Published online May 15, 2020. doi: 10.4239/wjd.v11.i5.202
Peer-review started: December 16, 2019
First decision: January 6, 2020
Revised: March 16, 2020
Accepted: March 23, 2020
Article in press: March 23, 2020
Published online: May 15, 2020
Processing time: 147 Days and 2.4 Hours
Type 2 diabetes mellitus (T2DM) has high morbidity and mortality worldwide, therefore there is of paramount importance to identify the risk factors in the populations at risk early in the course of illness. A strong correlation between severity of metabolic syndrome (MetS) and HbA1c, fasting insulin and insulin resistance has been reported. Accordingly, the MetS severity score (or MestS Z-score) can potentially be used to predict the risk of T2DM progression over time.
To evaluate the association the of MestS Z-score in first degree relatives (FDRs) of T2DM with the risk of prediabetes and type 2 diabetes in future.
A prospective open cohort study was conducted between 2003-2018. At baseline, the sample comprised of 1766 FDRs of patients with T2DM who had a normal glucose tolerance test. Relative risk (RR) and 95% confidence interval were calculated based on logistic regression. The receiver-operator characteristic analysis and area under the curve based on MetS Z-score were used to evaluate the risk of prediabetes and diabetes among the FDR population.
Baseline MetS Z-scores were associated with the its latest values (P < 0.0001). Compared with individuals who were T2DM free at the end of follow up, those who developed T2DM had higher MetS Z-score at baseline (P < 0.001). In multivariable logistic regression analyses for every unit elevation in MetS Z-score at the baseline, the RR for developing future T2DM and prediabetes was (RR = 1.94, RR = 3.84), (RR = 1.5, RR = 2.17) in total population and female group, respectively (P < 0.05). The associations remained significant after adjusting the potential confounding variables. A cut off value of 0.97 and 0.94 was defined in the receiver-operator characteristic curve based on the MetS Z-score for differentiating female patients with diabetes and prediabetes from the normal population, respectively.
The MetS Z-score was associated with an increased risk of future T2DM. Appropriate interventions at earlier stages for preventing and attenuating MetS effects may be considered as an effective strategy for FDR as at-risk population.
Core tip: This prospective cohort study showed that metabolic syndrome severity score at baseline, in first degree relative of type 2 diabetes mellitus patients with normal glucose tolerance, predicts the incidence of future diabetes and prediabetes. In this study, the cut off values of metabolic syndrome Z-score for predicting prediabetes and diabetes were 0.94 and 0.97, respectively. This negligible difference between two groups in terms of cut off values highlights the importance of intervention at prediabetes stage.
- Citation: Meamar R, Amini M, Aminorroaya A, Nasri M, Abyar M, Feizi A. Severity of the metabolic syndrome as a predictor of prediabetes and type 2 diabetes in first degree relatives of type 2 diabetic patients: A 15-year prospective cohort study. World J Diabetes 2020; 11(5): 202-212
- URL: https://www.wjgnet.com/1948-9358/full/v11/i5/202.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i5.202
Type 2 diabetes mellitus (T2DM) has high morbidity and mortality worldwide, therefore there is of paramount importance to identify risk factors in the populations at risk early in the course of illness[1].
One of the important factors in increasing the risk of acquiring DM is metabolic syndrome (MetS). MetS consists of having three of the five following abnormalities; central obesity, hypertension, high triacylglycerol, low high-density lipoproteins (HDL) cholesterol and elevated fasting glucose[2]. MetS is linked to insulin resistance[3] and obesity[4] due to abnormality in cellular function[5]. MetS is a risk factor for future cardiovascular disease (CVD)[6,7] and T2DM[7-9] both in children and adults[10,11]. Linear measurements of MetS Z-score is valuable not only for the identification of high-risk individuals but also for following up the disease development over time and evaluation of the response to treatment[12]. Recently the potential benefit of measuring MetS Z-score in childhood and predicting cardiovascular disease and diabetes later in life has been revealed[12-13].
The MetS not only is inherited but also influenced by lifestyle and genetic factors[14]. This provides an opportunity to modify lifestyle or intervene with treatments in the population who have been recognized to be at risk for prediabetes and diabetes mellitus such as the offspring of the patients with T2DM[15].
First degree relatives (FDRs) with T2DM are at higher risk of diabetes and pre-diabetes progression[16-18].
A study by Siewert et al[19] indicated that the prevalence of MetS is high in young FDR adults. Iran is a developing country and its population is adopting a more sedentary lifestyle with new diets resulting in a high prevalence of T2DM and prediabetes[20]. In this study, taking into account potential links between MetS Z-score as a marker for prediabetes or T2DM risk, we assessed the role of MetS Z-score for predicting prediabetes and T2DM in FDR population in a long-term follow-up cohort study to enable clinicians to identify and treat this high-risk population through conducting of interventions for preventing MetS or diminishing its side effects.
Data were drawn from the database of the Isfahan Diabetes Study on the first degree relatives (FDR), the details of the study have been presented elsewhere[21]. In summary, the study is an ongoing open cohort study started at 2003 on FDR of patients with T2DM in Isfahan, a large city in central Iran, to measure several possible risk factors of diabetes incidence. At baseline, our sample comprised of 3492 FDRs of T2DM patients. All participants were visited at the Isfahan Endocrine and Metabolism Research Center, affiliated to Isfahan University of Medical Sciences, Iran. The Bioethics Committee of Isfahan University of Medical Sciences approved the study and written informed consent was obtained from every participant based on the Declaration of Helsinki.
At the time of examination, subjects underwent anthropometric measurements and laboratory tests, including a standard 75-g 2-h oral glucose tolerance test (OGTT). For the current study, the analysis was limited to those normal glucose test (NGT) participants without missing data i.e. 1766 at baseline. NGT participants were followed from 2003 annually until 2018 and then classified to NGT, impaired glucose test and T2DM according to the American Diabetes Association criteria[22].
Anthropometric and demographic variables: All participants completed a demo-graphic questionnaire including age, gender, level of education, smoking and personal and medical history at baseline. Anthropometric and basic clinical measurements, including body mass index (kg/m2), waist circumference (WC, cm) and waist-to-hip ratio (WHR) were calculated according to standard methods[23] and blood pressure (BP) including both systolic and diastolic were recorded.
MetS Z-Score calculation: Traditional MetS was defined using the National Cholesterol Education Program Adult Treatment Panel-III criteria[2]. Participants had to meet three or more of the following five criteria: (1) Concentration of triacylglycerol ≥ 1.69 mmol/L (150 mg/dL); (2) HDL-cholesterol level < 1.04 mmol/L (40 mg/dL) for men and < 1.3 mmol/L (50 mg/dL) for women; (3) WC ≥ 102 cm for men and 88 cm for women; (4) Glucose concentration ≥ 5.55 mmol/L (100 mg/dL); and (5) systolic BP ≥ 130 mmHg or diastolic BP ≥ 85 mmHg. The MetS Z-score was calculated for adolescents at first visit using formulas published elsewhere[24]. Data collection was conducted at baseline and at follow-up according to the standards of Medical Care in Diabetes[25].
Laboratory parameters: All participants received a 75-g OGTT following a 12- hour overnight fasting period. Plasma glucose (PG) was measured at 0, 30, 60 and 120 min[26] (2-h PG) and fasting plasma glucose (FPG) (mg/dL) was measured by Pars Azmon kit Lot number: 94011 (a photometric method). HbA1c, cholesterol (LDL, HDL) and triglyceride were also measured.
Participants with FPG ≥ 200 mg/dL were considered diabetic. If FPG was ≥ 126 and < 200 mg/dL, a second FPG was measured on another day. If the second FPG was also ≥ 126 mg/dL, participants were classified as diabetic. Those with FPG ≥ 126 mg/dL or 2-h PG ≥ 200 mg/dL were also defined as diabetic. Impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) are intermediate stages in the natural history of type 2 diabetes and are called the pre-diabetes phase[22]. FPG < 126 mg/dL with a 2-h PG concentration ≥ 140 and < 200 mg/dL was interpreted as IGT. If FPG was in the range of 100–126 mg/dL and 2-h PG was < 140 mg/dL, it was considered as IFG. NGT was defined as FPG below 100 mg/dL and 2-h PG less than 140 mg/dL[27].
Quantitative variables are presented as mean ± SD or median [IQR], while qualitative as frequency (percentage). Depending on the normal or non-normal distribution of data, independent samples t-test or Mann-Whitney U tests were used for comparing continuous data between NPG and diabetes or prediabetes. Categorical data were compared with the χ2 test.
Pearson correlation coefficient was calculated for evaluating the correlation of MetS Z-score at first and last visit. The diagnostic accuracy of MetS Z-Score was evaluated by using the receiver operating characteristic (ROC) curve analysis and calculated area under the curve (AUC) and 95% confidence interval (CI) for AUC. We used binary logistic regression analysis for evaluating the predictive value of MetS Z-Score for diabetes and prediabetes incidence in the future in different models. In these analyses, after obtaining relative risk (RR) and 95%CI in the crude model, the adjustment was made for age and gender in the model 1. All statistical calculations were carried out with the SPSS 15 for Windows (SPSS Inc., Chicago, IL, United States) and P < 0.05 was used as a statistically significant level.
Over the fifteen years follow-up from the 1766 NGT participants at baseline, 78 participants developed DM (7.4%), 255 participants progressed to IFG (24.1%) and 89 participants developed IGT (8.4%). Overall, 344 (32.6%) developed pre-diabetes and 630 (59.7%) participants remained NGT. Of this total, 714 were missed to follow up due to moving geographically, withdrawing consent or changing contact details and being unavailable.
Table 1 presents the anthropometric, laboratory and clinical characteristics at the beginning of the study for those participants who developed to diabetes and those who remained normal at follow up periods. All glycemic variables including PG in 0, 30, 60 and 120 min, HbA1c, FPG, and total cholesterol and triglyceride as well as WHR were significantly different between diabetes and a normal group, particularly in total population and female group.
| Variables | Total | Male | Female | ||||||
| Diabetes (n = 78) | Normal (n = 630) | P value | Diabetes (n = 26) | Normal (n = 160) | P value | Diabetes (n = 52) | Normal (n = 470) | P value | |
| Age (yr) | 43.33 ± 6.15 | 42.01 ± 6.14 | 0.075 | 45.15 ± 7.33 | 42.83 ± 6.65 | 0.1 | 42.42 ± 5.31 | 41.71 ± 5.93 | 0.4 |
| BMI (kg/m2) | 29.33 ± 4.35 | 28.16 ± 4.20 | 0.02 | 27.6 ± 3.44 | 26.93 ± 3.64 | 0.38 | 30.16 ± 4.53 | 28.59 ± 4.31 | 0.01 |
| Smoking, yes [n (%)] | 2 (9.5%) | 17 (8.03%) | 0.84 | 1 (14.3%) | 12 (25%) | 0.53 | 1 (1.1%) | 5 (2.3%) | 0.43 |
| Education (diploma and more) | 33 (42.3%) | 317 (51.5%) | 0.12 | 13 (50%) | 108 (68.8%) | 0.06 | 20 (38.5%) | 208 (45.5%) | 0.33 |
| WHR | 0.83 ± 0.06 | 0.81 ± 0.07 | 0.01 | 0.9 ± 0.03 | 0.89 ± 0.05 | 0.62 | 0.8 ± 0.04 | 0.79 ± 0.05 | 0.04 |
| Blood glucose 0 (mg/dL) | 90.45 ± 6.92 | 87.05 ± 7.91 | 0.001 | 91.76 ± 5.58 | 87.79 ± 8.28 | 0.01 | 89.81 ± 7.45 | 86.8 ± 7.78 | 0.008 |
| Blood glucose 30 (mg/dL) | 143.92 ± 24.56 | 125.93 ± 24.91 | 0.001 | 144.32 ± 25.32 | 133.08 ± 27.98 | 0.06 | 143.73 ± 24.43 | 123.22 ± 23.14 | 0.001 |
| Blood glucose 60 (mg/dL) | 152.11 ± 34.83 | 121.58 ± 31.49 | 0.001 | 148.88 ± 38.67 | 129.25 ± 36.37 | 0.01 | 153.69 ± 33.06 | 118.74 ± 29.08 | 0.001 |
| Blood glucose 120 (mg/dL) | 108.93 ± 19.95 | 97.81 ± 21.05 | 0.001 | 102.93 ± 23.43 | 88.76 ± 22.44 | 0.003 | 111.88 ± 17.5 | 101.01 ± 19.6 | 0.001 |
| HbA1c | 5.2 ± 0.72 | 4.92 ± 0.78 | 0.006 | 5.28 ± 0.9 | 4.93 ± 0.63 | 0.03 | 5.16 ± 0.61 | 4.92 ± 0.82 | 0.05 |
| Triglyceride (mg/dL) | 181.42 ± 100.22 | 146.30 ± 81.10 | 0.001 | 203.48 ± 97.91 | 174.98 ± 96.79 | 0.17 | 170.6 ± 100.51 | 136.29 ± 72.42 | 0.002 |
| Total cholesterol (mg/dL) | 203.44 ± 44.55 | 189.96 ± 38.65 | 0.005 | 201.28 ± 40.22 | 192.09 ± 37.63 | 0.26 | 204.5 ± 46.87 | 189.37 ± 38.93 | 0.01 |
| HDL (mg/dL) | 44.21 ± 10.6 | 45.33 ± 11.57 | 0.43 | 43.34 ± 11.3 | 40.96 ± 10.82 | 0.32 | 44.6 ± 10.36 | 46.85 ± 11.46 | 0.18 |
| Systolic pressure (cmHg) | 11.58 ± 1.63 | 11.26 ± 1.48 | 0.08 | 12.14 ± 1.61 | 11.52 ± 1.49 | 0.06 | 11.32 ± 1.59 | 11.17 ± 1.47 | 0.48 |
| Diastolic pressure (cmHg) | 7.45 ± 1.24 | 7.41 ± 1.12 | 0.76 | 7.62 ± 1.16 | 7.55 ± 1.16 | 0.87 | 7.38 ± 1.28 | 7.36 ± 1.1 | 0.94 |
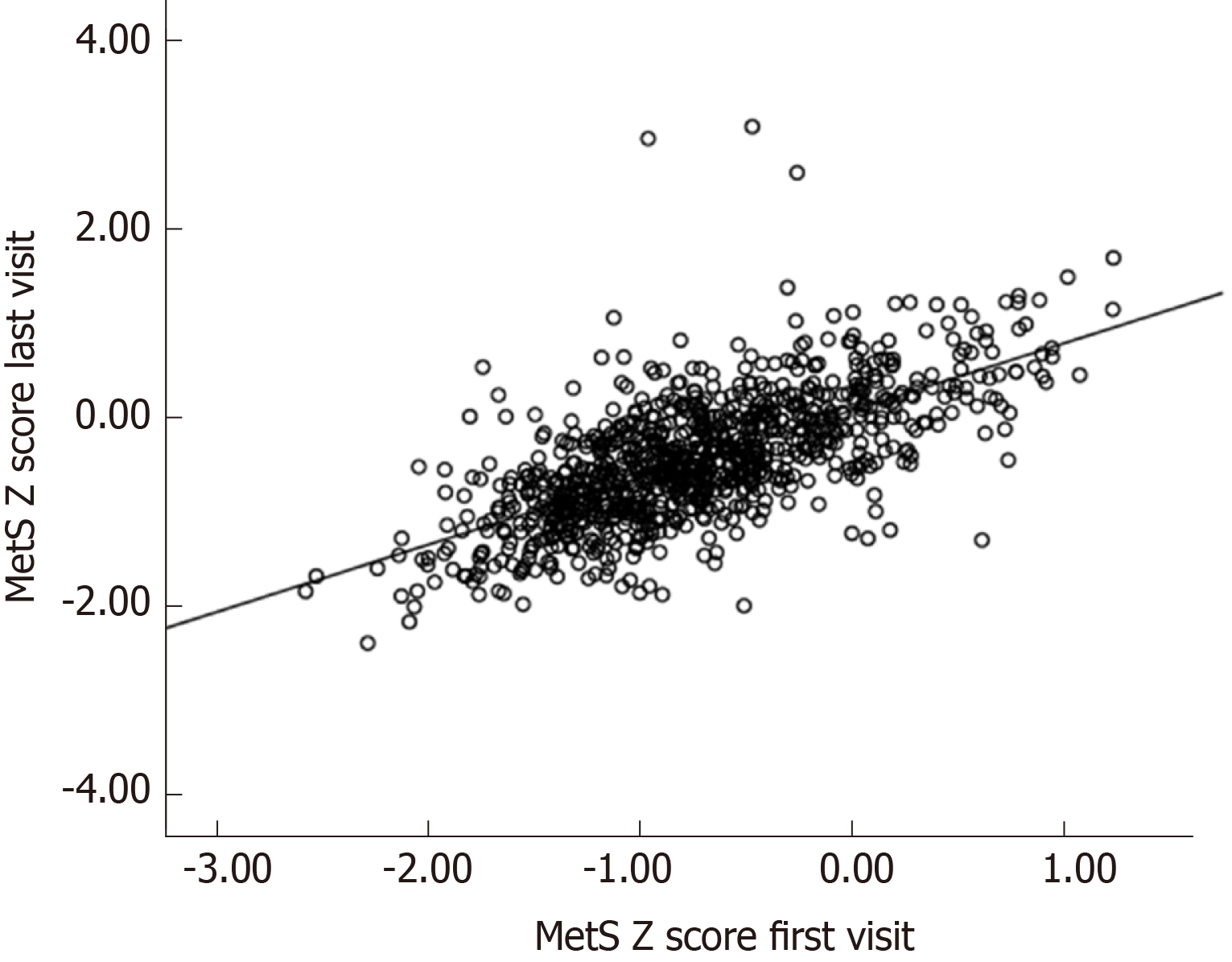
Table 2 presents the anthropometric measurements, laboratory and clinical characteristics at the beginning of the study for those participants who developed to pre-diabetes and those who remained normal. It illustrates a significant difference in glucose level at 0, 30, 60,120 min and triglyceride level, WHR and both systolic and diastolic blood pressure measurement in total population and female group when comparing pre-diabetes group with the normal group. There was a highly significant correlation in terms of MetS Z-score at first and last visits in the FDR population (r = 0.67; P < 0.001) (Figure 1).
| Variables | Total | Male | Female | ||||||
| Pre-diabetes (n = 344) | Normal (n = 630) | P value | Pre-diabetes (n = 94) | Normal (n = 161) | P value | Pre-diabetes (n = 250) | Normal (n = 470) | P value | |
| Age (yr) | 43.04 ± 6.33 | 42.01 ± 6.14 | 0.01 | 43.2 ± 6.12 | 42.83 ± 6.65 | 0.66 | 42.98 ± 6.42 | 41.71 ± 5.93 | 0.008 |
| BMI (kg/m2) | 28.44 ± 4.06 | 28.16 ± 4.2 | 0.32 | 26.84 ± 3.56 | 26.93 ± 3.64 | 0.84 | 29.04 ± 4.09 | 28.59 ± 4.31 | 0.17 |
| Smoking, yes [n (%)] | 8 (7.8%) | 17 (8.3%) | 0.88 | 7 (21.2%) | 12 (25%) | 0.69 | 1 (1.4%) | 5 (3.2%) | 0.45 |
| Education (diploma and more) | 154 (45.4%) | 317 (51.5%) | 0.07 | 61 (66.3%) | 108 (68.8%) | 0.68 | 93 (37.7%) | 208 (45.5%) | 0.05 |
| WHR | 0.82 ± 0.06 | 0.81 ± 0.07 | 0.04 | 0.9 ± 0.05 | 0.89 ± 0.05 | 0.83 | 0.8 ± 0.05 | 0.79 ± 0.05 | 0.01 |
| Blood glucose 0 (mg/dL) | 89.69 ± 6.9 | 87.05 ± 7.91 | 0.001 | 90.57 ± 6.7 | 87.79 ± 8.28 | 0.06 | 89.37 ± 6.96 | 86.8 ± 7.78 | 0.001 |
| Blood glucose 30 (mg/dL) | 136.63 ± 26 | 125.93 ± 24.91 | 0.001 | 141.44 ± 30.23 | 133.08 ± 27.98 | 0.02 | 134.74 ± 23.94 | 123.22 ± 23.14 | 0.001 |
| Blood glucose 60 (mg/dL) | 135.68 ± 31.23 | 121.58 ± 31.49 | 0.001 | 134.98 ± 33.37 | 129.25 ± 36.37 | 0.21 | 135.94 ± 30.45 | 118.74 ± 29.08 | 0.001 |
| Blood glucose 120 (mg/dL) | 104.2 ± 21.36 | 97.81 ± 21.05 | 0.001 | 94.72 ± 23.52 | 88.76 ± 22.44 | 0.04 | 107.7 ± 19.41 | 101.01 ± 19.6 | 0.001 |
| HbA1c | 5.06 ± 0.7 | 4.92 ± 0.78 | 0.009 | 5.09 ± 0.83 | 4.93 ± 0.63 | 0.1 | 5.05 ± 0.65 | 4.92 ± 0.82 | 0.04 |
| Triglyceride (mg/dL) | 157.78 ± 81.03 | 146.3 ± 81.1 | 0.03 | 176.98 ± 90.75 | 174.98 ± 96.79 | 0.87 | 150.56 ± 76.02 | 136.29 ± 72.42 | 0.01 |
| Total cholesterol (mg/dL) | 193.34 ± 38.2 | 189.96 ± 38.65 | 0.19 | 188.09 ± 36.58 | 192.09 ± 37.63 | 0.41 | 195.3 ± 38.67 | 189.37 ± 38.93 | 0.05 |
| HDL (mg/dL) | 44.37 ± 45.33 | 10.98 ± 11.57 | 0.21 | 41.26 ± 11.46 | 40.96 ± 10.82 | 0.83 | 45.51 ± 10.6 | 46.85 ± 11.46 | 0.13 |
| Systolic pressure (cmHg) | 11.67 ± 1.65 | 11.26 ± 1.48 | 0.001 | 11.75 ± 1.73 | 11.52 ± 1.49 | 0.26 | 11.64 ± 1.63 | 11.17 ± 1.47 | 0.001 |
| Diastolic pressure (cmHg) | 7.6 ± 1.14 | 7.41 ± 1.12 | 0.01 | 7.75 ± 1.19 | 7.55 ± 1.16 | 0.19 | 7.55 ± 1.12 | 7.36 ± 1.1 | 0.03 |
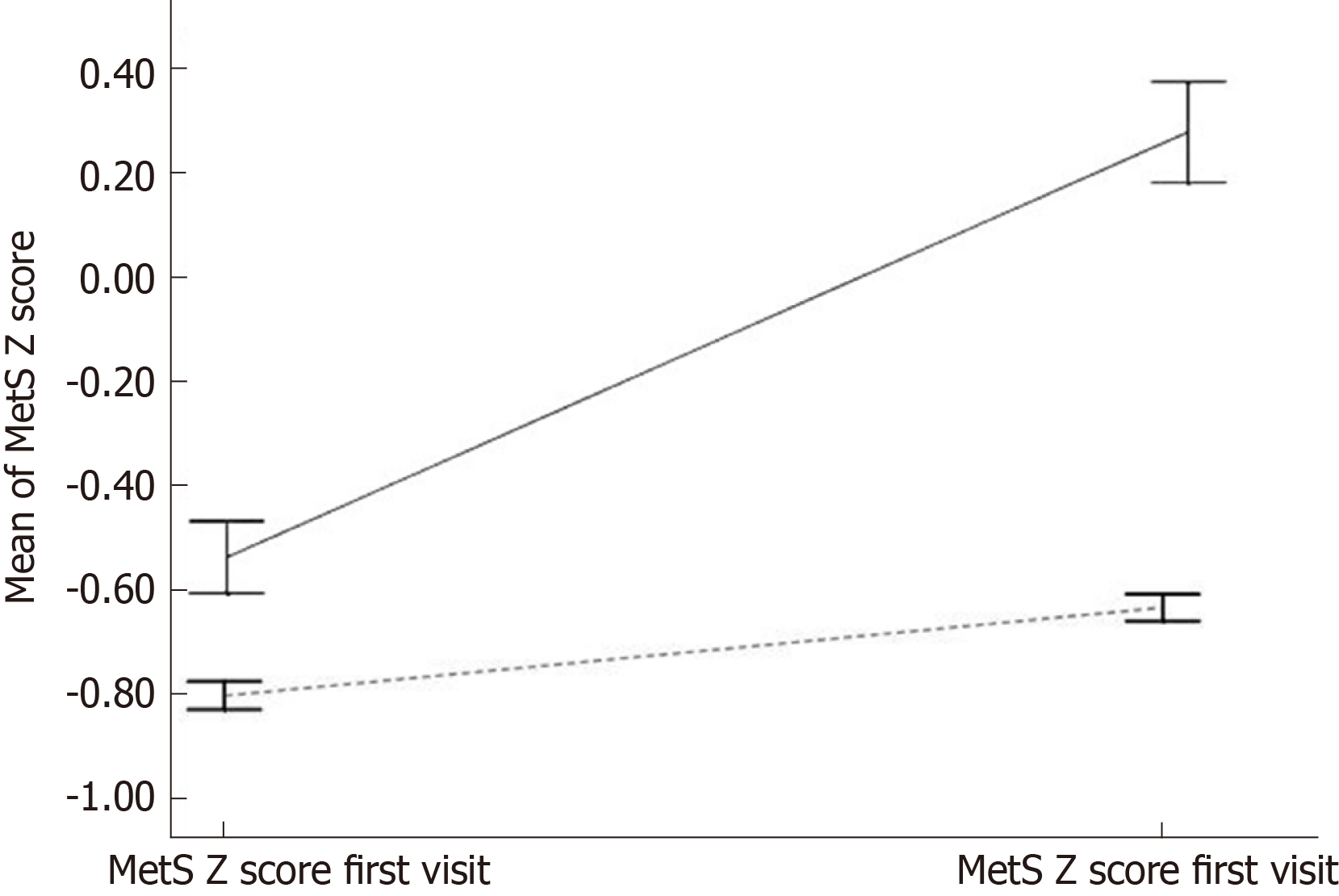
Figure 2 provides MetS Z-score at the first and final visits by diabetes disease status for two groups: Those who did not have diabetes at any of the two visits and those who developed T2DM between first and last visits.
Table 3 presents the results of crude and multivariable binary logistic regression analysis in different models for the association between MetS Z-score levels at baseline and diabetes and pre-diabetes risk at the future or at follow up. In crude logistic regression analysis, MetS Z-score level at baseline positively predicted the risk of future diabetes (RR = 1.94, RR = 3.84) and pre-diabetes (RR = 1.5, RR = 2.17) in total population and female group, respectively (all P < 0.05). However, significant results were not detected in the male group. In multivariable logistic regression analyses, the adjustment was made for age and gender as confounding factors (model). As illustrated in Table 3, the associations remained significant for diabetes (RR = 2.69, RR = 4.01) and prediabetes (RR = 1.76, RR = 2.07) in total population and female group, respectively (all P < 0.05). However, such significant results were not observed in the male group.
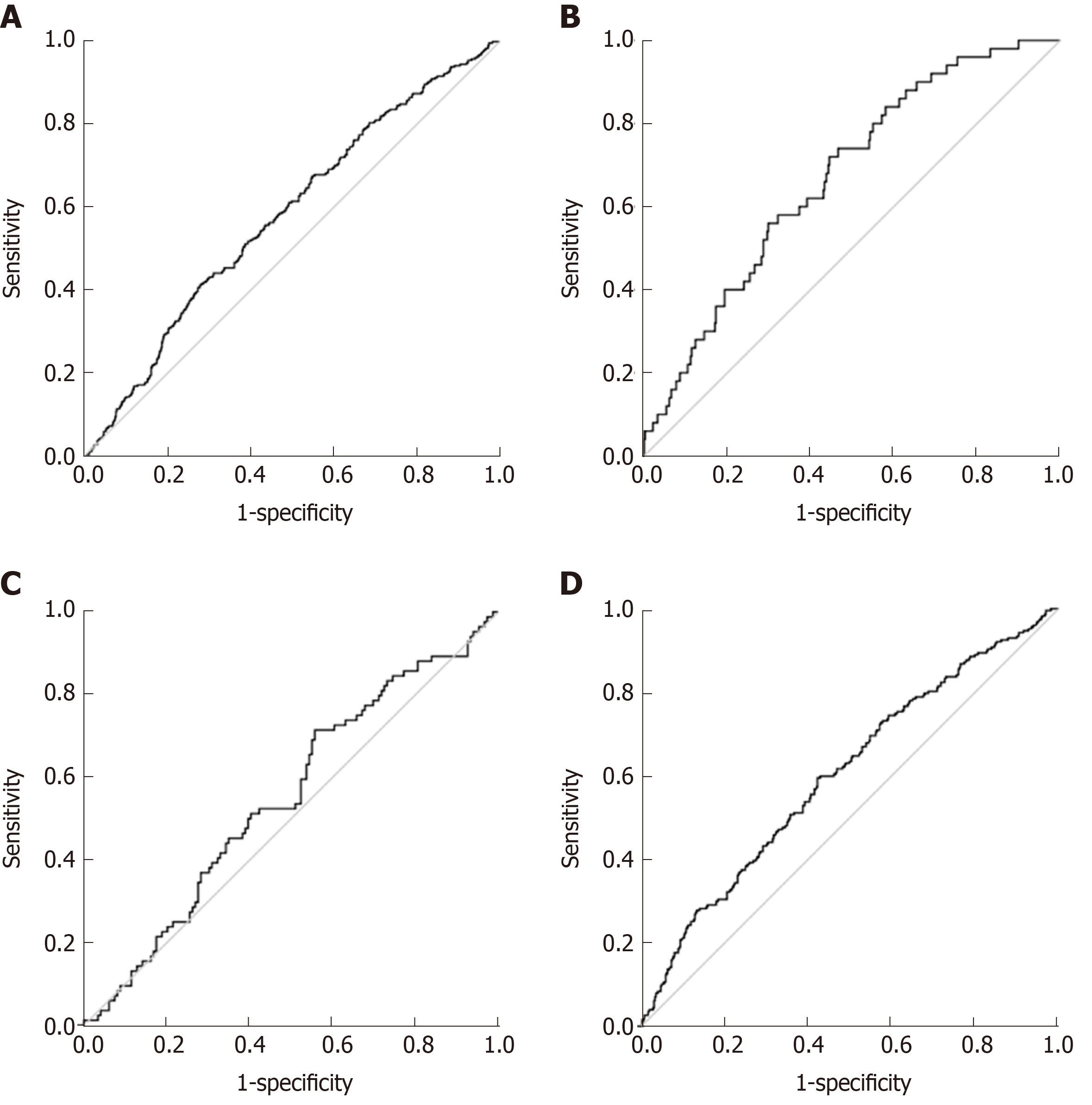
ROC curve analysis was used to determine the cutoff value of MetS Z-score at baseline for predicting diabetes and prediabetes in at follow up. The area under the ROC curve of MetS Z-score for predicting the incidence of diabetes and prediabetes is shown in Figure 3. A cutoff value 0.97 for MetS Z-score was obtained for differentiating the female patients with diabetes from normal with corresponding specificity of 56% and sensitivity of 72% and area under the ROC curve (AUC = 0.67, 95%CI: 0.59-0.74; P < 0.05). A cutoff value 0.78 for MetS Z-score was obtained for differentiating the total population with diabetes from normal with corresponding specificity of 56% and sensitivity of 66% and area under the ROC curve (AUC = 0.63, 95%CI: 0.57-0.69; P < 0.05). A cutoff value of Met Z-score at 0.94 was obtained for differentiating the female patients with prediabetes from normal people, with a corresponding specificity 58% and sensitivity 60% and area under the ROC curve of (AUC = 0.6, 95%CI: 0.55-0.64; P < 0.05) Also, a cutoff value of MetS Z-score 0.52 was obtained for differentiating total patients with prediabetes from normal people, with a corresponding specificity 72% and sensitivity 42% and area under the ROC curve of (AUC = 0.58, 95%CI: 0.54-0.61; P < 0.05) (Table 4).
To our knowledge, this is the first population-based study conducted to evaluate the association between MetS Z-score and the incidence of pre-diabetes/T2DM in the FDR population who were normal at first visit.
This study discovered that the degree of severity of MetS score as a linear measure is a predictive factor for the incidence of T2DM and prediabetes in the future. This association was overall moderate in the total population (AUC = 0.63) and mildly stronger in the females (AUC = 0.68) of the FDR population. Previously, MetS Z-score was correlated similarly to the prediction of CVD[13] and diabetes[12] in the non-FDR population. DeBoer et al[12] concluded that the severity of MetS in childhood could predict the incidence of adult T2DM in the future. In other studies, a strong correlation between MetS Z-score and HbA1c, fasting insulin and insulin resistance has been illustrated[28]. These findings suggest that MetS Z-score can potentially be used to detect risk and follow T2DM progression over time[12].
It has been reported that childhood MetS Z-score can predict diabetes risk in the future with an OR of 2.7 by the mean age of 38.5[12]. We found similar results: In the total FDR population, the RR for each 1.0 unit increase in the adulthood MetS Z-score in predicting diabetes and pre-diabetes by a mean age of 43 was 2.69 and 1.76 respectively.
Besides, MetS Z-score is associated with childhood obesity and is a significant risk factor for prediabetes and progression to T2DM[29]. Currently, there is an epidemic of obesity in the world which begins early in life. Tools such as MetS Z-score can be utilized to diagnose the population at higher risk for future disease and assisting primary prevention by suggesting lifestyle modifications[5].
Measuring MetS has its limitations. Firstly, it is difficult to monitor changes in MetS over time[30,31]. Secondly, despite evidence demonstrating that elevated WC or high triacylglycerol levels have a more important role and stronger association with MetS risk over time due to abnormal cellular processes involved, in this method of measurement equal importance is given to all of the components of MetS[32,33]. Thirdly, gender, race and ethnicity cause variation in the MetS value, for example, African American men have a low prevalence of MetS despite having high rates of T2DM and death from cardiovascular disease[34-36].
All of the available data establishes the necessity of using a continuous measurement of MetS for clinical applications. Gurka et al[37,38] have formulated sex- and race/ethnicity-specific MetS Z-score. The standardized Z-scores for each component coming together creates an overall estimate of the severity of MetS[24] with a linear association with future risk of T2DM and offers a tool for monitoring treatment efficacy[12].
The role of genetic factors in MetS cannot be ignored[15]. The association of oxidative stress with inflammatory processes and MetS Z-score has been studied[5,39]. In our study, the high correlation between these scores over 15 years suggests a degree of consistency of MetS in a given individual over time or a genetic susceptibility in the FDR population.
Several studies on FDR individuals indicated that alteration in carbohydrate and lipid metabolism including central obesity, dyslipidemia, glucose intolerance, and high blood pressure start at an early age[40,41]. Siewert et al[19] illustrated that the prevalence of MetS is high in young FDR adults, and since MetS and T2DM are closely related diseases and are driven by the same metabolic disturbances, preventive measures at an early age seem appropriate.
The prevalence of MetS is influenced by various factors such as gender, environmental and cultural in addition to genetic factors[42].
Similar to the USA the prevalence of MetS is higher in women than men in Iran[43,44]. This sex difference can be explained by a statistically significant higher prevalence of MetS components in women and agrees with the results of our study. In the FDR population, women with higher WHR, TG, and cholesterol than normal women had a higher chance to progress to diabetes or prediabetes while such a difference was not observed in male FDRs. The differences in lipid profile could be explained by hepatic lipase activity, patterns of diet and physical activity. Women show a sharp decline in physical activity at adolescence as compared to men, and this could explain the higher prevalence of obesity in women[43].
Recently, during one cohort study in Iran, the paternal history of T2DM was independently associated with increased risk for pre-diabetes/T2D in adolescence [HR = 1.63 (1.02–2.60)][45]. One of our other studies reported that the glycemic response to OGTT may predict the risk of development to T2DM in the FDR population[46].
In summary, our study has suggested that MetS Z-score at baseline, in FDR of T2DM patients with normal glucose tolerance, predicts the incidence of future diabetes and prediabetes. In this study, the cutoff values of MetS Z-score for predicting prediabetes and diabetes were 0.94 and 0.97, respectively. This negligible difference between two groups in terms of cutoff values highlights the importance of intervention at the prediabetes stage. Elevated levels may also be used to motivate patients to increase their physical activity or adopt a healthy diet to reverse the prediabetic state[47]. Appropriate interventions at an earlier stage in MetS may be considered as an effective strategy for preventing the development of diabetes and prediabetes in such a high-risk population.
There is potential links between MetS Z-score as a marker for prediabetes or type 2 diabetes mellitus (T2DM) risk.
Iran is a developing country and its population is adopting a more sedentary lifestyle with new diets resulting in a high prevalence of T2DM and prediabetes.
In this study, the association the of severity of MetS Z-score in FDRs of T2DM was assessed with the risk of prediabetes and type 2 diabetes in future.
In a prospective cohort study during a long-term follow-up period for the first time in Iran and as one of scare studies around the world we evaluated the predictive role of MetS Z-score for prediabetes and diabetes incidence risk in future among normal glucose tests. Our study results help clinicians to identify and treat this high-risk population through conducting of interventions for preventing MetS or diminishing its side effects.
MetS Z-score at the baseline, is a significant predictor for developing future T2DM and prediabetes in total population and female group. Reliable cut off values with high accuracy were obtained in the receiver operating characteristic curve analysis based on the MetS Z-score for differentiating patients with diabetes and prediabetes from the normal population.
MetS Z-score is a significant predictor for incidence of diabetes and prediabetes risk in future in high risk population of FDR and cut off value for MetS score was not notably different for those people who affected by diabetes and prediabetes. This negligible difference between two groups in terms of cut off values highlights the importance of intervention at the prediabetes stage.
The FDR people with high risk of developing diabetes and prediabetes are identifiable based on MetS Z-score. Accordingly, appropriate interventions at an earlier stage in MetS may be considered as an effective strategy for preventing the development of diabetes and prediabetes in such a high-risk population.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Avtanski D S-Editor: Wang YQ L-Editor: A E-Editor: Ma YJ
| 1. | Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2709] [Cited by in RCA: 2396] [Article Influence: 184.3] [Reference Citation Analysis (0)] |
| 2. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7515] [Cited by in RCA: 8294] [Article Influence: 414.7] [Reference Citation Analysis (0)] |
| 3. | Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP; San Antonio Heart Study. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 463] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 4. | Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2172] [Cited by in RCA: 2089] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 5. | DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition. 2013;29:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 6. | Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 402] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 7. | Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 454] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 8. | Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 459] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 9. | Lee AM, Gurka MJ, DeBoer MD. A metabolic syndrome severity score to estimate risk in adolescents and adults: current evidence and future potential. Expert Rev Cardiovasc Ther. 2016;14:411-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 663] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 11. | Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1233] [Cited by in RCA: 1279] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 12. | DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia. 2015;58:2745-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. J Am Coll Cardiol. 2015;66:755-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Bosy-Westphal A, Onur S, Geisler C, Wolf A, Korth O, Pfeuffer M, Schrezenmeir J, Krawczak M, Müller MJ. Common familial influences on clustering of metabolic syndrome traits with central obesity and insulin resistance: the Kiel obesity prevention study. Int J Obes (Lond). 2007;31:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Deboer MD. Ethnicity, obesity and the metabolic syndrome: implications on assessing risk and targeting intervention. Expert Rev Endocrinol Metab. 2011;6:279-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V. Cosegregation of obesity with familial aggregation of type 2 diabetes mellitus. Diabetes Obes Metab. 2000;2:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Li JK, Ng MC, So WY, Chiu CK, Ozaki R, Tong PC, Cockram CS, Chan JC. Phenotypic and genetic clustering of diabetes and metabolic syndrome in Chinese families with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2006;22:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Park HS, Yim KS, Cho SI. Gender differences in familial aggregation of obesity-related phenotypes and dietary intake patterns in Korean families. Ann Epidemiol. 2004;14:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Siewert S, Filipuzzi S, Codazzi L, Gonzalez I, Ojeda MS. Impact of metabolic syndrome risk factors in first-degree relatives of type 2 diabetic patients. Rev Diabet Stud. 2007;4:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Azizi F, Ghanbarian A, Momenan AA, Hadaegh F, Mirmiran P, Hedayati M, Mehrabi Y, Zahedi-Asl S; Tehran Lipid and Glucose Study Group. Prevention of non-communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials. 2009;10:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 585] [Cited by in RCA: 681] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 21. | Amini M, Afshin-Nia F, Bashardoost N, Aminorroaya A, Shahparian M, Kazemi M. Prevalence and risk factors of diabetes mellitus in the Isfahan city population (aged 40 or over) in 1993. Diabetes Res Clin Pract. 1997;38:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5513] [Cited by in RCA: 5463] [Article Influence: 195.1] [Reference Citation Analysis (0)] |
| 23. | Janghorbani M, Salamat MR, Aminorroaya A, Amini M. Utility of the Visceral Adiposity Index and Hypertriglyceridemic Waist Phenotype for Predicting Incident Hypertension. Endocrinol Metab (Seoul). 2017;32:221-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | DeBoer MD, Gurka MJ. Clinical utility of metabolic syndrome severity scores: considerations for practitioners. Diabetes Metab Syndr Obes. 2017;10:65-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism. 2011;60:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 26. | Hulman A, Wagner R, Vistisen D, Færch K, Balkau B, Manco M, Golay A, Häring HU, Heni M, Fritsche A, Witte DR. Glucose Measurements at Various Time Points During the OGTT and Their Role in Capturing Glucose Response Patterns. Diabetes Care. 2019;42:e56-e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Amini M, Janghorbani M. Diabetes and impaired glucose regulation in first-degree relatives of patients with type 2 diabetes in isfahan, iran: prevalence and risk factors. Rev Diabet Stud. 2007;4:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Lee AM, Fermin CR, Filipp SL, Gurka MJ, DeBoer MD. Examining trends in prediabetes and its relationship with the metabolic syndrome in US adolescents, 1999-2014. Acta Diabetol. 2017;54:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | DeBoer MD, Gurka MJ, Golden SH, Musani SK, Sims M, Vishnu A, Guo Y, Pearson TA. Independent Associations Between Metabolic Syndrome Severity and Future Coronary Heart Disease by Sex and Race. J Am Coll Cardiol. 2017;69:1204-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 30. | Gustafson JK, Yanoff LB, Easter BD, Brady SM, Keil MF, Roberts MD, Sebring NG, Han JC, Yanovski SZ, Hubbard VS, Yanovski JA. The stability of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2009;94:4828-4834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Li C, Ford ES, Huang TT, Sun SS, Goodman E. Patterns of change in cardiometabolic risk factors associated with the metabolic syndrome among children and adolescents: the Fels Longitudinal Study. J Pediatr. 2009;155:S5.e9-S5.16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Janiszewski PM, Janssen I, Ross R. Does waist circumference predict diabetes and cardiovascular disease beyond commonly evaluated cardiometabolic risk factors? Diabetes Care. 2007;30:3105-3109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1443] [Cited by in RCA: 1547] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 34. | DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the metabolic syndrome is associated with disproportionately high levels of high-sensitivity C-reactive protein in non-Hispanic black adolescents: an analysis of NHANES 1999-2008. Diabetes Care. 2011;34:734-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | DeBoer MD, Gurka MJ. Low sensitivity of the metabolic syndrome to identify adolescents with impaired glucose tolerance: an analysis of NHANES 1999-2010. Cardiovasc Diabetol. 2014;13:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the ability of metabolic syndrome criteria to predict elevations in fasting insulin levels in adolescents. J Pediatr. 2011;159:975-81.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Gurka MJ, Ice CL, Sun SS, Deboer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol. 2012;11:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism. 2014;63:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 39. | de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 494] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 40. | Groop L, Forsblom C, Lehtovirta M, Tuomi T, Karanko S, Nissén M, Ehrnström BO, Forsén B, Isomaa B, Snickars B, Taskinen MR. Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes. 1996;45:1585-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 197] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Vauhkonen I, Niskanen L, Vanninen E, Kainulainen S, Uusitupa M, Laakso M. Defects in insulin secretion and insulin action in non-insulin-dependent diabetes mellitus are inherited. Metabolic studies on offspring of diabetic probands. J Clin Invest. 1998;101:86-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Rochlani Y, Pothineni NV, Mehta JL. Metabolic Syndrome: Does it Differ Between Women and Men? Cardiovasc Drugs Ther. 2015;29:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 43. | Mabry RM, Reeves MM, Eakin EG, Owen N. Gender differences in prevalence of the metabolic syndrome in Gulf Cooperation Council Countries: a systematic review. Diabet Med. 2010;27:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Ostovar R, Kiani F, Sayehmiri F, Yasemi M, Mohsenzadeh Y, Mohsenzadeh Y. Prevalence of metabolic syndrome in Iran: A meta-analysis. Electron Physician. 2017;9:5402-5418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Mirbolouk M, Derakhshan A, Charkhchi P, Guity K, Azizi F, Hadaegh F. Incidence and predictors of early adulthood pre-diabetes/type 2 diabetes, among Iranian adolescents: the Tehran Lipid and Glucose Study. Pediatr Diabetes. 2016;17:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Feizi A, Meamar R, Eslamian M, Amini M, Nasri M, Iraj B. Area under the curve during OGTT in first-degree relatives of diabetic patients as an efficient indicator of future risk of type 2 diabetes and prediabetes. Clin Endocrinol (Oxf). 2017;87:696-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care. 2012;35:131-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |