OF group.
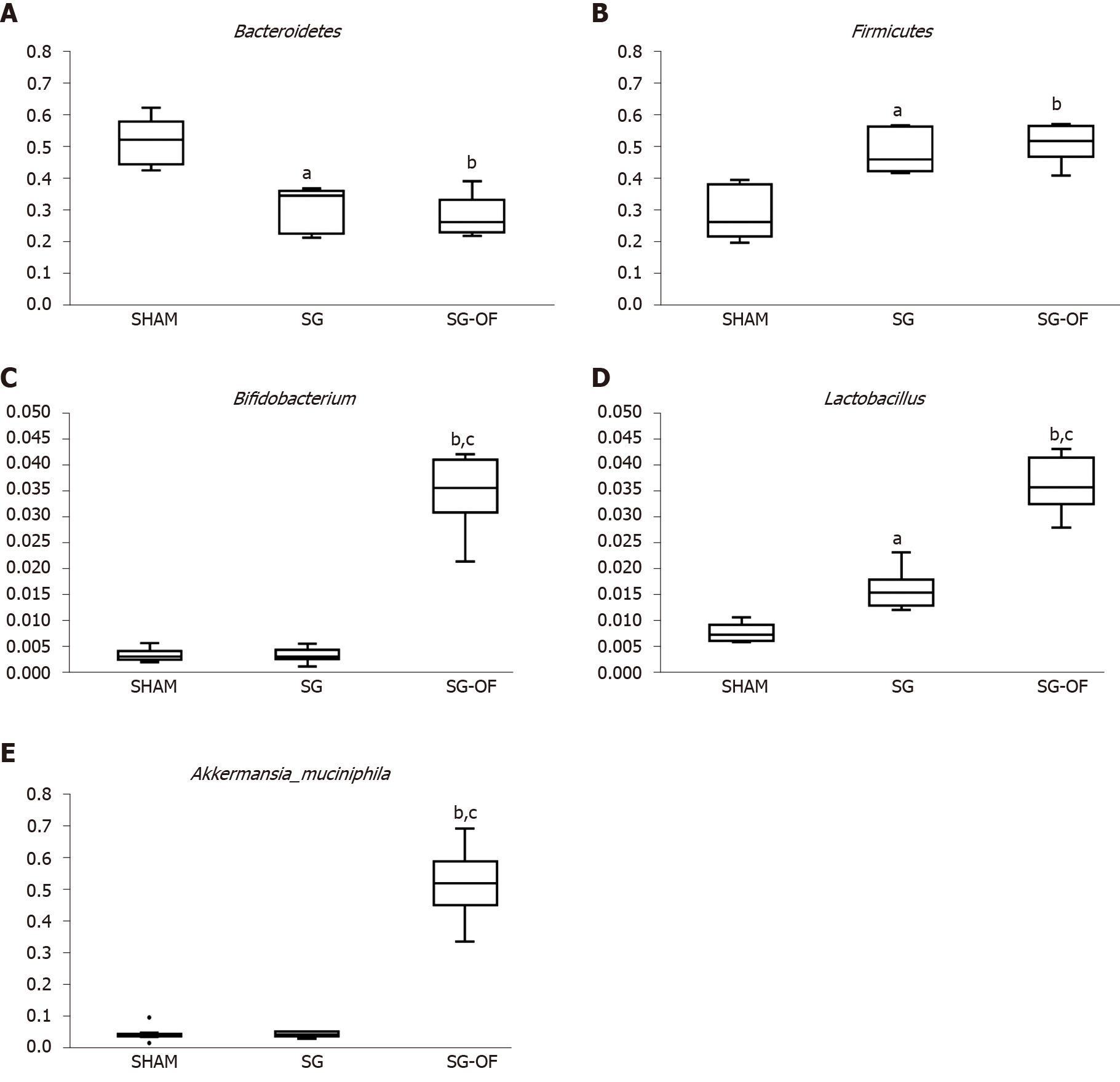
As shown in Figure 5A and B, Bacteroidetes were the predominant gut microbes in the SHAM group while firmicutes were dominant in the SG and SG-OF groups. The relative abundance of Bifidobacterium (Figure 5C), Lactobacillus (Figure 5D), and Akkermansia muciniphila (Figure 5E) in the SG-OF group was significantly higher than that in the SHAM and SG groups. There was no difference in relative abundance of Bifidobacterium and Akkermansia muciniphila between the SHAM group and SG group. The SG group showed a significantly higher relative abundance of lactobacillus than the SHAM group.
Compared with nonsurgical treatment, bariatric surgery has achieved a higher remission rate of T2DM[2,29]. In addition, SG is characterized by faster operation, fewer technical requirements, lower complication rate, and fewer postoperative nutritional problems[8]. In recent years, SG has been performed more frequently than RYGB in the United States/Canada and in Asian/Pacific regions[3]. However, as a novel surgical approach, the long-term effect of SG on diabetes is questionable. Suboptimal loss and regain of body weight are associated with noncompliant dietary and lifestyle habits[30]. Suboptimal loss and regain of body weight have been demonstrated as the factors for insufficient control of diabetes following bariatric surgery[31,32]. Prebiotics have been considered as an effective management tool to promote body weight loss and improve metabolism of glucose and lipid[11-14]. As shown in our previous study, HFD reverses the improvements in diabetes after bariatric surgery[21]. In the present study, we conducted SG on diabetic rats, and stimulated the reversion of improvements in diabetes with sustained postoperative HFD feeding. Meanwhile, a portion of the SG rats were provided with a specific HFD supplemented with 10% oligofructose (one kind of prebiotic) to explore whether oligofructose prevents the reversion of improvements in diabetes after SG.
In this study, at 2 wk after surgery, the AUCOGTT of the SG rats was significantly lower than that of the SHAM rats. Following postoperative HFD feeding, the AUCOGTT of the SG rats showed a gradually increasing trend. At 24 wk after surgery, the AUCOGTT of the SG group was significantly higher than that of the SG-OF group. The changing trends of insulin, HOMA-IR, triglyceride, and cholesterol levels were similar to that of the AUCOGTT. Among these factors, the difference in insulin secretion during the OGTT and triglyceride levels (at 12 wk after surgery) revealed an earlier appearance than others (at 24 wk after surgery), suggesting that, at the early stage after surgery, SG improves the metabolism of glucose and lipid obviously; postoperative HFD feeding reverses these metabolic improvements, and oligofructose feeding partially prevents the detrimental effect of HFD feeding. Factors such as body weight, calorie intake, GLP-1 levels, serum TBA levels, LPS levels, and the gut microbiota were measured to elucidate the possible mechanisms underlying the protective effect of oligofructose.
The body weight and calorie intake in the SG group were obviously lower than those in the SHAM group at all postoperative time points, and a lower body weight and calorie intake were observed in the SG-OF group but not in the SG group beginning at 12 wk after surgery. Body weight and calorie intake seem to be one of primary factors contributing to diabetes[20], so we hold the opinion that lower body weight and calorie intake may be one of the mechanisms for the protective effect of SG and oligofructose.
As one of the most important incretin hormones, GLP-1 is secreted by L-cells that are mainly located in the epithelium of the distal ileum and colon[33]. It regulates glucose homeostasis by stimulating insulin secretion, suppressing glucagon secretion, and promoting proliferation and inhibiting apoptosis of β cells[34]. In our study, a greater amount of GLP-1 was secreted in rats subjected to SG during the OGTT than in rats in the SHAM group at all postoperative time points, and a higher level of GLP-1 secretion in the SG-OF group than in the SG group was observed beginning at 12 wk after surgery. GLP-1 has been considered a mediator in the remission of diabetes after SG[35], and oligofructose has been demonstrated as the promoter for the production of endogenous GLP-1[36]. Based on these findings, we have deduced that the function of oligofructose for improving glucose metabolism after SG is partially attributed to the enhanced secretion of GLP-1. The mechanisms for the enhanced secretion of GLP-1 after oligofructose feeding may be due to the increased number of L-cells and colonic fermentation[36,37]. Short-chain fatty acids [(SCFAs); butyrate, propionate, and acetate)] are produced through the fermentation of nondigestible carbohydrates in the colon, and it has been reported that the oral administration of butyrate and propionate in mice can significantly increase plasma levels of GLP-1, thus leading to an improvement in insulin sensitivity[38], which is consistent with another report showing that SCFAs stimulate free fatty acid receptor 2 that is colocalized in L-cells to increase the secretion of GLP-1[39]. Unfortunately, SCFAs were not determined in our study.
Bile acids can act as signaling molecules to regulate the metabolism of lipid, glucose, and energy, and these regulatory functions of bile acids are predominantly mediated by farnesoid X receptor and the G-protein-coupled receptor TGR5[40]. In this study, at 2 wk after surgery, fasting serum TBA of the rats subjected to SG was significantly higher than that in the SHAM group. This result is coincident with the previous report in human[41], showing that the increased TBA levels contribute to the improvements in the metabolism of glucose and lipid after SG. However, we did not observe a significant difference in TBA levels between the SG group and SG-OF group at 12 and 24 wk after surgery, indicating that the protective effect of oligofructose may be independent of the increased TBA levels.
LPS is a component of the cell wall in Gram-negative intestinal microbiota, which triggers low-grade inflammation and results in insulin resistance. A chronic infusion of LPS over a 4-wk period in chow-fed mice leads to increased adiposity, increased macrophage infiltration in adipose tissues, hepatic inflammation, and hepatic insulin resistance[42]. Clinical trials have shown that SG decreases LPS levels[43], consistent with the outcome reported in our study. What is more, we found that LPS levels in the SG-OF group were lower than those in the SG group at 12 and 24 wk after surgery. A similar effect of oligofructose has also been observed in obese mice without surgery[15]. Therefore, we consider that the protective effect of oligofructose might partially be ascribed to the reduced LPS level.
The gut microbiota has been verified as an environmental factor affecting obesity and diabetes, and can modulate the metabolism of host glucose and lipid[44]. A recent study has revealed a significantly decreased Firmicutes abundance in T2DM patients when compared with nondiabetic individuals[45]. Meanwhile, a remarkably higher relative abundance of Firmicutes and a lower relative abundance of Bacteroidetes in rats receiving SG were observed. These results are consistent with the report from Ryan et al[46]. However, no difference in the relative abundances of Firmicutes and Bacteroidetes between the SG group and SG-OF group were observed, indicating that the benefit of oligofructose is not associated with the relative abundance of Firmicutes and Bacteroidetes. Notably, the rats from the SG-OF group had remarkably higher relative abundances of Bifidobacterium, Lactobacillus, and Akkermansia muciniphila. And the increased relative abundance of these bacteria has been observed in oligofructose-fed diabetic rodents by other research groups[19,47]. The advantage of oligofructose in the control of diabetes may be associated with an improvement in inflammation. Therefore, the effect of oligofructose in protecting the recurrence of diabetes after SG may be partially mediated by the increased abundance of Bifidobacterium, Lactobacillus and Akkermansia muciniphila, accompanied by the decrease in the abundance of Bacteroides, thus reducing the concentration of LPS.
There are still some limitations in this study. First, although oligofructose partially prevented the HFD-induced glucose and lipid metabolism damage after SG in rats, further clinical studies are highly desired to validate these results in humans. Second, the effects of oligofructose on calorie intake, insulin levels, GLP-1 levels, TBA levels, LPS levels, and the gut microbiota after SG were analyzed in this study; however, the intrinsic mechanism underlying the protective function of oligofructose should be further explored. We plan to further study the internal mechanism of these changes, especially focusing on the changes of the gut microbiota. At the same time, we are applying for relevant clinical trials to further observe the effects and side effects of probiotics, and we hope that oligofructose will become a novel target to reduce or delay the recurrence of diabetes after bariatric surgery.
Oligofructose can partially prevent the HFD-induced glucose and lipid metabolism damage in rats after SG, and the effects of oligofructose on calorie intake, insulin levels, GLP-1 levels, LPS levels, and the gut microbiota may contribute to this protective function.
Type 2 diabetes mellitus is one of the important complications of obesity. Bariatric surgery can treat obesity and diabetes effectively, but the recurrence of diabetes after surgery is still one of the problems to be solved. However, whether oligofructose has an effect on metabolism after bariatric surgery remains to be further studied.
Prebiotics promote the weight loss and improve the metabolism of glucose and lipid. The mechanism of these benefits may be due to reduced energy intake, regulation of gut microbiota, improvement of low-grade inflammation, and increase of intestinal hormones, such as glucagon like peptide-1 (GLP-1) and peptide YY, which prompted us to explore whether prebiotics can reduce or delay the recurrence of diabetes after surgery.
The present study aimed to find the effect and mechanism of oligofructose on diabetic remission in diabetic rats after sleeve gastrectomy (SG).
SG and SHAM operation were performed on diabetes rats induced with a high-fat diet (HFD), nicotinamide, and low-dose streptozotocin. Then the rats in SHAM and SG groups were continuously provided with the HFD, and the rats in the SG-oligofructose group were provided with a specific HFD containing 10% oligofructose. Body weight, calorie intake, oral glucose tolerance test, homeostasis model assessment of insulin resistance, lipid profile, serum insulin, GLP-1, total bile acids, lipopolysaccharide (LPS), and colonic microbiota levels were determined and compared at the designated time points. All statistical analyses was performed using Solutionsstatistical Package for the Social Sciences version 19.0 (IBM, United States), and the statistically significant difference was considered at P < 0.05.
Oligofructose treatment reduced body weight, energy intake, and LPS levels, increased GLP-1 levels, and improved insulin resistant and lipid profiles. Oligofructose also altered the gut microbiota in the SG-oligofructose group.
Oligofructose partially prevents HFD-induced glucose and lipid metabolism damage after SG in rats, and the effects of oligofructose on calorie intake, insulin, GLP-1, LPS, and gut microbiota may contribute to protective function for damaged metabolism.
The results of this study provide evidence that oligofructose could be a promising agent for the treatment in diabetic rats after SG. Further studies that assess the effect of oligofructose on the mechanism of preventing HFD-induced glucose and lipid metabolism damage after SG may substantiate our findings and pave the path for clinical translation of the therapeutic effects of oligofructose.
The authors would like to acknowledge Liu SZ, Wang KX, and Sun D for their skillful technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee MW, Sharma M S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Buchwald H, Buchwald JN. Metabolic (Bariatric and Nonbariatric) Surgery for Type 2 Diabetes: A Personal Perspective Review. Diabetes Care. 2019;42:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden Å, Bouchard C, Carlsson B, Karason K, Lönroth H, Näslund I, Sjöström E, Taube M, Wedel H, Svensson PA, Sjöholm K, Carlsson LM. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297-2304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 743] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 3. | Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27:2279-2289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 580] [Cited by in RCA: 550] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 4. | Aminian A, Vidal J, Salminen P, Still CD, Nor Hanipah Z, Sharma G, Tu C, Wood GC, Ibarzabal A, Jimenez A, Brethauer SA, Schauer PR, Mahawar K. Late Relapse of Diabetes After Bariatric Surgery: Not Rare, but Not a Failure. Diabetes Care. 2020;43:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 5. | Aminian A, Brethauer SA, Andalib A, Punchai S, Mackey J, Rodriguez J, Rogula T, Kroh M, Schauer PR. Can Sleeve Gastrectomy "Cure" Diabetes? Ann Surg. 2016;264:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | McTigue KM, Wellman R, Nauman E, Anau J, Coley RY, Odor A, Tice J, Coleman KJ, Courcoulas A, Pardee RE, Toh S, Janning CD, Williams N, Cook A, Sturtevant JL, Horgan C, Arterburn D; PCORnet Bariatric Study Collaborative. Comparing the 5-Year Diabetes Outcomes of Sleeve Gastrectomy and Gastric Bypass: The National Patient-Centered Clinical Research Network (PCORNet) Bariatric Study. JAMA Surg. 2020;155:e200087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 7. | Toh BC, Chan WH, Eng AKH, Lim EKW, Lim CH, Tham KW, Fook-Chong S, Tan JTH. Five-year long-term clinical outcome after bariatric metabolic surgery: A multi-ethnic Asian population in Singapore. Diabetes Obes Metab. 2018;20:1762-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Gagner M. Effect of sleeve gastrectomy on type 2 diabetes as an alternative to Roux-en-Y gastric bypass: a better long-term strategy. Surg Obes Relat Dis. 2015;11:1280-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Cutolo PP, Nosso G, Vitolo G, Brancato V, Capaldo B, Angrisani L. Clinical efficacy of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass in obese type 2 diabetic patients: a retrospective comparison. Obes Surg. 2012;22:1535-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Cho JM, Kim HJ, Lo Menzo E, Park S, Szomstein S, Rosenthal RJ. Effect of sleeve gastrectomy on type 2 diabetes as an alternative treatment modality to Roux-en-Y gastric bypass: systemic review and meta-analysis. Surg Obes Relat Dis. 2015;11:1273-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Mischke M, Arora T, Tims S, Engels E, Sommer N, van Limpt K, Baars A, Oozeer R, Oosting A, Bäckhed F, Knol J. Specific synbiotics in early life protect against diet-induced obesity in adult mice. Diabetes Obes Metab. 2018;20:1408-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Angelino D, Martina A, Rosi A, Veronesi L, Antonini M, Mennella I, Vitaglione P, Grioni S, Brighenti F, Zavaroni I, Fares C, Torriani S, Pellegrini N. Glucose- and Lipid-Related Biomarkers Are Affected in Healthy Obese or Hyperglycemic Adults Consuming a Whole-Grain Pasta Enriched in Prebiotics and Probiotics: A 12-Week Randomized Controlled Trial. J Nutr. 2019;149:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Trautwein EA, Rieckhoff D, Erbersdobler HF. Dietary inulin lowers plasma cholesterol and triacylglycerol and alters biliary bile acid profile in hamsters. J Nutr. 1998;128:1937-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 15. | van der Beek CM, Canfora EE, Kip AM, Gorissen SHM, Olde Damink SWM, van Eijk HM, Holst JJ, Blaak EE, Dejong CHC, Lenaerts K. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism. 2018;87:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 16. | Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev. 2018;31:35-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 17. | Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 592] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 19. | Calikoglu F, Barbaros U, Uzum AK, Tutuncu Y, Satman I. The Metabolic Effects of Pre-probiotic Supplementation After Roux-en-Y Gastric Bypass (RYGB) Surgery: a Prospective, Randomized Controlled Study. Obes Surg. 2021;31:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3146] [Cited by in RCA: 3641] [Article Influence: 202.3] [Reference Citation Analysis (0)] |
| 21. | Liu SZ, Sun D, Zhang GY, Wang L, Liu T, Sun Y, Li MX, Hu SY. A high-fat diet reverses improvement in glucose tolerance induced by duodenal-jejunal bypass in type 2 diabetic rats. Chin Med J (Engl). 2012;125:912-919. [PubMed] |
| 22. | Wu D, Yan ZB, Cheng YG, Zhong MW, Liu SZ, Zhang GY, Hu SY. Deactivation of the NLRP3 inflammasome in infiltrating macrophages by duodenal-jejunal bypass surgery mediates improvement of beta cell function in type 2 diabetes. Metabolism. 2018;81:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Côté CD, Rasmussen BA, Duca FA, Zadeh-Tahmasebi M, Baur JA, Daljeet M, Breen DM, Filippi BM, Lam TK. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat Med. 2015;21:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 24. | Duca FA, Côté CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 328] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 25. | Sun D, Liu S, Zhang G, Chen W, Yan Z, Hu S. Type 2 diabetes control in a nonobese rat model using sleeve gastrectomy with duodenal-jejunal bypass (SGDJB). Obes Surg. 2012;22:1865-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Bruinsma BG, Uygun K, Yarmush ML, Saeidi N. Surgical models of Roux-en-Y gastric bypass surgery and sleeve gastrectomy in rats and mice. Nat Protoc. 2015;10:495-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24517] [Article Influence: 612.9] [Reference Citation Analysis (1)] |
| 28. | Salonen A, Nikkilä J, Jalanka-Tuovinen J, Immonen O, Rajilić-Stojanović M, Kekkonen RA, Palva A, de Vos WM. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J Microbiol Methods. 2010;81:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 424] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 29. | Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery vs non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 940] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 30. | Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 570] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 31. | Belligoli A, Bettini S, Segato G, Busetto L. Predicting Responses to Bariatric and Metabolic Surgery. Curr Obes Rep. 2020;9:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C; Endocrine Society. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95:4823-4843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 321] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 33. | Stemmer K, Finan B, DiMarchi RD, Tschöp MH, Müller TD. Insights into incretin-based therapies for treatment of diabetic dyslipidemia. Adv Drug Deliv Rev. 2020;159:34-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 445] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 35. | Madsbad S, Holst JJ. GLP-1 as a mediator in the remission of type 2 diabetes after gastric bypass and sleeve gastrectomy surgery. Diabetes. 2014;63:3172-3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Verhoef SP, Meyer D, Westerterp KR. Effects of oligofructose on appetite profile, glucagon-like peptide 1 and peptide YY3-36 concentrations and energy intake. Br J Nutr. 2011;106:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Piche T, des Varannes SB, Sacher-Huvelin S, Holst JJ, Cuber JC, Galmiche JP. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology. 2003;124:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Lin HV, Frassetto A, Kowalik EJ Jr, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G, Marsh DJ. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 760] [Cited by in RCA: 926] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 39. | Kaji I, Karaki S, Tanaka R, Kuwahara A. Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J Mol Histol. 2011;42:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 696] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 41. | Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 42. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4564] [Article Influence: 253.6] [Reference Citation Analysis (1)] |
| 43. | Clemente-Postigo M, Roca-Rodriguez Mdel M, Camargo A, Ocaña-Wilhelmi L, Cardona F, Tinahones FJ. Lipopolysaccharide and lipopolysaccharide-binding protein levels and their relationship to early metabolic improvement after bariatric surgery. Surg Obes Relat Dis. 2015;11:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Meijnikman AS, Gerdes VE, Nieuwdorp M, Herrema H. Evaluating Causality of Gut Microbiota in Obesity and Diabetes in Humans. Endocr Rev. 2018;39:133-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 45. | Barlow GM, Yu A, Mathur R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr Clin Pract. 2015;30:787-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 46. | Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 694] [Cited by in RCA: 749] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 47. | Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066-9071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2639] [Cited by in RCA: 3316] [Article Influence: 276.3] [Reference Citation Analysis (0)] |