Published online Dec 15, 2021. doi: 10.4239/wjd.v12.i12.2050
Peer-review started: July 7, 2021
First decision: July 28, 2021
Revised: August 9, 2021
Accepted: December 8, 2021
Article in press: December 8, 2021
Published online: December 15, 2021
Processing time: 161 Days and 21.5 Hours
Diabetic retinopathy (DR) is a serious and potentially blinding complication of diabetes mellitus. Retinal neovascularization is one of the main pathological features of proliferative DR, and inhibiting retinal neovascularization is a research focus.
The aim was to evaluate the effect of intravitreal injection of recombinant human maspin on neovascularization in DR.
An oxygen-induced retinopathy (OIR) mouse model was used to simulate neovascularization in DR. New born C57BL/6J mice were randomly divided to a normal control group, a maspin injection OIR group, and an OIR group. The mice in the maspin injection OIR group were injected with recombinant human maspin in the bilateral vitreous cavity on postnatal day P12, and those in the OIR group were injected with sterile phosphate buffered saline. The protein expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1-alpha (HIF-1α) in the retina was measured by western blotting, and the mRNA expression of VEGF and HIF-1α was measured by real-time polymerase chain reaction. The vascular cell nuclei that broke through the inner limiting membrane (ILM) were counted in haematoxylin-eosin stained retinal sections.
It was found that the number of vascular cell nuclei breaking through the ILM was 31.8 ± 8.75 in the OIR group, which was significantly more than that in the normal control group (P < 0.001). The number of vascular cell nuclei breaking through the ILM was 6.19 ± 2.91 in the maspin injection OIR group, which was significantly less than that in OIR group (P < 0.01). The relative protein and mRNA expression of VEGF and HIF-1α was significantly lower in the retinas in the maspin injection OIR group than in those in the OIR group (P < 0.01).
Maspin inhibited neovascularization in DR by modulating the HIF-1α/VEGF pathway, which provides a potential and effective strategy for the treatment of DR.
Core Tip: The aim of our study was to evaluate the effectiveness of intravitreal injection of recombinant human maspin on neovascularization in diabetic retinopathy. A mouse model of oxygen-induced retinopathy was used to simulate neovascularization in diabetic retinopathy. Maspin inhibited neovascularization in this model by modulating the hypoxia-inducible factor 1-alpha/vascular endothelial growth factor pathway, which provides a potential and effective strategy for the treatment of diabetic retinopathy.
- Citation: Qiu F, Tong HJ. Inhibitory effect of maspinon neovascularization in diabetic retinopathy. World J Diabetes 2021; 12(12): 2050-2057
- URL: https://www.wjgnet.com/1948-9358/full/v12/i12/2050.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i12.2050
Diabetic retinopathy (DR) is a serious and potentially blinding complication of diabetes mellitus[1]. The prevalence of DR in patients with diabetes is 34.6% worldwide[2]. The global prevalence of diabetes is estimated to be 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045[3], and the prevalence of DR is expected to rise accordingly. DR progresses in stages from a non-proliferative to a more vision-threatening proliferative (PDR) form. Retinal neovascularization is a key pathological feature of PDR, and inhibiting retinal neovascularization is a research focus[4].
Maspin is a member of the serine protease inhibitor (serpin)family. Studies have shown that maspin, which is a class II tumour suppressor gene, can induce tumour cell apoptosis, reduce the movement of tumour cells, and increase adhesion to inhibit tumour invasion and metastasis. Maspin can also directly induce endothelial cell apoptosis and inhibit the endothelial cell signalling pathway to inhibit the development of tumour angiogenesis. Recombinant maspin inhibits corneal endothelial neovascularization by inhibiting the expression of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor[5]. Recombinant maspin was found to inhibit tumour growth and angiogenesis in an animal model of prostate cancer[6]. Injection of adenovirus carrying maspin into the left ventricle was found to disrupt angiogenesis in developing and mature tumours[7]. Maspin is highly expressed during keratinocyte senescence and has antiangiogenic activity[8]. Maspin-mimetic nanostructures can inhibit angiogenesis in tubulogenesis assays with human umbilical vein endothelial cells and in vivo assays in the chick chorioallantoic membrane[9].
The mouse model of oxygen-induced retinopathy (OIR) has much in common with human ischemic retinopathy and effectively simulates retinal neovascularization in vivo. The model is widely used to study neovascularization in DR[10,11]. At present, the effect of maspin on retinal neovascularization in animal models is not clear. In this study, we investigated whether maspin could inhibit retinal neovascularization in a mouse OIR model. Through the results of this study, we hope to find agents that inhibit neovascularization in DR and provide a theoretical basis for clinical treatments.
All mouse procedures were performed following the guidelines of the Chinese Ministry of Science and Technology Guidelines on the Humane Treatment of Laboratory Animals.
New born C57BL/6J mice were randomly divided into three groups of 25 each, a normal control group, a maspin injection OIR group, and an OIR group. The mice were housed in a specific pathogen free animal laboratory. On postnatal day P17, 10 mice (20 eyes) were randomly selected from each group for haematoxylin-eosin (HE) staining and 15 mice (30 eyes) were selected for RNA extraction from the left eye for real-time polymerase chain reaction (PCR) and protein extraction from the right eye for western blotting.
The mouse OIR model was established as previous described[12]. On day P7 new-born C57BL/6J mice and their mothers were transferred to a constant hyper oxygen chamber with a volume fraction of 75% ± 2% and were then returned to normal air on day 12. Mice in the normal control group were housed in normal air.
On day 12, 0.5 µL of 0.05 mg/mL recombinant human maspin was injected into the vitreous cavities of mice in the maspin injection OIR group mice with a microsyringe (Hamilton Company, Reno, NV, United States). In the OIR group, 0.5 µL of sterile phosphate buffered saline was injected.
Ten mice (20 eyes) in each group were used to prepare tissue sections of eyeball specimens. The eyeballs were fixed in 4% paraformaldehyde for 24 h and embedded in paraffin. Sagittal sections parallel to the cornea and optic disc were prepared at a thickness of 6 µm. One of every six sections was selected at random; five sections were randomly selected from each eye. The sections were dewaxed and rehydrated in a graduated ethanol series for HE staining. The number of vascular cell nuclei that broke through the inner limiting membrane (ILM) was counted with an optical microscope, and the average number that broke through the ILM in each section was calculated. Vascular cell nuclei in the vitreous cavity that were not associated with the ILM were not counted).
Mouse retinal tissue was lysed in RIPA lysis buffer (Beyotime, Jiangsu, China) and the concentration of the extracted protein was determined with a bicinchoninic acid protein assay kit (Beyotime). Forty micrograms of total protein extract from each sample was separated by 5%–14% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and electrophoretically transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, United States). The membranes were incubated with primary antibodies (anti-VEGF, 1:500 dilution, BIOSS, Beijing, China; anti-HIF-1α, 1:500 dilution, Proteintech, Rosemont, IL, United States). Proteins were analysed with an enhanced chemiluminescence kit (Beyotime). β-actin served as an internal control.
mRNA was extracted from mouse retinal tissue with RNA pure total RNA extraction kits (Bioteke, Jiangsu, China). cDNA was generated with super M-MLV transcriptase (Bioteke). The primers were as follows: HIF-1α, forward: 5′-AGT GTACCC TAA CTA GCC GA-3′, reverse: 5′-CAC AAA TCAGCA CCA AGC -3′; VEGF, forward: 5′-ACA CACCCA CCC ACA TAC ATA-3′, reverse: 5′-ACT CAA GTCCAC AGC AGT CAA-3′. Relative expression of VEGF and HIF-1α mRNAs was calculated by the comparative cycle threshold method. β-actin served as an internal control.
The results were reported as means ± SD. All assays were repeated at least three times. SPSS software (Version 20.0, IBM, Armonk, NY, United States) was used for the statistical analysis. Between-group differences were compared by analysis of variance, and P value < 0.05 were considered to be statistically significant.
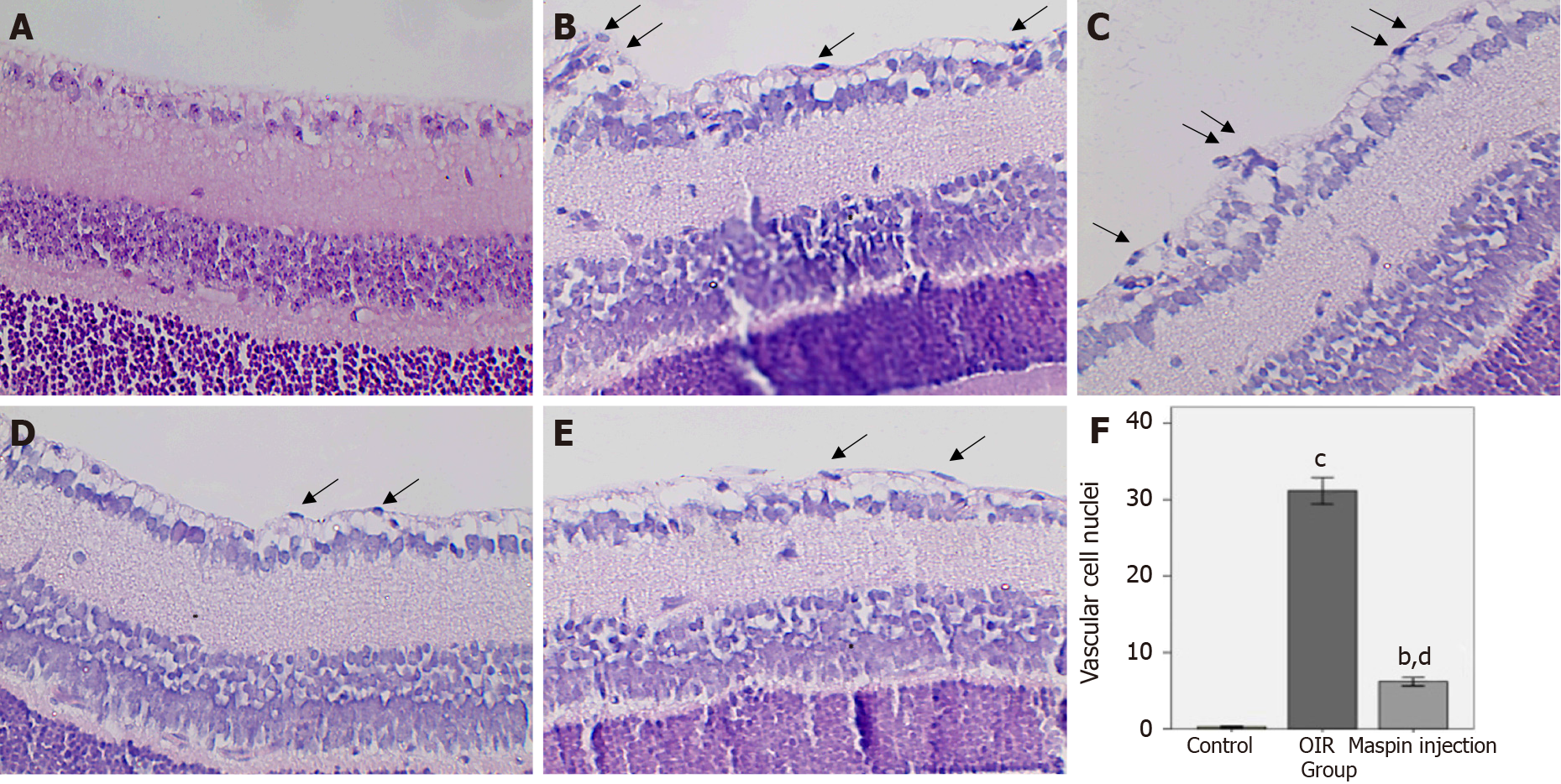
In the normal control group, the ILM was smooth, with vascular cell nuclei breaking through the ILM in only a few places (Figure 1A). Many pathologic neovascular tufts broke through the ILM in the OIR group (Figure 1B and C). The number of vascular cell nuclei in the maspin injection OIR group was significantly decreased compared with that in the OIR group (Figure 1D and E). In the normal control group, 0.52 ± 0.10 vascular cell nuclei broke through the ILM in each tissue section. The number that broke through the ILM in the OIR group was 31.8 ± 8.75, which was significantly higher than that in the normal control group (P < 0.001). In the maspin injection OIR group, 6.19 ± 2.91 vascular cell nuclei broke through the ILM in each tissue section, which was significantly less than that in OIR group (P < 0.01) and more than that in the normal control group (P < 0.01; Figure 1F).
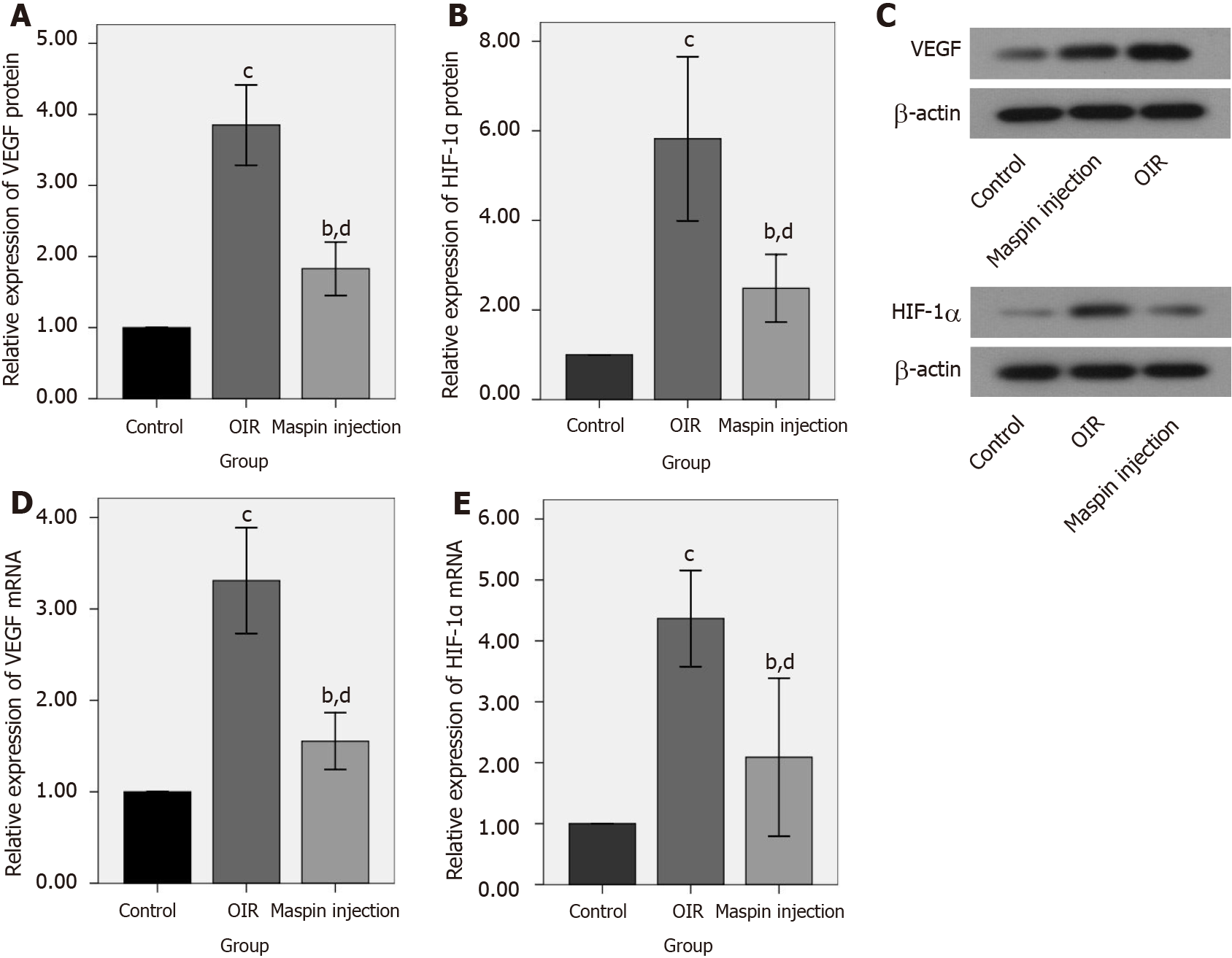
The western blot results found that relative protein expression of VEGF and HIF-1α in retinas from the OIR group was significantly higher than that in the normal control group (both, P < 0.001). The expression of VEGF and HIF-1α protein in retinas from the maspin injection OIR group was significantly lower than that in the OIR group (P < 0.01), and higher than that in the normal control group (P < 0.01; Figure 2A, B, and C).
The relative mRNA expression of VEGF and HIF-1α mRNA in retinas from the OIR group was significantly higher than that in the normal control group (both P < 0.001). The relative mRNA expression of VEGF and HIF-1α mRNA in retinas from the maspin injection OIR group was significantly lower than that in the OIR group (P < 0.01), and higher than that in the normal control group (P < 0.01; Figure 2D and E).
Our previous studies showed that maspin inhibited high glucose-induced angiogenesis in human retinal microvascular endothelial cells[13]. The inhibitory effect of maspin on retinal neovascularization in vivo has not been reported. Intravitreal injection is the main method of studying and treating DR[4,14-16]. The mouse OIR model is widely used to simulate neovascularization and to study the prevention of neovascularization in ischemic retinal diseases such as DR. The retinal blood vessels of 7-d-old mice are not yet mature. Inhalation of high concentration oxygen stimulates retinal blood vessels to undergo reversible spasmodic changes[17]. Continuous hyperoxia causes small vessel occlusion and areas of retinal nonperfusion. After the mice returned to normal air on day P12, the central avascular retina was in a state of hypoxia, leading to both normal vessel regrowth and the formation of extraretinal pathological neovascularization[12,18]. On day P17, retinal neovascularization reached the most advanced stage[19-22]. The model shares many characteristics with DR, and the number of neovascular sites can be measured by counting the nuclei that break through the ILM in HE-stained retinal tissue sections[19,21,22]. We found that significantly fewer nuclei broke through the ILM in the maspin injection OIR group than in the OIR group, indicating that maspin inhibited the development of neovascularization in the DR model.
VEGF promotes the division and proliferation of vascular endothelial cells and increases vascular permeability[23] and is a key angiogenic factor that induces retinal neovascularization[24]. Clinical studies have found that inhibiting VEGF effectively inhibited retinal neovascularization; anti-VEGF therapy has become the main method of treating DR and other retinal neovascular conditions[25-27]. Under hypoxic conditions, HIF-1α is produced in the nucleus and binds to the HIF-1α binding site on the target gene to initiate transcription and promote angiogenesis[28]. HIF-1α can regulate the expression of VEGF, and is active in maintaining energy metabolism and angiogenesis of tumour cells. The activation of VEGF transcription and maintenance of VEGF mRNA stability in hypoxic tissues is mainly regulated by HIF-1α. VEGF is one of the target genes of HIF-1α[29]. HIF-1α and VEGF are abnormally upregulated in PDR, and HIF-1α regulates the expression of VEGF and promotes retinal neovascularization[30-32]. Interfering RNA targeting VEGF and HIF-1a was effective in inhibiting retinal neovascularization[33,34]. Inhibiting VEGF and HIF-1α is an effective method to treat DR[35,36]. Our previous studies showed that maspin could inhibit HIF-1α and VEGF expression in HG-treated human retinal microvascular endothelial cells. In this study, the retinal expression of VEGF and HIF-1α in was significantly lower in the maspin injection OIR group than that in the OIR group, suggesting that maspin may inhibit neovascularization in DR by modulating the HIF-1α/VEGF pathway. In our study, we observed the inhibitory effect of one dose of recombinant human maspin on retinal neovascularization in OIR on day 17. The effect of different doses of maspin on retinal neovascularization in OIR at different times is planned in future research.
In conclusion, our study showed that maspin inhibited neovascularization in DR by modulating the HIF-1α/VEGF pathway, providing a potential and effective strategy for the treatment of DR.
Diabetic retinopathy (DR) is a serious and potentially blinding complication of diabetes mellitus.
We used an experimental animal model to find a more effective strategy for the treatment of DR.
The study aim was to evaluate the effect of intravitreal injection of recombinant human maspin on neovascularization in DR.
An oxygen-induced retinopathy (OIR) model in mice was used to simulate neovascularization in diabetic retinopathy. On postnatal day P12, 0.5 µL of 0.05 mg/mL recombinant human maspin was injected into the vitreous cavity of maspin injection OIR group mice. The protein and mRNA expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1-alpha (HIF-1α) in the retina were assayed. The numbers of vascular cell nuclei that broke through the inner limiting membrane were counted.
The results revealed that intravitreal injection of maspin inhibited neovascularization and reduced protein and mRNA expression of VEGF, HIF-1α in the retinal tissue of OIR model mice.
Maspin inhibited neovascularization of DR by modulating the VEGF/HIF-1α pathway, providing a potential and effective strategy for the treatment of DR.
Retinal neovascularization is one of the main pathological features of PDR. Inhibiting retinal neovascularization is a research focus.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karalliedde J, Ortega AL, Yoon S S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes. 2015;6:489-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 281] [Cited by in RCA: 317] [Article Influence: 31.7] [Reference Citation Analysis (4)] |
| 2. | Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3524] [Cited by in RCA: 3102] [Article Influence: 238.6] [Reference Citation Analysis (3)] |
| 3. | Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5345] [Cited by in RCA: 5895] [Article Influence: 982.5] [Reference Citation Analysis (8)] |
| 4. | Stewart MW. Treatment of diabetic retinopathy: Recent advances and unresolved challenges. World J Diabetes. 2016;7:333-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 117] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (2)] |
| 5. | Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 323] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Cher ML, Biliran HR Jr, Bhagat S, Meng Y, Che M, Lockett J, Abrams J, Fridman R, Zachareas M, Sheng S. Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis. Proc Natl Acad Sci U S A. 2003;100:7847-7852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Li Z, Shi HY, Zhang M. Targeted expression of maspin in tumor vasculatures induces endothelial cell apoptosis. Oncogene. 2005;24:2008-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Nickoloff BJ, Lingen MW, Chang BD, Shen M, Swift M, Curry J, Bacon P, Bodner B, Roninson IB. Tumor suppressor maspin is up-regulated during keratinocyte senescence, exerting a paracrine antiangiogenic activity. Cancer Res. 2004;64:2956-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Zha RH, Sur S, Boekhoven J, Shi HY, Zhang M, Stupp SI. Supramolecular assembly of multifunctional maspin-mimetic nanostructures as a potent peptide-based angiogenesis inhibitor. Acta Biomater. 2015;12:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Chen Q, Qiu F, Zhou K, Matlock HG, Takahashi Y, Rajala RVS, Yang Y, Moran E, Ma JX. Pathogenic Role of microRNA-21 in Diabetic Retinopathy Through Downregulation of PPARα. Diabetes. 2017;66:1671-1682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Liu CH, Wang Z, Sun Y, Chen J. Animal models of ocular angiogenesis: from development to pathologies. FASEB J. 2017;31:4665-4681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Vähätupa M, Järvinen TAH, Uusitalo-Järvinen H. Exploration of Oxygen-Induced Retinopathy Model to Discover New Therapeutic Drug Targets in Retinopathies. Front Pharmacol. 2020;11:873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Qiu F, Tong H, Wang Y, Tao J, Wang H, Chen L. Recombinant human maspin inhibits high glucose-induced oxidative stress and angiogenesis of human retinal microvascular endothelial cells via PI3K/AKT pathway. Mol Cell Biochem. 2018;446:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Han N, Xu H, Yu N, Wu Y, Yu L. MiR-203a-3p inhibits retinal angiogenesis and alleviates proliferative diabetic retinopathy in oxygen-induced retinopathy (OIR) rat model via targeting VEGFA and HIF-1α. Clin Exp Pharmacol Physiol. 2020;47:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Su L, Ren X, Wei H, Zhao L, Zhang X, Liu J, Su C, Tan L, Li X. Intravitreal Conbercept (KH902) for Surgical Treatment of Severe Proliferative Diabetic Retinopathy. Retina. 2016;36:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Simunovic MP, Maberley DA. Anti-Vascular Endothelial Growth Factor Therapy for Proliferative Diabetic Retinopathy: A Systematic Review and Meta-Analysis. Retina. 2015;35:1931-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Vähätupa M, Jääskeläinen N, Cerrada-Gimenez M, Thapa R, Järvinen T, Kalesnykas G, Uusitalo-Järvinen H. Oxygen-Induced Retinopathy Model for Ischemic Retinal Diseases in Rodents. J Vis Exp. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Löfqvist C, Hellström A, Smith LE. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813-2826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 499] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 19. | Xu W, Cheng W, Cui X, Xu G. Therapeutic effect against retinal neovascularization in a mouse model of oxygen-induced retinopathy: bone marrow-derived mesenchymal stem cells versus Conbercept. BMC Ophthalmol. 2020;20:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Zhu Y, Zhang L, Lu Q, Gao Y, Cai Y, Sui A, Su T, Shen X, Xie B. Identification of different macrophage subpopulations with distinct activities in a mouse model of oxygen-induced retinopathy. Int J Mol Med. 2017;40:281-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Wang W, Li Z, Sato T, Oshima Y. Tenomodulin inhibits retinal neovascularization in a mouse model of oxygen-induced retinopathy. Int J Mol Sci. 2012;13:15373-15386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Madamanchi A, Capozzi M, Geng L, Li Z, Friedman RD, Dickeson SK, Penn JS, Zutter MM. Mitigation of oxygen-induced retinopathy in α2β1 integrin-deficient mice. Invest Ophthalmol Vis Sci. 2014;55:4338-4347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Carmeliet P, Collen D. Molecular analysis of blood vessel formation and disease. Am J Physiol. 1997;273:H2091-H2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Moravski CJ, Kelly DJ, Cooper ME, Gilbert RE, Bertram JF, Shahinfar S, Skinner SL, Wilkinson-Berka JL. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000;36:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Pham B, Thomas SM, Lillie E, Lee T, Hamid J, Richter T, Janoudi G, Agarwal A, Sharpe JP, Scott A, Warren R, Brahmbhatt R, Macdonald E, Straus SE, Tricco AC. Anti-vascular endothelial growth factor treatment for retinal conditions: a systematic review and meta-analysis. BMJ Open. 2019;9:e022031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Stewart MW. A Review of Ranibizumab for the Treatment of Diabetic Retinopathy. Ophthalmol Ther. 2017;6:33-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Sarwar S, Bakbak B, Sadiq MA, Sepah YJ, Shah SM, Ibrahim M, Do DV, Nguyen QD. Fusion Proteins: Aflibercept (VEGF Trap-Eye). Dev Ophthalmol. 2016;55:282-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Roberts AM, Ohh M. Beyond the hypoxia-inducible factor-centric tumour suppressor model of von Hippel-Lindau. Curr Opin Oncol. 2008;20:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 471] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 30. | Zhang D, Lv FL, Wang GH. Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur Rev Med Pharmacol Sci. 2018;22:5071-5076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 31. | Ling S, Birnbaum Y, Nanhwan MK, Thomas B, Bajaj M, Ye Y. MicroRNA-dependent cross-talk between VEGF and HIF1α in the diabetic retina. Cell Signal. 2013;25:2840-2847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Behl T, Kotwani A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res. 2015;99:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Forooghian F, Das B. Anti-angiogenic effects of ribonucleic acid interference targeting vascular endothelial growth factor and hypoxia-inducible factor-1alpha. Am J Ophthalmol. 2007;144:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Jiang J, Xia XB, Xu HZ, Xiong Y, Song WT, Xiong SQ, Li Y. Inhibition of retinal neovascularization by gene transfer of small interfering RNA targeting HIF-1alpha and VEGF. J Cell Physiol. 2009;218:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Han XX, Guo CM, Li Y, Hui YN. Effects of bevacizumab on the neovascular membrane of proliferative diabetic retinopathy: reduction of endothelial cells and expressions of VEGF and HIF-1α. Mol Vis. 2012;18:1-9. [PubMed] |
| 36. | Wei J, Jiang H, Gao H, Wang G. Blocking Mammalian Target of Rapamycin (mTOR) Attenuates HIF-1α Pathways Engaged-Vascular Endothelial Growth Factor (VEGF) in Diabetic Retinopathy. Cell Physiol Biochem. 2016;40:1570-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |