Copyright
©The Author(s) 2015.
World J Diabetes. Feb 15, 2015; 6(1): 136-144
Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.136
Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.136
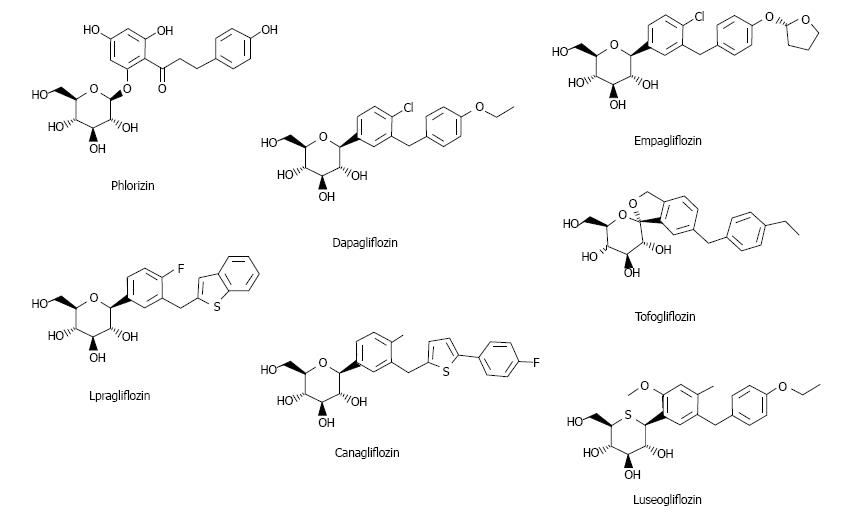
Figure 1 Chemical structure of sodium-glucose cotransporter 2 inhibitors in late-stage clinical trials.
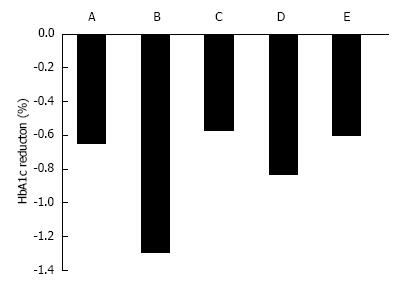
Figure 2 The adjusted mean difference in HbA1c from baseline to 12 wk between placebo and the standard dose of sodium-glucose cotransporter 2 inhibitors.
A: 50 mg ipragliflozin in Westerners; B: 50 mg ipragliflozin in Japanese; C: 100 mg canagliflozin in Westerners; D: 10 mg dapagliflozin in Westerners; E: 25 mg empagliflozin in Westerners.
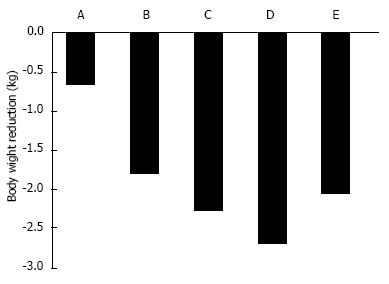
Figure 3 The adjusted mean difference in body weight from baseline to 12 wk between placebo and the standard dose of sodium-glucose cotransporter 2 inhibitors.
A: 50 mg ipragliflozin in Westerners; B: 50 mg ipragliflozin in Japanese; C: 100 mg canagliflozin in Westerners; D: 10 mg dapagliflozin in Westerners; E: 25 mg empagliflozin in Westerners.
- Citation: Ohkura T. Ipragliflozin: A novel sodium-glucose cotransporter 2 inhibitor developed in Japan. World J Diabetes 2015; 6(1): 136-144
- URL: https://www.wjgnet.com/1948-9358/full/v6/i1/136.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i1.136