Copyright
©The Author(s) 2024.
World J Diabetes. Oct 15, 2024; 15(10): 2022-2035
Published online Oct 15, 2024. doi: 10.4239/wjd.v15.i10.2022
Published online Oct 15, 2024. doi: 10.4239/wjd.v15.i10.2022
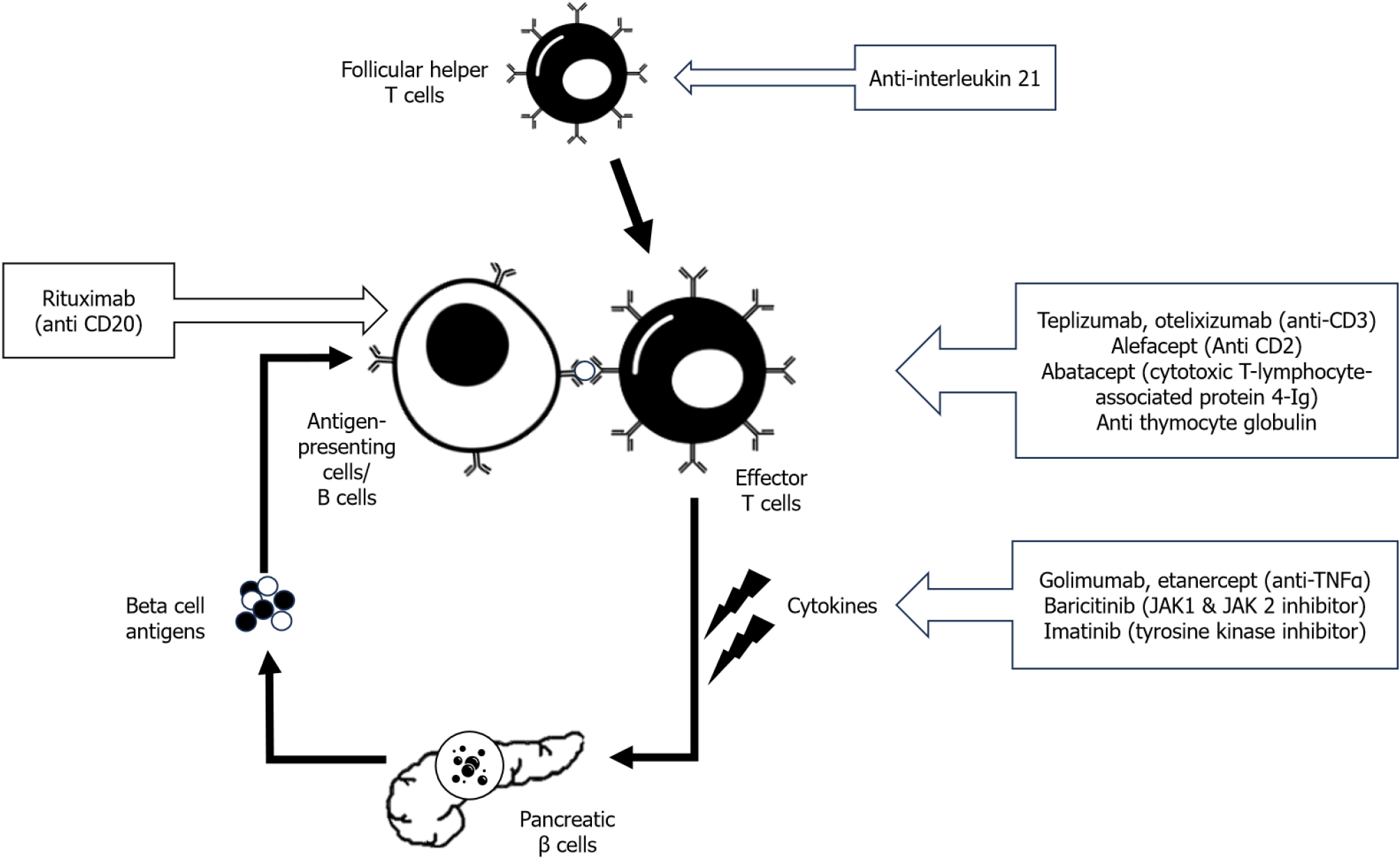
Figure 1 Outline of antibody-based immunotherapy in type 1 diabetes.
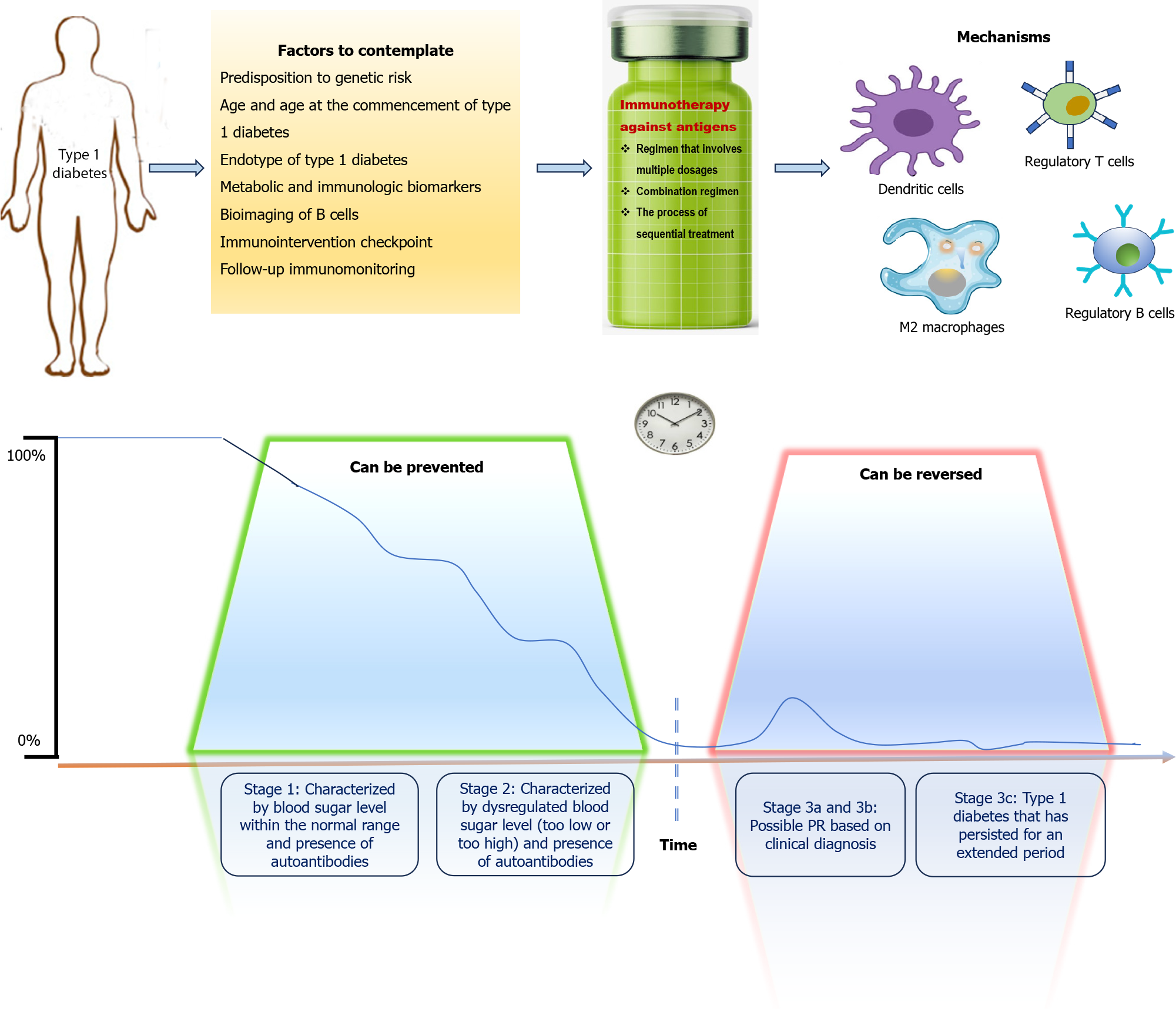
Figure 2 Considerations and requirements for successful immunotherapy using antigen-specific approaches in type 1 diabetes.
If the antigen-specific therapy is administered before the clinical diagnosis of type 1 diabetes (T1D) (stage 1 and 2), the development of T1D could be revoked; otherwise, if T1D has already been diagnosed (stage 3), the intervention should be accompanied by a β cell regenerative agent to restore the β cell mass and its functionality. One should consider the genetic risk susceptibility of the patient, age and age at T1D onset, autoreactivity profile and T1D endotype, metabolic and immunologic biomarkers, and ideal antigen-specific therapy time point for the treatment to be as personalized as possible. On this basis, the partial remission (PR) – when β cell tolerance is thought to be restored transiently – and exploring subsequent but milder PR phases could be of value as immune intervention checkpoints (in stage 3a and 3b). In addition, the antigen-specific immunotherapy should enforce the generation of tolerogenic dendritic cells, M2 macrophages, regulatory T (Treg) cells and regulatory B cells, thus reeducating the immune response against β cell autoantigens and re-establishing β cell immunological tolerance.
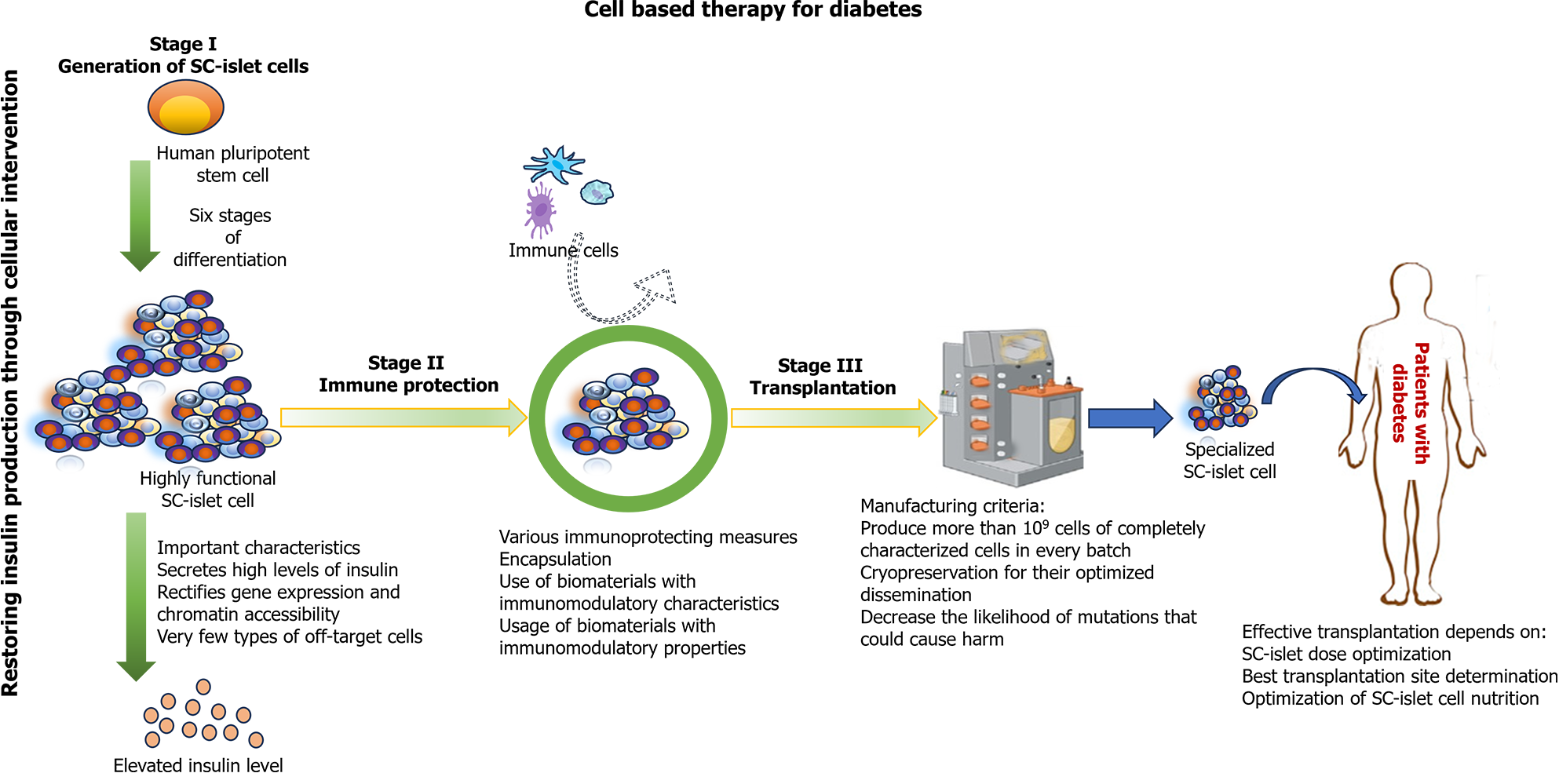
Figure 3 Key components of successful stem cell derived islet therapy for treating type 1 diabetes.
- Citation: Ray S, Palui R. Immunotherapy in type 1 diabetes: Novel pathway to the future ahead. World J Diabetes 2024; 15(10): 2022-2035
- URL: https://www.wjgnet.com/1948-9358/full/v15/i10/2022.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i10.2022