Published online Jun 15, 2020. doi: 10.4239/wjd.v11.i6.239
Peer-review started: November 7, 2019
First decision: December 12, 2019
Revised: March 30, 2020
Accepted: April 18, 2020
Article in press: April 18, 2020
Published online: June 15, 2020
Processing time: 207 Days and 18.9 Hours
Type I diabetes (T1D) is characterized by insulin loss caused by inflammatory cells that excessively infiltrate and destroy the pancreas, resulting in dysregulation of tissue homeostasis, mechanobiological properties, and the immune response. The streptozotocin (STZ)-induced mouse model exhibits multiple features of human T1D and enables mechanistic analysis of disease progression. However, the relationship between the mechanochemical signaling regulation of STZ-induced T1D and macrophage migration and phagocytosis is unclear.
To study the mechanochemical regulation of STZ-induced macrophage response on pancreatic beta islet cells to gain a clearer understanding of T1D.
We performed experiments using different methods. We stimulated isolated pancreatic beta islet cells with STZ and then tested the macrophage migration and phagocytosis.
In this study, we discovered that the integrin-associated surface factor CD47 played a critical role in immune defense in the STZ-induced T1D model by preventing pancreatic beta islet inflammation. In comparison with healthy mice, STZ-treated mice showed decreased levels of CD47 on islet cells and reduced interaction of CD47 with signal regulatory protein α (SIRPα), which negatively regulates macrophage-mediated phagocytosis. This resulted in weakened islet cell immune defense and promoted macrophage migration and phagocytosis of target inflammatory cells. Moreover, lipopolysaccharide-activated human acute monocytic leukemia THP-1 cells also exhibited enhanced phagocytosis in the STZ-treated islets, and the aggressive attack of the inflammatory islets correlated with impaired CD47-SIRPα interactions. In addition, CD47 overexpression rescued the pre-labeled targeted cells.
This study indicates that CD47 deficiency promotes the migration and phagocytosis of macrophages and provides mechanistic insights into T1D by associating the interactions between membrane structures and inflammatory disease progression.
Core tip: Type I diabetes (T1D) has caused worldwide public health concerns. The mechanochemical regulation of the disease-induced immune response raised more considerations. We provide mechanistic insights into T1D, associating interactions between membrane structures and inflammatory disease progression. The immune response could be a novel section for preventing the T1D progression.
- Citation: Zhang J, Tan SB, Guo ZG. CD47 decline in pancreatic islet cells promotes macrophage-mediated phagocytosis in type I diabetes. World J Diabetes 2020; 11(6): 239-251
- URL: https://www.wjgnet.com/1948-9358/full/v11/i6/239.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i6.239
Infiltration of inflammatory cells into pancreatic islets and selective destruction of insulin-secreting cells are characteristics of type I diabetes[1,2]. A similar process occurs in autoimmune diabetes, as well as in multiple low-dose streptozotocin (STZ)-treated mice[3,4]. Macrophages play a critical role in the development and pathogenesis of autoimmune or inflammatory disease. Macrophage recruitment strongly correlates with the progression of autoimmune diabetes, as these are the first immune cells to infiltrate the pancreatic islet, where they act as antigen-presenting and effector cells[5-9]. To maintain tissue integrity, tissue macrophages must prevent phagocytosis of healthy endogenous cells. By differentiating “self” and “non-self” cells, macrophages exert their phagocytic function. Immunogenic proteins play critical roles in this process. CD47-signal regulatory protein α (SIRPα) interaction-mediated inhibition is a critical mechanism that prevents macrophages from phagocytizing healthy endogenous cells[10-13]. CD47 is an integrin-associated molecule that is an essential marker of ‘‘self’’[14]. The inhibitory receptor SIRPα is an extracellular ligand of CD47. In this mechanism, CD47 acts as a marker of self, along with SIRPα, and inhibits signaling in macrophages related to migration and phagocytosis of abnormal cells or other antigens[13,15]. Through its extracellular IgV-like loops, SIRPα binds to CD47, which induces phosphorylation of the SIRPα immunoreceptor tyrosine-based inhibitory motifs (ITIMs), leading to association with the SH2 domain–containing protein tyrosine phosphatases SHP-1 or SHP-2. Then, negative signaling cascades are initiated, resulting in the inhibition of macrophage function[16,17]. The end result of CD47-SIRPα-mediated signaling is the inhibition of inappropriate phagocytosis of endogenous cells[18,19]. Previous studies have reported the CD47-SIRPα inhibition mechanism in red blood cell (RBC) transfusion experiments, which demonstrated that wild-type mice rapidly eliminate syngeneic CD47-null RBCs through erythrophagocytosis in the spleen. The lack of tyrosine phosphorylation in SIRPα ITIMs was associated with macrophage aggressiveness[20,21]. Later, other experiments showed that CD47-deficient circulating cells were rapidly cleared by splenic macrophages. A lack of this inhibitory signaling promoted blood cell binding to macrophages and was sufficient to trigger a phagocytic signal[22,23]. Consistent with the CD47-SIRPα interaction inhibiting macrophage phagocytosis, studies of cancer pathogenesis indicated that increased CD47 expression commonly occurred on tumor cells and acted as a pathway to evade immunological eradication[24-26]. However, perturbation of the CD47-SIRPα interaction provides opportunities for cancer eradication, especially in conjunction with therapeutic anticancer antibodies[27-31]. Other studies showed that by binding to SIRPα, lung factors, such as surfactant protein A (SP-A) and surfactant protein D (SP-D), act as surveillance molecules to suppress macrophage phagocytic function and lung inflammation[32,33]. Recent research indicated that both the CD47-SIRPα interaction and IL-10 constrain inflammation-induced macrophage phagocytosis of healthy endogenous cells[10]. Multiple low-dose STZ (MLD-STZ) treatment induces infiltration of macrophages in the islets and then leads to insulitis and diabetes[34]. Thus, the core question remains: What is the mechanism that initiates macrophage infiltration and phagocytosis of endogenous cells under inflammatory stimulation? In this mouse model, the results revealed that CD47 expression by pancreatic islet beta cells was reduced, leading to increased interest in discovering whether CD47 downregulation induces macrophage recruitment to pancreatic islets. As a protective factor, CD47 expression in pancreatic islet beta cells prevents macrophage phagocytic function when inflammatory lesions occur[35,36]. Ablating CD47 might be sufficient to contribute to autoimmune disease progression. Enhancing the expression of CD47 might improve the survival of pancreatic islet beta cells in the development of autoimmune diabetes.
The primary antibody against insulin was obtained from Cell Signaling Technology (Boston, CA, United States). Primary antibodies against CD47 and GAPDH were obtained from Santa Cruz BioTechnology (San Diego, CA, United States). The anti-F4/80 primary antibody was purchased from eBioscence (San Diego, CA, United States). STZ was purchased from Sigma. The insulin ELISA kit was purchased from BD Biosciences. Carboxy fluorescein succinimidyl ester (CFSE) was purchased from Invitrogen. RAW264.7 and Min6 cells were purchased from the China Cell Culture Center (Shanghai, China). The cells were cultured in low-glucose RPMI 1640 supplemented with 10% fetal bovine serum (FBS) (Gibco), penicillin, and streptomycin in a water-saturated atmosphere with 5% CO2.
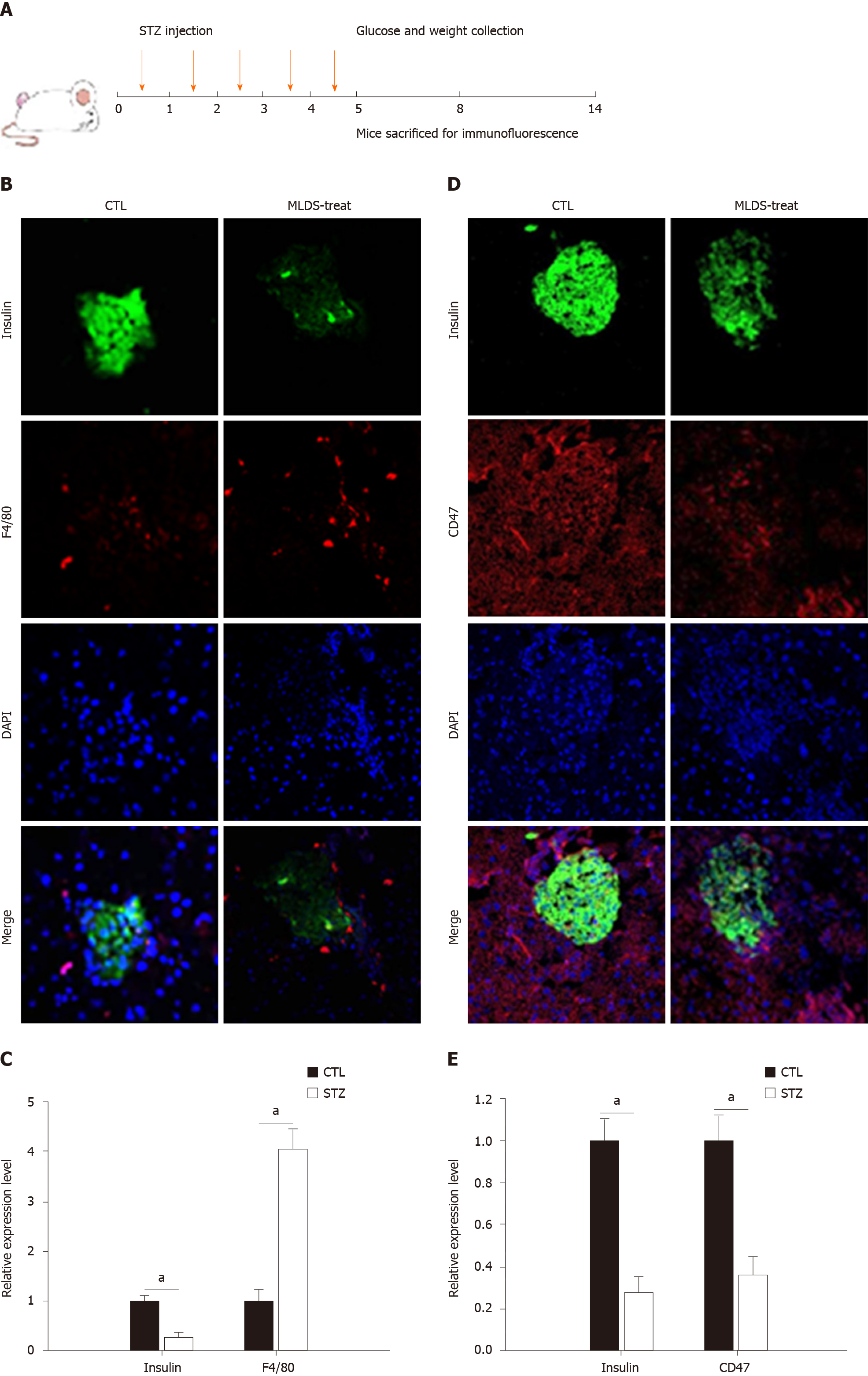
Male C57BL/6J mice (7-8 wk old, weighing 20-25 g) were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China) and were maintained in a pathogen-free animal facility on a 12 h light/dark cycle. STZ-induced murine diabetes was established as previously described. Briefly, to establish diabetes, the mice received five daily intraperitoneal injections of STZ (40 mg/kg body weight) dissolved in citrate buffer, pH 4.5. Control mice were given equal volumes of citrate buffer. The mice were monitored for blood glucose and body weight changes.
To detect pancreatic islet CD47 protein expression and macrophage infiltration, 5 mm sections of frozen pancreas were labeled with anti-insulin, anti-CD47, and anti-F4/80 primary antibodies. The corresponding fluorescent secondary antibodies were used to label the primary antibodies. Fluorescence microscopy was then used for imaging. Min6 cells were labeled with a CD47 primary antibody and subsequently with a secondary FITC antibody. The labeled Min6 cells were detected by flow cytometry to evaluate CD47 expression changes.
Macrophage activation and in vitro phagocytosis assays were performed as previously described. In brief, lipopolysaccharide (LPS) was added to the medium to stimulate macrophage activation for 8 h. Min6 cells, treated with and without STZ, were labeled with CFSE and co-incubated with activated macrophages for 2 h, after which phagocytosis was analyzed by fluorescence microscopy.
The mice were anesthetized with chloral hydrate and euthanized. Pancreatic islets were isolated by collagenase digestion and were hand-picked according to the method described above. The islets were cultured in RPMI 1640 medium containing 5.5 mmol/L glucose and supplemented with 1% penicillin-streptomycin, 10% fetal bovine serum (all from Gibco/BRL, Burlington, ON), and 10 mmol/L HEPES (Sigma). Serum insulin concentrations were assessed using specific insulin ELISA kits according to the manufacturer’s instructions.
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Ethics Committee on the Care and Use of Laboratory Animals of Nanjing Normal University.
Min6 cell lysates were analyzed by Western blot to detect changes in CD47 expression after treatment with STZ. Western blot analysis was conducted using an antibody specific for CD47. The antigen was visualized using an ECL plus detection system (Amersham Pharmacia Biotech). Normalization was performed by probing the same samples with an anti-GAPDH antibody. Student's ttest was used for comparisons.
A diabetic mouse model was established by MLD-STZ treatment as previously described[37]. Seven- to eight-wk-old male C57/BL6 mice received five daily intraperitoneal injections of STZ (40 mg/kg body weight), which was dissolved in citrate buffer (pH 4.5) (Figure 1A). Control animals received equal volumes of citrate buffer. Daily blood samples were taken from the tail vein for glucose detection. Then, any changes in blood glucose were tested with a glucometer. The results revealed that plasma glucose values in STZ-injected mice were significantly elevated after the fourth STZ injection (mean plasma glucose value of 13.6 mmol/L, n = 10) compared to those of the citrate buffer group (mean plasma glucose value of 8.7 mmol/L, n = 10), and a substantially high level was maintained for the subsequent 9 d (Supplementary Figure 1A). Additionally, the body weights were recorded. The control group displayed a normal body weight, while the STZ-treated mice showed slower growth (Supplementary Figure 1B).
To determine macrophage infiltration, pancreatic tissue was fixed and labeled for F4/80[38,39]. As shown in Figure 1B and 1D, a large number of macrophages surrounded and infiltrated the islets, accompanied by reduced insulin secretion in STZ-treated mice. The statistical analysis is shown in Figure 1C and 1D. Significant insulin reduction was associated with pancreatic islet beta cell necrosis and pancreatic architecture damage. The anti-CD47 antibody was used to detect CD47 expression in pancreatic islet cells. CD47 expression on pancreatic islets was significantly reduced after five daily doses of STZ (Figure 1D). These results clearly highlight that macrophage activation and invasiveness were increased with a reduction in pancreatic islet cell CD47 expression in the STZ-treated group.
The use of Min6 cells that were treated with STZ further confirmed the effect of STZ on CD47 expression, as well as macrophage phagocytosis. For these experiments, Min6 cells were stimulated with 1 mmol/L STZ for 18 h. As expected, CD47 expression on the Min6 cell surface strongly decreased after STZ treatment, as measured by a FAC scan flow cytometer (Figure 2A and 2B). These results further confirmed that STZ affected CD47 expression both in vitro and in vivo. To determine the function of Min6 cells after STZ treatment, we incubated Min6 cells with 1 mmol/L STZ for 1 h. After a 24-h recovery, insulin secretion was detected under 5 mmol/L or 25 mmol/L glucose conditions. Insulin secretion was reduced under both low and high glucose conditions in STZ-treated cells (Figure 2C). STZ impaired Min6 cell function with CD47 expression reduction. These results suggest that the reduction in CD47 is a critical factor in the process of macrophage accumulation in pancreatic islet cells, resulting in a series of immune responses, as well as in the clinical development and pathogenesis of autoimmune diabetes. To detect CD47 expression-mediated regulation of the phagocytic function of macrophages, Min6 cells were labeled with CFSE and subsequently cocultured with LPS-activated macrophages. Accelerated Min6 cell phagocytosis was observed under STZ treatment compared with that of the control group. The results revealed that macrophages had a greater tendency to phagocytose CFSE-labeled Min6 cells, and the STZ-induced downregulation of CD47 vastly improved macrophage phagocytic activity (Figure 3D).
To imitate a similar situation and study the direct effect of STZ on pancreatic islet beta cells, mouse pancreatic islet cells were isolated as previously described[40]. Then, the islet cells were cultured in dishes with or without 1 mmol/L STZ stimulation for 12 h. Consistent with the in vitro test, we found that CD47 expression in pancreatic islet beta cells was significantly reduced under STZ treatment (Supplementary Figure 2A and B). Insulin secretion was also detected at 5 mmol/L or 25 mmol/L glucose. There was a similar result as that of treatment of Min6 cells, and insulin secretion was greatly reduced by STZ stimulation (Supplementary Figure 2C). These results indicate that the effect of STZ on pancreatic islet beta cells and macrophages might be related to the reduction in CD47 expression, which may provide a new mechanism of diabetes pathogenesis.
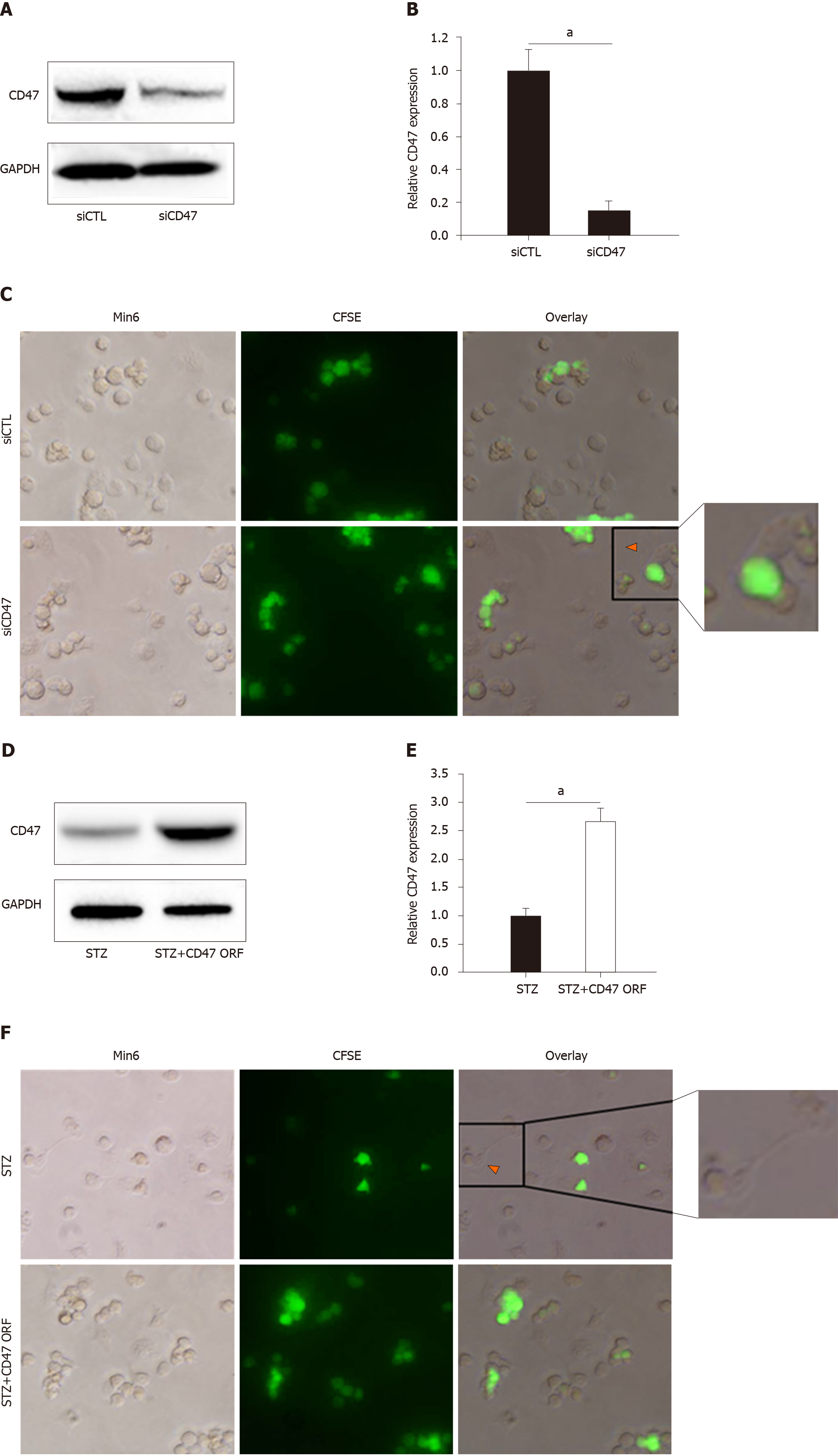
To confirm the direct effect of CD47 in regulating macrophage phagocytosis, we transfected Min6 cells with CD47 siRNA, followed by coculture of CFSE-labeled Min6 cells and LPS-activated macrophages to detect macrophage phagocytic activity. As shown in Figure 3A and 3B, relative CD47 expression was significantly downregulated with siCD47 transfection compared with siCTL transfection. Correspondingly, enhanced macrophage phagocytic activity was observed with CD47 siRNA transfection (Figure 3C). The CD47 open reading frame (ORF) was also transfected to further confirm that CD47-SIRPα negatively mediates phagocytosis. The CD47 expression level was tested by Western blot (Figure 3D and 3E). As shown in Figure 3F, cells transfected with the CD47 ORF displayed decreased macrophage phagocytosis compared to those treated with STZ alone. This demonstrates that the CD47-SIRPα interaction plays a key inhibitory role in the process of pancreatic islet beta cell clearance. These results confirmed that the absence of CD47-SIRPα-mediated inhibition improved macrophage phagocytic activity, and stronger inhibitory signaling triggered by CD47 ligation under STZ conditions is needed to effectively block macrophage phagocytosis.
Figure 4 shows the hypothetical model of CD47-SIRPα-regulated macrophage phagocytosis in pancreatic islets with or without STZ stimulation. CD47-SIRPα-mediated inhibition is relevant and indispensable when phagocytosis occurs toward the “self”. Under normal conditions, the CD47-SIRPα interaction provides inhibitory signals that prevent macrophage phagocytosis. In inflammatory conditions, such as STZ stimulation, macrophage phagocytosis is activated due to weakened CD47-SIRPα interactions. Reduced SIRPα might promote macrophage phagocytosis of CD47-low target cells, such as STZ-induced pancreatic islet beta cells.
Macrophages are critical in the development and pathogenesis of autoimmune disease. They are the first immune cells to infiltrate the pancreatic islet when an “eat me” signal is present[3,7,41]. The balance between activating and inhibitory signals[42,43] regulates macrophage activation. CD47 is one of the inhibitory signals through its interaction with SIRPα, which is expressed on the surface of macrophages and other immune cells. Cells that express CD47 are recognized as “self”; otherwise, they will be eliminated as “non-self” targets[23]. Although the CD47-SIRPα mechanism may be dispensable under normal conditions, it becomes extremely important under inflammatory conditions and infection, during which macrophages enhance phagocytosis toward endogenous cells[44]. Findings from SIRPα-/- and CD47-/- mice show that the animals rapidly develop anemia under inflammatory challenges, which suggests that the lack of SIRPα or CD47 significantly reduces the threshold of this condition[45,46]. As reported previously[47,48], SIRPα expression in macrophages decreased following LPS stimulation, suggesting the dynamic nature of CD47-SIRPα-mediated inhibition, especially in inflammation and infection. In addition, data presented in recent studies showed that CD47-SIRPα-mediated inhibition controls not only phagocytic target selection but also the phagocytic robustness of the chosen target[10].
In the present study, we report for the first time that pancreatic islet beta cell surface CD47 reduction plays a critical role in pancreatic islet beta cell depletion in STZ-induced diabetes. This conclusion was supported by the data derived from both in vivo and in vitro experiments. First, concurrent pancreatic islet beta cell depletion and reduction in CD47 expression was observed in STZ-injected mice compared with citrate buffer-treated mice. Macrophage infiltration in pancreatic islets was also increased, as determined by F4/80 labeling. The reduction in CD47 expression was specific to pancreatic islet beta cells, since there was no change in CD47 surface expression in other cells in the pancreas after STZ treatment. This might explain why STZ specifically attacks pancreatic islet beta cells and leads to diabetes. Second, we confirmed the direct effect of STZ on pancreatic islet beta cells in vitro. After STZ treatment, both Min6 cells and isolated pancreatic islet beta cells displayed reduced CD47 expression levels and reduced insulin secretion. Enhanced phagocytosis of STZ-stimulated Min6 cells was also observed in an in vitro phagocytosis experiment. In addition, the enhanced macrophage phagocytosis of cells with downregulated CD47 expression was confirmed by direct transfection of CD47 siRNA. These results confirm our hypothesis that macrophages phagocytize pancreatic islet beta cells by recognizing CD47 signaling in the MLD-STZ-induced diabetes mouse model, which might provide an explanation of the mechanism of STZ-induced diabetes. CD47 is a novel therapeutic factor aimed at attenuating and preventing macrophage-regulated target cell depletion[49]. Moreover, these findings also encourage us to prevent the progression of diabetes or other autoimmune and inflammatory diseases clinically by improving CD47 expression. Further studies are needed to define the mechanism that controls receptor-mediated macrophage phagocytic recognition of endogenous cells.
Type I diabetes (T1D) is characterized by insulin loss, accompanied by excessive inflammatory cell infiltration like macrophages and the destruction of the pancreas. Regarding the mechanochemical signaling regulation of T1D, the relationship between macrophage migration and phagocytosis is still unclear. In this study, we provided a new insight into the immune response occurring in the pancreas.
We try to provide a new insight into the mechanism of immune response occurring in the pancreas of T1D patients.
Our aim was to provide a new strategy to prevent T1D progression.
This study was performed both in vivo and in vitro. Macrophage migration and infiltration were assayed to study the mechanism of T1D immune response. The statistical analysis was performed using SPSS statistical software (version 16.0).
In this study, we found a significant decrease of CD47 in pancreatic beta islet cells stimulated with STZ and enhanced migration and infiltration of macrophages. As an integrin-associated surface factor, CD47 expression level is strongly related to the microphage immune response to inflamed pancreas beta islet.
Our study shows a new mechanistic insight into T1D from view of immune response.
This study could provide a new strategy to prevent the progression of T1D.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pirola L, Vorobjova T S-Editor: Wang YQ L-Editor: Wang TQ E-Editor: Li X
| 1. | Niu S, Bian Z, Tremblay A, Luo Y, Kidder K, Mansour A, Zen K, Liu Y. Broad Infiltration of Macrophages Leads to a Proinflammatory State in Streptozotocin-Induced Hyperglycemic Mice. J Immunol. 2016;197:3293-3301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 671] [Cited by in RCA: 591] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 3. | Catanzaro OL, Dziubecki D, Labal E, Sirois P. Activation of peritoneal macrophages during the evolution of type 1 diabetes (insulitis) in streptozotocin-treated mice. Peptides. 2010;31:1884-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Zhao Y, Shen Z, Zhang D, Luo H, Chen J, Sun Y, Xiao Q. Ghrelin ameliorates nerve growth factor Dysmetabolism and inflammation in STZ-induced diabetic rats. Metab Brain Dis. 2017;32:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Feduska JM, Tse HM. The proinflammatory effects of macrophage-derived NADPH oxidase function in autoimmune diabetes. Free Radic Biol Med. 2018;125:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 524] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 7. | Cantor J, Haskins K. Recruitment and activation of macrophages by pathogenic CD4 T cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1. J Immunol. 2007;179:5760-5767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Bian Z, Guo Y, Ha B, Zen K, Liu Y. Regulation of the inflammatory response: enhancing neutrophil infiltration under chronic inflammatory conditions. J Immunol. 2012;188:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3290] [Cited by in RCA: 3114] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 10. | Bian Z, Shi L, Guo YL, Lv Z, Tang C, Niu S, Tremblay A, Venkataramani M, Culpepper C, Li L, Zhou Z, Mansour A, Zhang Y, Gewirtz A, Kidder K, Zen K, Liu Y. Cd47-Sirpα interaction and IL-10 constrain inflammation-induced macrophage phagocytosis of healthy self-cells. Proc Natl Acad Sci USA. 2016;113:E5434-E5443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Wong AS, Mortin-Toth S, Sung M, Canty AJ, Gulban O, Greaves DR, Danska JS. Polymorphism in the innate immune receptor SIRPα controls CD47 binding and autoimmunity in the nonobese diabetic mouse. J Immunol. 2014;193:4833-4844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Wang H, Wu X, Wang Y, Oldenborg PA, Yang YG. CD47 is required for suppression of allograft rejection by donor-specific transfusion. J Immunol. 2010;184:3401-3407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Tsai RK, Rodriguez PL, Discher DE. Self inhibition of phagocytosis: the affinity of 'marker of self' CD47 for SIRPalpha dictates potency of inhibition but only at low expression levels. Blood Cells Mol Dis. 2010;45:67-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Alvey C, Discher DE. Engineering macrophages to eat cancer: from "marker of self" CD47 and phagocytosis to differentiation. J Leukoc Biol. 2017;102:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Lv Z, Bian Z, Shi L, Niu S, Ha B, Tremblay A, Li L, Zhang X, Paluszynski J, Liu M, Zen K, Liu Y. Loss of Cell Surface CD47 Clustering Formation and Binding Avidity to SIRPα Facilitate Apoptotic Cell Clearance by Macrophages. J Immunol. 2015;195:661-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Gavrieli M, Watanabe N, Loftin SK, Murphy TL, Murphy KM. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem Biophys Res Commun. 2003;312:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Zen K, Guo Y, Bian Z, Lv Z, Zhu D, Ohnishi H, Matozaki T, Liu Y. Inflammation-induced proteolytic processing of the SIRPα cytoplasmic ITIM in neutrophils propagates a proinflammatory state. Nat Commun. 2013;4:2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Barclay AN. Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr Opin Immunol. 2009;21:47-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, Sykes M, Yang YG, Ohdan H. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci USA. 2007;104:5062-5066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | Gerstenkorn C, Robertson H, Mohamed MA, O'Donnell M, Ali S, Talbot D. Detection of cytomegalovirus (CMV) antigens in kidney biopsies and transplant nephrectomies as a marker for renal graft dysfunction. Clin Chem Lab Med. 2000;38:1201-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Yi T, Li J, Chen H, Wu J, An J, Xu Y, Hu Y, Lowell CA, Cyster JG. Splenic Dendritic Cells Survey Red Blood Cells for Missing Self-CD47 to Trigger Adaptive Immune Responses. Immunity. 2015;43:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg PA. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005;105:3577-3582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 167] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Wang H, Madariaga ML, Wang S, Van Rooijen N, Oldenborg PA, Yang YG. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci USA. 2007;104:13744-13749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PØ, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662-6667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 1285] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 25. | Li F, Lv B, Liu Y, Hua T, Han J, Sun C, Xu L, Zhang Z, Feng Z, Cai Y, Zou Y, Ke Y, Jiang X. Blocking the CD47-SIRPα axis by delivery of anti-CD47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology. 2018;7:e1391973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Sun FJ, Zhang CQ, Chen X, Wei YJ, Li S, Liu SY, Zang ZL, He JJ, Guo W, Yang H. Downregulation of CD47 and CD200 in patients with focal cortical dysplasia type IIb and tuberous sclerosis complex. J Neuroinflammation. 2016;13:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Chao MP, Weissman IL, Majeti R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 493] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 28. | Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1313] [Cited by in RCA: 1235] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 29. | Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, Park CY, Weissman IL, Majeti R. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374-1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 30. | Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, Otten MA, Finetti P, Van Egmond M, Matozaki T, Kraal G, Birnbaum D, van Elsas A, Kuijpers TW, Bertucci F, van den Berg TK. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci USA. 2011;108:18342-18347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 31. | Ishikawa-Sekigami T, Kaneko Y, Okazawa H, Tomizawa T, Okajo J, Saito Y, Okuzawa C, Sugawara-Yokoo M, Nishiyama U, Ohnishi H, Matozaki T, Nojima Y. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood. 2006;107:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Geunes-Boyer S, Oliver TN, Janbon G, Lodge JK, Heitman J, Perfect JR, Wright JR. Surfactant protein D increases phagocytosis of hypocapsular Cryptococcus neoformans by murine macrophages and enhances fungal survival. Infect Immun. 2009;77:2783-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Jäkel A, Clark H, Reid KB, Sim RB. The human lung surfactant proteins A (SP-A) and D (SP-D) interact with apoptotic target cells by different binding mechanisms. Immunobiology. 2010;215:551-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Mensah-Brown E, Shahin A, Parekh K, Hakim AA, Shamisi MA, Hsu DK, Lukic ML. Functional capacity of macrophages determines the induction of type 1 diabetes. Ann N Y Acad Sci. 2006;1084:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Slee JB, Alferiev IS, Nagaswami C, Weisel JW, Levy RJ, Fishbein I, Stachelek SJ. Enhanced biocompatibility of CD47-functionalized vascular stents. Biomaterials. 2016;87:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Sick E, Boukhari A, Deramaudt T, Rondé P, Bucher B, André P, Gies JP, Takeda K. Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway. Glia. 2011;59:308-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Lukić ML, Stosić-Grujicić S, Shahin A. Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev Immunol. 1998;6:119-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Caminschi I, Lucas KM, O'Keeffe MA, Hochrein H, Laâbi Y, Köntgen F, Lew AM, Shortman K, Wright MD. Molecular cloning of F4/80-like-receptor, a seven-span membrane protein expressed differentially by dendritic cell and monocyte-macrophage subpopulations. J Immunol. 2001;167:3570-3576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Gentek R, Molawi K, Sieweke MH. Tissue macrophage identity and self-renewal. Immunol Rev. 2014;262:56-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 40. | Hani H, Ibrahim TA, Othman AM, Lila MA, bt Allaudin ZN. Isolation, density purification, and in vitro culture maintenance of functional caprine islets of Langerhans as an alternative islet source for diabetes study. Xenotransplantation. 2010;17:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | McCracken MN, Cha AC, Weissman IL. Molecular Pathways: Activating T Cells after Cancer Cell Phagocytosis from Blockade of CD47 "Don't Eat Me" Signals. Clin Cancer Res. 2015;21:3597-3601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 42. | A-Gonzalez N, Castrillo A. Origin and specialization of splenic macrophages. Cell Immunol. 2018;330:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 557] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 44. | Legrand N, Huntington ND, Nagasawa M, Bakker AQ, Schotte R, Strick-Marchand H, de Geus SJ, Pouw SM, Böhne M, Voordouw A, Weijer K, Di Santo JP, Spits H. Functional CD47/signal regulatory protein alpha (SIRP(alpha)) interaction is required for optimal human T- and natural killer- (NK) cell homeostasis in vivo. Proc Natl Acad Sci USA. 2011;108:13224-13229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 45. | Vaeteewoottacharn K, Kariya R, Pothipan P, Fujikawa S, Pairojkul C, Waraasawapati S, Kuwahara K, Wongkham C, Wongkham S, Okada S. Attenuation of CD47-SIRPα Signal in Cholangiocarcinoma Potentiates Tumor-Associated Macrophage-Mediated Phagocytosis and Suppresses Intrahepatic Metastasis. Transl Oncol. 2019;12:217-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 46. | Barros MM, Yamamoto M, Figueiredo MS, Cançado R, Kimura EY, Langhi DM, Chiattone CS, Bordin JO. Expression levels of CD47, CD35, CD55, and CD59 on red blood cells and signal-regulatory protein-alpha,beta on monocytes from patients with warm autoimmune hemolytic anemia. Transfusion. 2009;49:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Kong XN, Yan HX, Chen L, Dong LW, Yang W, Liu Q, Yu LX, Huang DD, Liu SQ, Liu H, Wu MC, Wang HY. LPS-induced down-regulation of signal regulatory protein {alpha} contributes to innate immune activation in macrophages. J Exp Med. 2007;204:2719-2731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Lo J, Lau EY, Ching RH, Cheng BY, Ma MK, Ng IO, Lee TK. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology. 2015;62:534-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 49. | Soto-Pantoja DR, Stein EV, Rogers NM, Sharifi-Sanjani M, Isenberg JS, Roberts DD. Therapeutic opportunities for targeting the ubiquitous cell surface receptor CD47. Expert Opin Ther Targets. 2013;17:89-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |