Published online May 15, 2020. doi: 10.4239/wjd.v11.i5.193
Peer-review started: January 1, 2020
First decision: January 15, 2020
Revised: March 24, 2020
Accepted: March 28, 2020
Article in press: March 28, 2020
Published online: May 15, 2020
Processing time: 130 Days and 20.5 Hours
Obesity and diabetes are associated with high levels of oxidative stress. In Romanian patients with obesity and (or) diabetes, this association has not been sufficiently explored.
To evaluate oxidative stress in obese and (or) diabetic subjects and to investigate the possible correlations between oxidative stress and anthropometric/biochemical parameters.
Oxidative stress was evaluated from a single drop of capillary blood. Reactive oxygen species (ROS) were evaluated using the free oxygen radical test (FORT). The free oxygen radical defence (FORD) assay was used to measure antioxidant levels.
FORT levels were higher in obese subjects (3.04 ± 0.36 mmol/L H2O2) vs controls (2.03 ± 0.14 mmol/L H2O2) (P < 0.0001). FORD levels were lower in obese subjects (1.27 ± 0.13 mmol/L Trolox) vs controls (1.87 ± 1.20 mmol/L Trolox) (P = 0.0072). Obese diabetic subjects had higher FORT values (3.16 ± 0.39 mmol/L H2O2) vs non-diabetic counterparts (2.99 ± 0.33 mmol/L H2O2) (P = 0.0233). In obese subjects, FORT values correlated positively with body mass index (BMI) (r = 0.48, P = 0.0000), waist circumference (WC) (r = 0.31, P = 0.0018), fasting plasma glucose (FPG) (r = 0.31, P = 0.0017), total cholesterol (TC) (r = 0.27, P = 0.0068) and uric acid (r = 0.36, P = 0.0001). FORD values correlated negatively with BMI (r = -0.43, P = 0.00001), WC (r = -0.28, P = 0.0049), FPG (r = -0.25, P = 0.0130), TC (r = -0.23, P = 0.0198) and uric acid (r = -0.35, P = 0.0002). In obese diabetic subjects, FORT values correlated positively with BMI (r = 0.49, P = 0.0034) and TC (r = 0.54, P = 0.0217). FORD values were negatively associated with BMI (r = -0.54, P = 0.0217) and TC (r = -0.58, P = 0.0121).
Oxidative stress levels, as measured by the FORT and FORD assays, were higher in obese subjects vs controls. ROS levels were elevated in diabetic obese patients vs obese non-diabetic patients and controls.
Core tip: Oxidative stress is involved in obesity, diabetes, and subsequently, diabesity (the co-occurrence of obesity and diabetes). In our study, oxidative stress levels were increased in patients with obesity, diabetes and diabesity. We suggest that the free oxygen radical test and the free oxygen radical defence assays are useful in evaluating the levels of oxidative stress in obesity, diabetes and diabesity. In this study, free oxygen radical test and free oxygen radical defence values also correlated with anthropometric and laboratory parameters in patients with obesity, diabetes and diabesity.
- Citation: Găman MA, Epîngeac ME, Diaconu CC, Găman AM. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World J Diabetes 2020; 11(5): 193-201
- URL: https://www.wjgnet.com/1948-9358/full/v11/i5/193.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i5.193
Worldwide, obesity and type 2 diabetes mellitus (T2DM) have emerged in epidemic proportions. This problematic issue has affected both high- and low-income countries alike, and by 2030 the global prevalence of T2DM is expected to surpass 7.5% (from 6.2% in 2010)[1,2]. According to the country profile released in 2016 by the World Health Organization (WHO), the prevalence of obesity and diabetes in Romania was estimated at 23.4% and 8.4%, respectively[3]. Thus, understanding the molecular mechanisms that drive the development and the evolution of obesity and T2DM are of the utmost importance in our country as well as worldwide.
Both obesity and T2DM are associated with increased levels of oxidative stress. These disorders are characterized by the excessive production of reactive oxygen species (ROS), as well as dysfunctional antioxidant systems[4,5]. Adipose tissue is a veritable endocrine organ, capable of producing adipokines which stimulate the generation of ROS and pro-inflammatory molecules, such as interleukin 1β (IL-1β) and 6 (IL-6) and tumour necrosis factor-alpha (TNF-α)[5]. In T2DM, chronic hyperglycaemia is also a source of ROS, and the ROS-hyperglycaemia crosstalk is involved in the development of the micro- and macro-vascular complications of T2DM[4]. Thus, a vicious cycle in which oxidative stress generates oxidative stress commences. In this context, the increased levels of oxidative stress in obese and diabetic subjects might explain the development of diabesity, i.e., the occurrence of T2DM in obese individuals[2].
Due to the limited knowledge regarding the crosstalk between obesity, T2DM and oxidative stress in Romanian patients, in the current study, we aimed to evaluate oxidative stress levels in obese non-diabetic and obese diabetic subjects, and to identify possible correlations between biochemical and oxidative stress parameters in these patients.
A total of 102 obese subjects were recruited from several outpatient clinics in Craiova, a city in southwest Romania, for inclusion in the study group. The patients were classified as obese and stratified into obesity classes in accordance with the WHO definition of obesity: Body mass index (BMI) > 30 kg/m2[6]. T2DM was diagnosed based on the American Diabetes Association criteria[7]. Thirty healthy individuals were selected for the control group.
Oxidative stress was evaluated from a single drop of capillary blood using the CR3000 analyser (Callegari, the Catellani group, Parma, Italy). The CR3000 analyser uses two colorimetric assays to evaluate oxidative stress: The free oxygen radical test (FORT) and the free oxygen radical defence (FORD). The FORT reflects the levels of ROS in the blood, and in normal individuals should have a value ≤ 2.3 mmol/L H2O2. The FORD assay reflects the levels of antioxidants in the blood, and in normal individuals has a value in the 1.07-1.53 mmol/L Trolox range. Both FORT and FORD are valuable tests in assessing oxidative stress levels in patients with T2DM and obesity and have been used in research for more than 10 years. The detailed principles of the assays are described elsewhere[8]. Reagents were also purchased from Callegari, the Catellani group, Parma, Italy.
The following demographic and clinical parameters were evaluated: Age, sex, weight and height to calculate the BMI and waist circumference (WC). The following laboratory variables were evaluated by standard methods: Fasting plasma glucose (FPG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), triglycerides (TG) and uric acid (UA). The estimated glomerular filtration rate (eGFR) was calculated using the MDRD formula.
Categorical variables are reported as frequencies and percentages and continuous variables as the mean ± SD. Categorical variables were compared using Fisher’s exact test. Continuous variables were compared using independent samples t-test. All variables were tested to check the normal distribution of the data. The Pearson and the Spearman correlation coefficients were employed for parametric and nonparametric variables to investigate the possible associations between FORT or FORD and other biochemical parameters. The level of significance was presented as P values and the analysis was performed at the 5% level of significance using GraphPad QuickCalcs (https://www.graphpad.com), MedCalc (https://www.medcalc.org) and Microsoft Excel (Microsoft Office Professional Plus 2013).
The demographic, clinical and laboratory parameters of the study population are reported in Table 1. In this study, we included 102 obese patients (mean age: 62.14 ± 10.19 years, 79.41% female) and 30 healthy controls (mean age: 44.60 ± 18.76 years, 70.00% female). Obese patients had higher BMI (35.75 ± 3.75 kg/m2 vs 24.56 ± 1.78 kg/m2, P < 0.0001), WC (106.58 ± 13.27 cm vs 96.38 ± 3.76 cm, P < 0.0001), FPG (117.56 ± 42.13 mg/dL vs 92.63 ± 7.60 mg/dL, P = 0.0016), TC (228.66 ± 45.22 mg/dL vs 137.70 ± 20.46 mg/dL, P < 0.0001), LDL-c (141.78 ± 42.43 mg/dL vs 106.93 ± 15.93 mg/dL, P < 0.0001), TG (156.98 ± 92.25 mg/dL vs 95.77 ± 20.22 mg/dL, P = 0.0005), UA (4.44 ± 1.18 mg/dL vs 3.80 ± 0.92 mg/dL, P = 0.0073), and lower HDL-c (47.72 ± 12.68 mg/dL vs 66.23 ± 10.85 mg/dL, P < 0.0001) and eGFR (68.49 ± 18.84 mL/min/1.73 m2vs 106.77 ± 17.75 mL/min/1.73 m2, P < 0.0001) vs controls. FORT values were higher (3.04 ± 0.36 mmol/L H2O2 vs 2.03 ± 0.14 mmol/L H2O2, P < 0.0001) and FORD levels were lower (1.27 ± 0.13 mmol/L Trolox vs 1.87 ± 1.20 mmol/L Trolox, P = 0.0072) in obese patients as compared to healthy controls.
| Obese subjects | Controls | P value | |
| Male/Female (n) | 21/81 | 9/21 | 0.3234 |
| Age (yr) | 62.14 (10.19) | 44.60 (18.76) | < 0.0001 |
| BMI (kg/m2) | 35.75 (3.75) | 24.56 (1.78) | < 0.0001 |
| WC (cm) | 106.58 (13.27) | 96.38 (3.76) | < 0.0001 |
| FPG (mg/dL) | 117.56 (42.13) | 92.63 (7.60) | 0.0016 |
| TC (mg/dL) | 228.66 (45.22) | 137.70 (20.46) | < 0.0001 |
| HDL-c (mg/dL) | 47.72 (12.68) | 66.23 (10.85) | < 0.0001 |
| LDL-c (mg/dL) | 141.78 (42.43) | 106.93 (15.93) | < 0.0001 |
| TG (mg/dL) | 156.98 (92.25) | 95.77 (20.22) | 0.0005 |
| UA (mg/dL) | 4.44 (1.18) | 3.80 (0.92) | 0.0073 |
| eGFR (mL/min/1.73 m2) | 68.49 (18.84) | 106.77 (17.75) | < 0.0001 |
| FORT (mmol/L H2O2) | 3.04 (0.36) | 2.03(0.14) | < 0.0001 |
| FORD (mmol/L Trolox) | 1.27 (0.13) | 1.87 (1.20) | 0.0072 |
The study population included 33 (32.35%) obese patients diagnosed with T2DM and 69 (67.65%) obese patients without T2DM. The demographic, clinical and laboratory parameters of the obese T2DM subjects vs the obese non-diabetic patients are shown in Table 2.
| T2DM | Obese non-diabetic subjects | P value | |
| Male/Female (n) | 6/27 | 15/54 | 0.7965 |
| Age (yr) | 66.45 (9.78) | 60.07 (9.79) | 0.0027 |
| BMI (kg/m2) | 36.66 (4.01) | 35.32 (3.57) | 0.0920 |
| WC (cm) | 107.24 (13.91) | 106.26 (13.04) | 0.7286 |
| FPG (mg/dL) | 153.32 (56.94) | 100.45 (13.91) | < 0.0001 |
| TC (mg/dL) | 231.92 (46.75) | 227.11 (44.74) | 0.6180 |
| HDL-c (mg/dL) | 43.98 (10.48) | 49.51 (13.30) | 0.0384 |
| LDL-c (mg/dL) | 137.54 (38.10) | 143.80 (44.47) | 0.4885 |
| TG (mg/dL) | 149.41 (111.44) | 160.60 (82.18) | 0.5690 |
| UA (mg/dL) | 4.70 (1.33) | 4.32 (1.10) | 0.1319 |
| FORT (mmol/L H2O2) | 3.16 (0.39) | 2.99 (0.33) | 0.0233 |
| FORD (mmol/L Trolox) | 0.67 (0.15) | 0.72 (0.14) | 0.0649 |
Obese diabetic subjects were older (66.45 ± 9.78 years vs 60.07 ± 9.79 years, P = 0.0027), had higher FPG (153.32 ± 56.94 mg/dL vs 100.45 ± 13.91 mg/dL, P < 0.0001) and lower HDL-c (43.98 ± 10.48 mg/dL vs 49.51 ± 13.30 mg/dL, P = 0.0384) vs obese subjects without diabetes. Also, obese subjects with T2DM recorded higher FORT values (3.16 ± 0.39 mmol/L H2O2 vs 2.99 ± 0.33 mmol/L H2O2, P = 0.0233) vs obese subjects without diabetes.
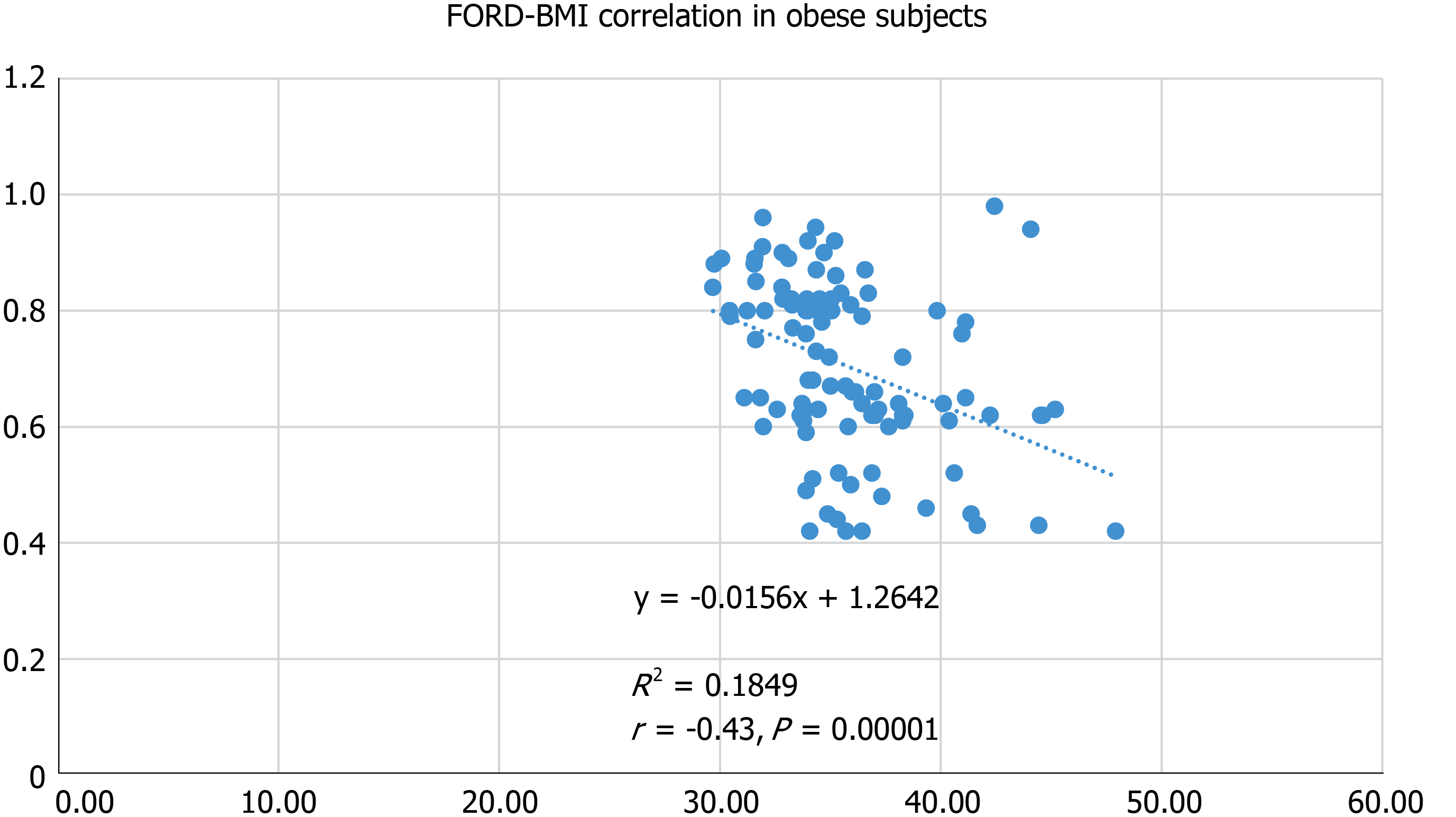
In obese subjects, we recorded positive correlations between FORT and BMI (r = 0.48, P = 0.0000), WC (r = 0.31, P = 0.0018), FPG (r = 0.31, P = 0.0017), TC (r = 0.27, P = 0.0068) and UA (r = 0.36, P = 0.0001). Also, in obese subjects, FORD correlated negatively with the BMI (r = -0.43, P = 0.00001) (Figure 1), WC (r = -0.28, P = 0.0049), FPG (r = -0.25, P = 0.0130), TC (r = -0.23, P = 0.0198) and UA (r = -0.35, P = 0.0002).
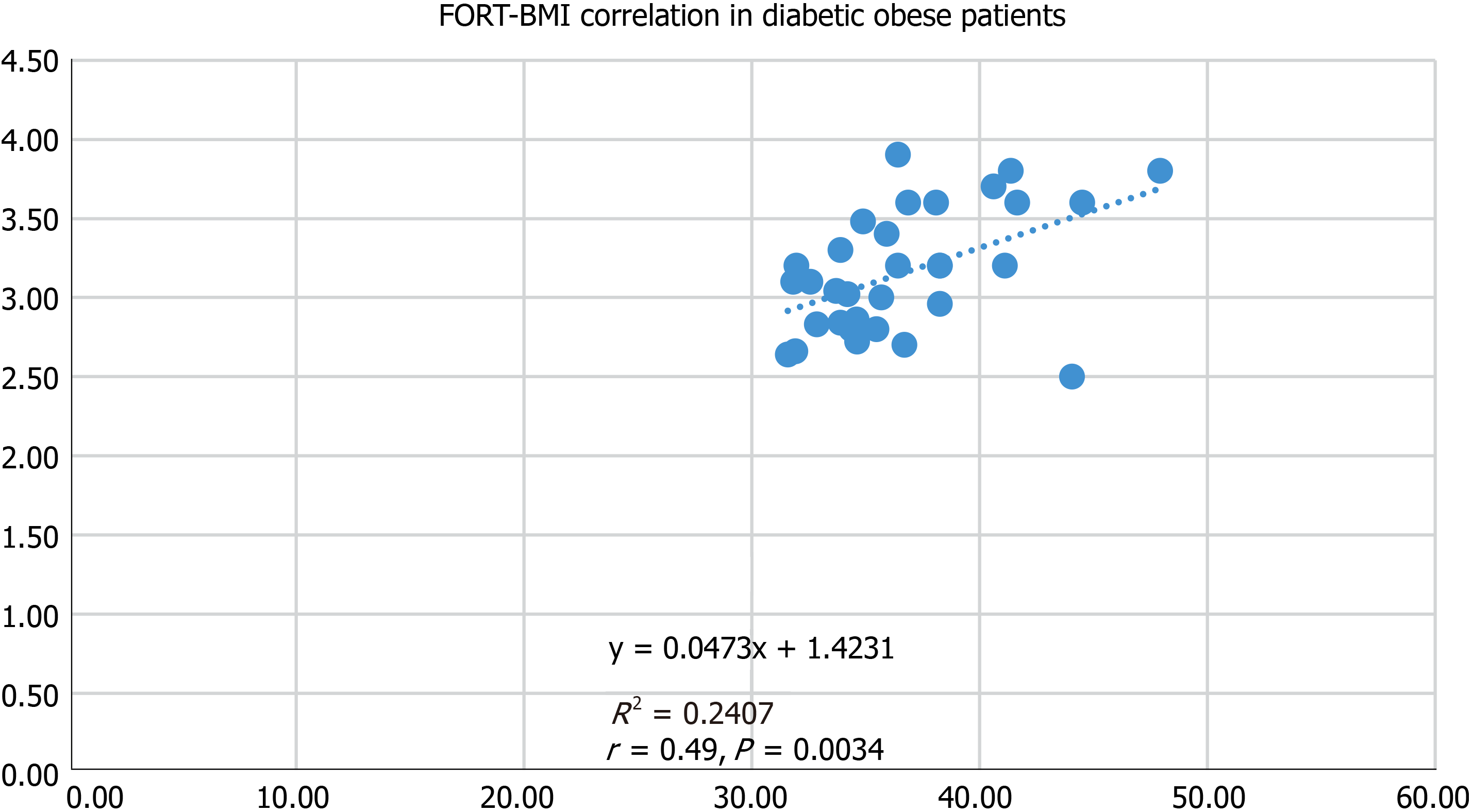
In obese diabetic subjects, we detected strong positive associations between FORT and BMI (r = 0.49, P = 0.0034) (Figure 2), and FORT and TC (r = 0.54, P = 0.0217). Also, FORD was negatively associated with BMI (r = -0.54, P = 0.0217) and TC (r = -0.58, P = 0.0121).
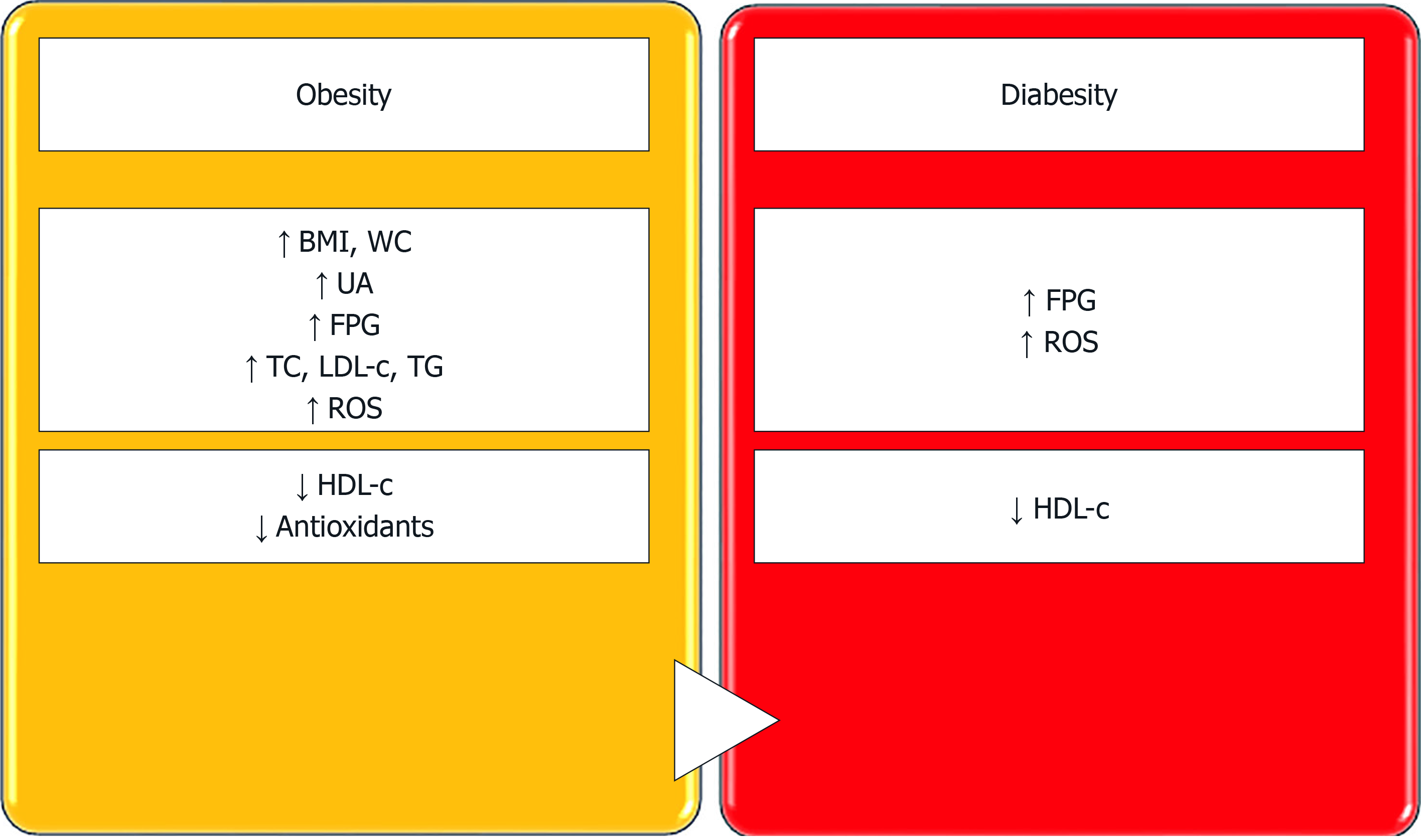
Our study focussed on the evaluation of oxidative stress in obese (diabetic and non-diabetic) patients, and showed that ROS levels (assessed by the FORT assay) are increased and antioxidant levels (assessed by the FORD assay) are decreased in patients diagnosed with obesity vs healthy controls. Moreover, obese patients who also had T2DM had higher ROS levels vs obese non-diabetic subjects and healthy controls. In obese subjects with T2DM, FORD levels were not significantly decreased as compared to non-diabetic obese patients; thus, we may assume that, in T2DM, the body might produce supplementary amounts of antioxidants to scavenge the excessive amount of free oxygen radicals (Figure 3).
Our study reinforced that ROS levels are increased in subjects with obesity and that the elevation in ROS is more pronounced in obese patients who have T2DM. Moreover, in obese patients, we detected positive correlations between FORT, which measures ROS levels in the body, and BMI, WC, FPG, TC and UA. Also, in patients diagnosed with diabesity (the association between obesity and T2DM), we recorded higher FORT values vs subjects with obesity and healthy controls. Similarly, Pavlatou et al[8] also evaluated oxidative stress levels in T2DM subjects using the FORT and FORD assays, and reported increased FORT values in diabetic patients vs healthy controls. Furthermore, their study demonstrated positive correlations between FORT and BMI, WC, LDL-c and TG. However, in T2DM patients, we found positive correlations between FORT and BMI (r = 0.49, P = 0.0034). Positive correlations between FORT and WC (r = 0.31, P = 0.0018) were only seen in obese non-diabetic subjects in our research. Thus, our data support the hypothesis that excessive body weight is associated with increased levels of oxidative stress, as ROS values increased and antioxidant levels decreased with increased BMI. Obesity is characterized by redox alterations induced and linked with excessive dietary intake, chronic fat cell inflammation, mitochondrial dysfunction, glycoxidation and oxidation of fatty acids. Moreover, oxidative stress seems to be related to the development of insulin resistance in T2DM. Obese subjects have insulin resistance which, in turn, causes compensatory hyperinsulinaemia and can explain the development of diabesity[9,10].
In our study, we detected decreased FORD values in obese patients. Also, FORD, which reflects the levels of antioxidants in the body, was negatively correlated with BMI (r = -0.54, P = 0.0217). Surprisingly, the difference in FORD levels between patients with obesity and those with diabesity was rather unremarkable. Thus, we may assume that, in T2DM, the body is forced to produce larger amounts of endogenous antioxidants to counteract the increase in ROS. Similar to our findings, Pavlatou et al[8] also reported decreased FORD levels in patients diagnosed with T2DM. However, in their research, the mean BMI of the patients was 29.3 ± 5.7 kg/m2 as opposed to 28.7 ± 4.2 kg/m2 in controls (P = 0.62). Thus, T2DM patients included in the aforementioned study were mostly overweight or were diagnosed with class I obesity. Despite the excessive generation of oxidative stress in obesity and T2DM, antioxidant supplementation remains controversial. Current evidence recommends lifestyle changes as a first step in the management of these disorders, with physical exercise and low-calorie, antioxidant-rich diets as key elements of the therapeutic armamentarium[11].
In the present study, TC correlated positively with FORT and TC (r = 0.27, P = 0.0068) and negatively with FORD (r = -0.23, P = 0.0198) in obese patients. Moreover, TC also correlated positively with FORT (r = 0.54, P = 0.0217) and negatively with FORD (r = -0.58, P = 0.0121) in diabetic obese patients. However, Pavlatou et al[8] reported a positive correlation between LDL-c and FORT (r = 0.03, P = 0.05). The FORT-TC and FORD-TC correlations reported in our study might be explained by the accumulation of toxic lipids which lead to lipotoxicity in diabetic and (or) obese patients[10]. In T2DM, dyslipidaemia is highly prevalent, with nearly 70% of patients having high TG and LDL-c values and low HDL-c[12]. Hypercholesterolaemia and obesity are recognized risk factors in the development of T2DM, but adherence to lipid-lowering drugs in patients with T2DM remains low[13-15]. HDL-c is a major candidate in the antioxidant defence against ROS-induced damage, and its myriad of positive effects in health (anti-inflammatory, antioxidant, anti-atherogenic etc.) are related to its strong cooperation with antioxidant enzymes, such as superoxide dismutase (SOD) or paraoxonase-1 (PON-1). The activity of PON-1 and SOD is reduced in obese dyslipidaemic patients[5]. Moreover, studies have shown that the activity of antioxidant enzymes, such as SOD or catalase, is also decreased in T2DM. It seems that the risk of developing T2DM is higher amongst catalase-deficient subjects[4]. On the other hand, Picu et al[16] reported only slightly lower SOD and total antioxidant status values, and slightly higher UA levels in T2DM patients as compared to controls. However, they did report a higher total oxidant status in T2DM patients vs healthy controls. Increased levels of UA might be related to development of the metabolic syndrome. In the metabolic syndrome, UA has been shown to stimulate the generation of ROS by fat cells and stimulates lipid peroxidation[17]. The role of oxidative stress in T2DM is complex, and experimental studies have concluded that chronic oxidative stress levels in pancreatic beta cells lead, via chronic hyperglycaemia-caused glucotoxicity, to a loss of expression of the endogenous insulin gene[18]. In addition, the contribution of high ROS levels, low antioxidant defences and lipid abnormalities in carcinogenesis should not be forgotten[19-21]. We previously reported that diffuse large B-cell lymphoma is associated with increased ROS levels and low HDL-c and antioxidant values[22]. Moreover, low HDL-c levels have also been linked to other types of non-Hodgkin’s lymphoma, breast, lung, gynaecological or prostate cancers, as well as other malignancies[23].
Our research has some strengths. Firstly, the involvement of oxidative stress in T2DM and (or) obesity in the population from southwest Romania has received little attention in studies. We believe this is the first report to evaluate oxidative stress in obese and (or) diabetic patients using the FORT and FORD assays in Romania. Moreover, we evaluated ROS and antioxidant levels using a point-of-care method which has been employed in research for over ten years, and we reported not only that oxidative stress levels are increased in obese and (or) diabetic subjects, but also the correlations between FORT or FORD and anthropometric/biochemical parameters. The current study confirms our previous research findings, i.e., that obesity is associated with increased oxidative stress levels[24]. However, our research initiative has several limitations. We included a relatively small number of patients and we were unable to recruit a control group of diabetic non-obese subjects. In addition, the CR3000 was not designed to evaluate urinary oxidative stress parameters. We will work on addressing these limitations in the near future.
In conclusion, oxidative stress levels, as measured by the FORT and FORD assays, were higher in obese subjects vs healthy controls. ROS levels were elevated in diabetic obese patients vs obese non-diabetic patients and healthy controls. Obese patients had higher BMI, WC, FPG, TC, LDL-c, TG, UA and lower HDL-c vs healthy controls. In obese subjects, FORT levels correlated positively with BMI, FPG, TC and UA, and FORD levels correlated negatively with BMI, WC, FPG, TC and UA. Obese diabetic subjects were older, had higher FPG and lower HDL-c. In obese diabetic subjects, FORT levels correlated positively with BMI and TC, and FORD levels correlated negatively with BMI and TC. Taken together, these findings indicate that the management of obesity and (or) diabetes should also take into consideration strategies to reduce ROS levels and increase the antioxidant capacity of the body, in addition to the treatment of lipid abnormalities. However, further studies are needed to clarify the crosstalk between oxidative stress, obesity and diabesity.
Oxidative stress is a key player in health and disease, and its particular involvement in the development of obesity, type 2 diabetes mellitus, cardiovascular disorders, neurodegeneration and cancer have attracted much attention from the scientific community in recent years.
The motivation for our research was to contribute to the study of oxidative stress involvement in obesity, diabetes and their co-occurrence (diabesity), and to improve the current knowledge regarding the development of these public health problems.
The main objectives of this study were to evaluate oxidative stress levels in obesity, diabetes and diabesity using the free oxygen radical test (FORT) and the free oxygen radical defence (FORD) tests. In addition, we investigated whether FORT and (or) FORD values correlated with anthropometric and laboratory parameters.
Oxidative stress was evaluated from a single drop of capillary blood using the CR3000 analyser by two colorimetric assays: The free oxygen radical test (FORT) and the free oxygen radical defence (FORD) assays. Demographic, clinical and biochemical parameters were assessed by standard methods.
FORT levels were higher in obese subjects vs healthy controls and correlated positively with body mass index, waist circumference, fasting plasma glucose, total cholesterol and uric acid. FORD levels were lower in obese subjects vs healthy controls and correlated negatively with body mass index, waist circumference, fasting plasma glucose, total cholesterol and uric acid. Patients with diabesity had higher FORT values vs non-diabetic counterparts. In these subjects, FORT levels correlated positively with body mass index and total cholesterol, and FORD levels was negatively associated with body mass index and total cholesterol.
Oxidative stress levels are increased in obese subjects. In patients with diabesity, reactive oxygen species are elevated vs obese non-diabetic subjects and controls.
Further studies are needed to clarify the role of oxidative stress in obesity, diabetes and diabesity, and to transpose these results from bench to bedside. The value of antioxidants in the management of these public health problems needs further clarification.
Manuscript source: Invited Manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aureliano M, Nakhoul F S-Editor: Wang YQ L-Editor: Webster JR E-Editor: Liu MY
| 1. | Haghighatdoost F, Amini M, Aminorroaya A, Abyar M, Feizi A. Different metabolic/obesity phenotypes are differentially associated with development of prediabetes in adults: Results from a 14-year cohort study. World J Diabetes. 2019;10:350-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Riobó Serván P. Obesity and diabetes. Nutr Hosp. 2013;28 Suppl 5:138-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 3. | WHO Diabetes Country Profiles 2016. Geneva, Switzerland: World Health Organization, 2016. Available from: https://www.who.int/diabetes/country-profiles/diabetes_profiles_explanatory_notes.pdf. |
| 4. | Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PI. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J. 2016;473:4527-4550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 592] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 5. | Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, Di Rosa G, Gitto E, Arrigo T. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. 2014;16:378-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 684] [Cited by in RCA: 629] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 6. | Measuring Obesity—Classification and Description of Anthropometric Data. Report on a WHO Consultation of the Epidemiology of Obesity. Copenhagen: World Health Organization, 1987. |
| 7. | American Diabetes Association. Executive summary: Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (1)] |
| 8. | Pavlatou MG, Papastamataki M, Apostolakou F, Papassotiriou I, Tentolouris N. FORT and FORD: two simple and rapid assays in the evaluation of oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2009;58:1657-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Rani V, Deep G, Singh RK, Palle K, Yadav UC. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016;148:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 759] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 10. | Niemann B, Rohrbach S, Miller MR, Newby DE, Fuster V, Kovacic JC. Oxidative Stress and Cardiovascular Risk: Obesity, Diabetes, Smoking, and Pollution: Part 3 of a 3-Part Series. J Am Coll Cardiol. 2017;70:230-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 241] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 11. | Abdali D, Samson SE, Grover AK. How effective are antioxidant supplements in obesity and diabetes? Med Princ Pract. 2015;24:201-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Jialal I, Singh G. Management of diabetic dyslipidemia: An update. World J Diabetes. 2019;10:280-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (2)] |
| 13. | Boles A, Kandimalla R, Reddy PH. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1026-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 14. | Găman MA, Dobrică EC, Pascu EG, Cozma MA, Epîngeac ME, Găman AM, Pantea SA, Bratu OG, Diaconu CC. Cardio metabolic risk factors for atrial fibrillation in type 2 diabetes mellitus: Focus on hypertension, metabolic syndrome and obesity. J Mind Med Sci. 2019;6:157-161. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Alwhaibi M, Altoaimi M, AlRuthia Y, Meraya AM, Balkhi B, Aldemerdash A, Alkofide H, Alhawassi TM, Alqasoumi A, Kamal KM. Adherence to Statin Therapy and Attainment of LDL Cholesterol Goal Among Patients with Type 2 Diabetes and Dyslipidemia. Patient Prefer Adherence. 2019;13:2111-2118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Picu A, Petcu L, Ştefan S, Mitu M, Lixandru D, Ionescu-Tîrgovişte C, Pîrcălăbioru GG, Ciulu-Costinescu F, Bubulica MV, Chifiriuc MC. Markers of Oxidative Stress and Antioxidant Defense in Romanian Patients with Type 2 Diabetes Mellitus and Obesity. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Hopps E, Noto D, Caimi G, Averna MR. A novel component of the metabolic syndrome: the oxidative stress. Nutr Metab Cardiovasc Dis. 2010;20:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 18. | Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005;280:11107-11113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Moisă C, Găman MA, Pascu EG, Assani AD, Drăgusin OC, Epîngeac ME, Găman AM. The role of oxidative stress in essential thrombocythemia. Arch Balk Med Union. 2018;53:70-75. |
| 20. | Moisă C, Găman MA, Diaconu CC, Assani AD, Găman AM. The evaluation of oxidative stress in patients with essential thrombocythemia treated with risk-adapted therapy. Arch Balk Med Union. 2018;53:529-534. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Wen J, Dong Q, Liu G, Gao Y, Li XL, Jin JL, Li JJ, Guo YL. Improvement of oxidative stress status by lipoprotein apheresis in Chinese patients with familial hypercholesterolemia. J Clin Lab Anal. 2019;e23161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Găman MA, Epîngeac ME, Găman AM. The evaluation of oxidative stress and high-density lipoprotein cholesterol levels in diffuse large B-cell lymphoma. Rev Chim (Bucharest). 2019;70:977-980. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Vílchez JA, Martínez-Ruiz A, Sancho-Rodríguez N, Martínez-Hernández P, Noguera-Velasco JA. The real role of prediagnostic high-density lipoprotein cholesterol and the cancer risk: a concise review. Eur J Clin Invest. 2014;44:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Epîngeac ME, Găman MA, Diaconu CC, Gad M, Găman AM. The evaluation of oxidative stress levels in obesity. Rev Chim (Bucharest). 2019;70:2241-2244. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |