Copyright
©The Author(s) 2024.
World J Diabetes. Sep 15, 2024; 15(9): 1847-1852
Published online Sep 15, 2024. doi: 10.4239/wjd.v15.i9.1847
Published online Sep 15, 2024. doi: 10.4239/wjd.v15.i9.1847
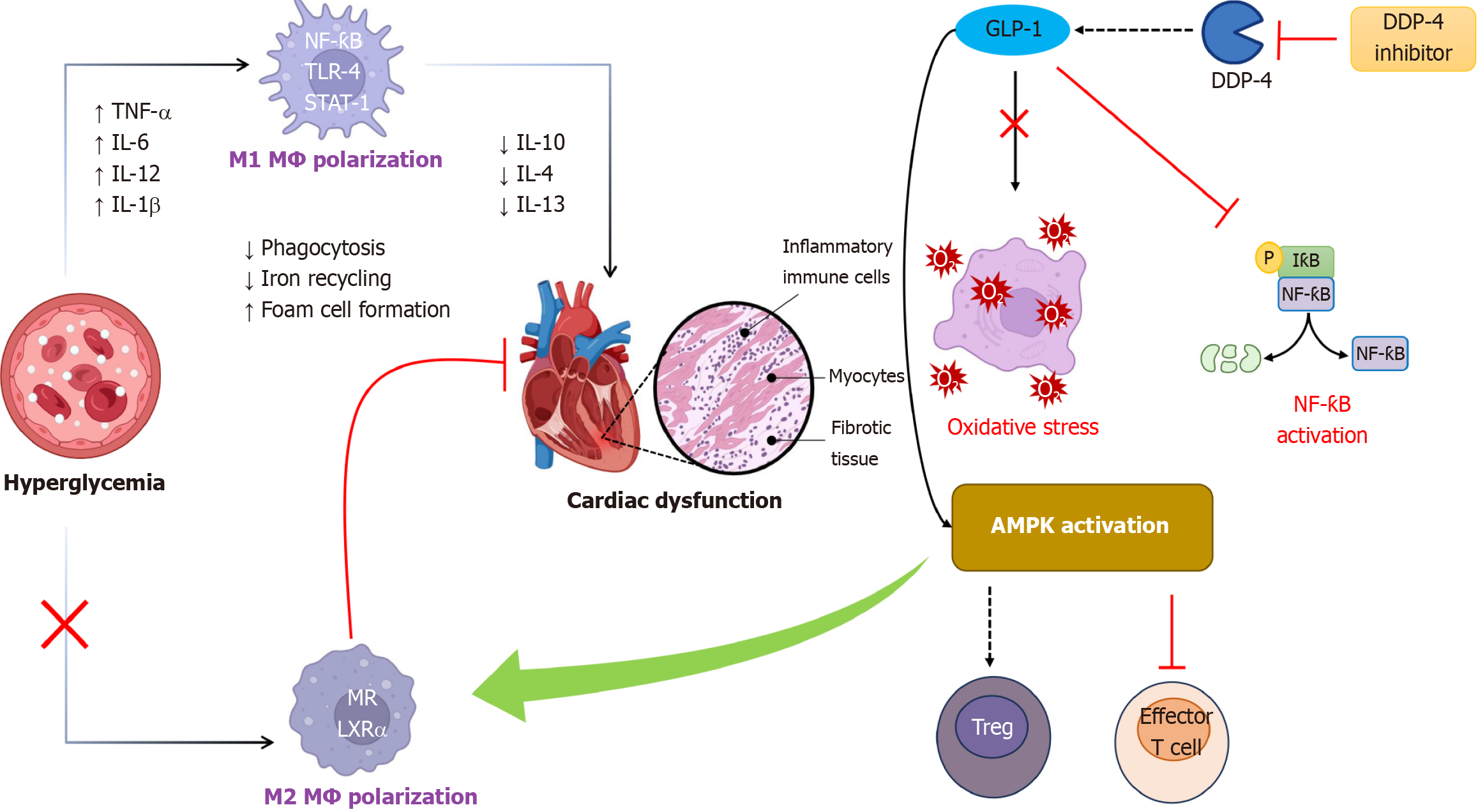
Figure 1 Potential mechanisms by which dipeptidyl peptidase-4 inhibitors modulate macrophage function and protect against diabetic cardiomyopathy.
Dipeptidyl peptidase-4 (DPP-4) inhibition elevates glucagon-like peptide-1 (GLP-1) levels, which promotes an anti-inflammatory M2 macrophage phenotype and reduces pro-inflammatory cytokine production. This shift dampens inflammation and promotes tissue repair. GLP-1 signaling reduces oxidative stress in macrophages, protecting them from damage and promoting a healthier phenotype. DPP-4 inhibitors might influence macrophage function by suppressing pro-inflammatory signaling pathways (e.g., nuclear factor kappa B) and enhancing AMP-activated protein kinase activation, which promotes fatty acid oxidation and energy production. GLP-1 signaling may influence cholesterol transporters in macrophages, improving their ability to efflux excess cholesterol and reducing atherogenic potential. NF-κB: Nuclear factor kappa B; AMPK: AMP-activated protein kinase; TNF: Tumor necrosis factor; IL: Interleukin; GLP-1: Glucagon-like peptide-1; DDP-4: Dipeptidyl peptidase-4; Treg: Regulatory T cell; MR: Mannose receptor; LXRα: Liver X receptor alpha; TLR-4: Toll-like receptor 4; STAT-1: Signal transducer and activator of transcription 1; IκB: Inhibitor of kappa B.
- Citation: Mohammadi S, Al-Harrasi A. Macrophage modulation with dipeptidyl peptidase-4 inhibitors: A new frontier for treating diabetic cardiomyopathy? World J Diabetes 2024; 15(9): 1847-1852
- URL: https://www.wjgnet.com/1948-9358/full/v15/i9/1847.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i9.1847