Published online Feb 15, 2019. doi: 10.4239/wjd.v10.i2.133
Peer-review started: January 4, 2019
First decision: January 12, 2019
Revised: February 13, 2019
Accepted: February 13, 2019
Article in press: February 14, 2019
Published online: February 15, 2019
Processing time: 46 Days and 8.6 Hours
Sodium glucose cotransporter 2 (SGLT2) inhibitors use has been associated with toe amputations and non-healing ulcers and gangrene mostly of lower extremities. There are no case reports about association of Empagliflozin with finger ulcers or gangrene. This is the first case report of Empagliflozin (Jardiance) an SGLT2 inhibitor causing gangrene of fingers and second case in literature about any SGLT2 inhibitor causing gangrene of upper extremity.
A 76-year-old man with type 2 diabetes mellitus sustained minimal trauma to both middle fingers, which started healing. He was started on empagliflozin a week later for management of type 2 diabetes mellitus and started developing gangrene to both middle finger tips along with neuropathic pain which worsened over the course of next four months. Investigations were negative for vascular insufficiency, infection and vasculitis and imaging of hand was normal. Discontinuation of empagliflozin slowed progression of gangrene and caused symptomatic improvement with reduction in neuropathic pain.
This case report suggests possible association of empagliflozin and finger gangrene and recommends that more research and awareness among clinicians is needed in this area.
Core tip: Empagliflozin can cause finger gangrene in patients with type 2 diabetes mellitus. Empaglifozin has gained popularity recently as a newer anti diabetic agent with improved cardiovascular outcomes and better glycemic control in addition to lowering blood pressure and helping with weight loss. Lack of proper awareness about this condition can lead to progression of disease if not identified early on and can result in amputations. This medication should be used with caution in patients who have high risk of gangrene such as that on prednisone and in those with diabetic neuropathy.
- Citation: Ramachandra Pai RP, Kangath RV. Bilateral gangrene of fingers in a patient on empagliflozin: First case report. World J Diabetes 2019; 10(2): 133-136
- URL: https://www.wjgnet.com/1948-9358/full/v10/i2/133.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i2.133
This is the first ever case reported in literature about empaglifozin (Jardiance) as a possible cause of finger gangrene. Sodium glucose cotransporter 2 (SLGT2) inhibitors inhibit sodium and glucose cotransport at proximal renal tubules. SLGT2 inhibitors have been associated with an increased risk of genital infections secondary to increased glycosuria. According to the results of CANVAS trial, Dapagliflozin, another SGLT2 inhibitor of the same class as empagliflozin, has been shown to significantly reduce the risk of cardiovascular events by 14% but it doubled the risk of amputation in patients with type 2 diabetes mellitus[1]. In a similar study conducted on patients with type 2 diabetes mellitus at high risk for cardiovascular events, patients were given empaglifozin vs placebo and those on empagliflozin had lesser adverse cardiovascular events and lower all-cause mortality. Among patients receiving empagliflozin, there was an increased rate of genital infections but there was no increase in lower limb amputations[2]. In another study of over eight million case safety reports, increased risk of lower-limb amputations especially toe amputations were reported with empagliflozin[3].
A data analysis conducted based on data from US Food and Drug Administration adverse event Reporting System showed a total of 66 cases of SGLT2 inhibitor-associated amputations[3]. Among these, there was only one case of hand amputation which was from Dapagliflozin. All others were lower extremity gangrene and ulcers, most commonly of toes[4]. There are two case reports of empagliflozin related Fournier’s gangrene in literature[5,6] which pointed the benefit of keeping a high index of suspicion and early cessation of SGLT2 inhibitors could potentially prevent the progression of these infections requiring surgical debridement later. Empagliflozin has also been associated with vulvovaginal candidiasis along with other SGLT2 inhibitors[7].
SGLT2 inhibitors are used in general, cautiously in patients with vascular insufficiency, neuropathy, risk of amputations and very high hemoglobin A1C over 11. However, there are no case reports to date about an empagliflozin as a possible cause of non-healing finger ulcers or gangrene. Ours is the first reported case of empagliflozin (a SGLT2 inhibitor) as likely cause of gangrene of fingers.
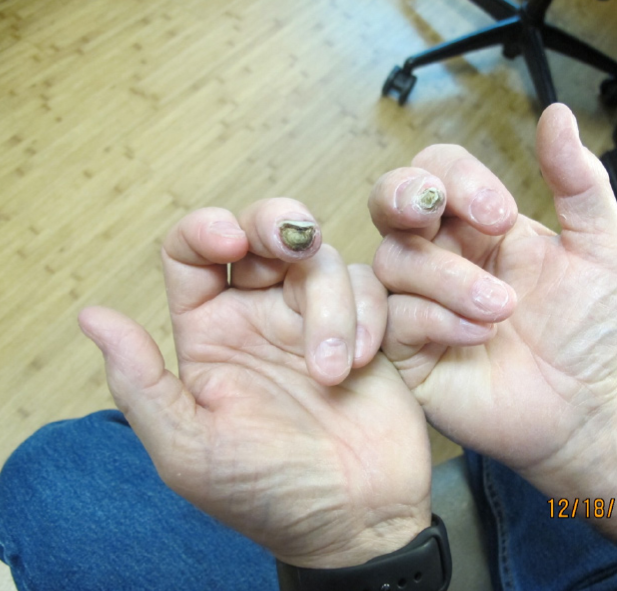
Gangrene both middle fingers.
A 76-year-old man with moderately controlled type 2 diabetes mellitus (hba1c of 8.6) sustained minor injury to the tip of both middle fingers while doing some mechanical work. He had no burns or exposure to heat. Initially, the fingers were healing well with minimal scarring. A week after the injury, he was started on empagliflozin 10 mg for better glycemic control in addition to his other medications. Three weeks after the injury (two weeks after being started on empagliflozin), he started noticing significant pain on tip of both middle fingers which also started changing color to brown and then to black (Figure 1).
No history of previous vasculitis. He has history of polymyalgia rheumatica and was on prednisone 3 mg daily for the past few years. His other medications included aspirin, atorvastatin, metformin and saxagliptin. No history of diabetic neuropathy.
He is a nonsmoker with no alcohol use. No family history of diabetes, gangrene or significant illnesses.
He was seen and evaluated in the emergency room twice in the following four months due to worsening symptoms and investigations were done. On exam during both times, he was afebrile, and physical exam was normal except for gangrenous changes tips of both middle fingers. There was no area of erythema around the region of gangrene on either side. Ankle brachial pressure index was normal and filling pressures were normal in both upper extremities.
Blood counts, erythrocyte sedimentation rate, C reactive protein were within normal limits. Tests for vasculitis were negative including Anti-nuclear cytoplasmic antibody and anti-nuclear antibody.
Hand X-rays were normal. Echocardiogram showed no evidence of embolic sources.
Possible etiology was concluded to be from microvascular damage of unclear etiology.
Plastic surgery, vascular surgery, dermatology and rheumatology referrals were completed. Biopsy was withheld as there was no surrounding erythema. Patient was seen in endocrinology outpatient for diabetes management and his endocrinologist suspected empagliflozin as a possible cause and discontinued the medication. He was switched to alternate medications for better glycemic control.
After a week of stopping empagliflozin, patient started noticing improvement in his pain as well as slowing of blackish discoloration near tip of fingers.
Occurrence of finger gangrene or upper extremity gangrene in individuals with type 2 diabetes on treatment with empaglifozin has not been described previously in the literature. We suggest this adverse event could be under reported due to low index of suspicion.
Patient mentioned in this case presented with gangrene at the same site where he sustained minimal trauma initially, therefore the suspicion was more for vasculitis. But the patient had noticed that the sites were healing well initially. Starting of empagliflozin coincided with onset of symptoms of neuropathic pain and worsening of non-healing ulcers and development of gangrene tip of fingers and vasculitis markers were negative.
Even though this patient has polymyalgia rheumatica and was on prednisone at the time of symptoms, markers for vasculitis were negative and he was on consistent dose of low dose prednisone for few years before onset of symptoms. Addition of empagliflozin again was the only other contributing factor for development of symptoms.
The timing of empaglifozin and onset of symptoms as well as improvement after stopping empaglifozin point towards a likely association of the medication with finger gangrene.
This first case report of empaglifozin causing finger gangrene suggests the possibility that upper extremity gangrene with use of empaglifozin could go undiagnosed as occurred initially in this case. Prescribers need to be aware of this association and future studies are warranted to clarify if upper extremity ulcers or gangrene are associated with SGLT2 inhibitor use.
Increased awareness among primary care physicians and surgeons about this association could prevent progression of non-healing upper extremity ulcers, gangrene and resultant amputations.
Conflict of interest statement: The authors disclose no relevant financial conflicts of interest.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Saisho Y, Koch TR S- Editor: Wang JL L- Editor: A E- Editor: Song H
| 1. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5388] [Article Influence: 673.5] [Reference Citation Analysis (0)] |
| 2. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8279] [Article Influence: 827.9] [Reference Citation Analysis (1)] |
| 3. | Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: Is this a class effect? Diabetes Obes Metab. 2018;20:1531-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA Adverse Event Reporting System. Diabetologia. 2017;60:1385-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 5. | Kumar S, Costello AJ, Colman PG. Fournier’s gangrene in a man on empagliflozin for treatment of Type 2 diabetes. Diabetic Medicine. 2017;1646-1648. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Voelker R. Rare Infection Linked With SGLT-2 Inhibitor Use. JAMA. 2018;320:1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Li D, Wang T, Shen S, Fang Z, Dong Y, Tang H. Urinary tract and genital infections in patients with type 2 diabetes treated with sodium-glucose co-transporter 2 inhibitors: A meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |