Copyright
©The Author(s) 2021.
World J Diabetes. Oct 15, 2021; 12(10): 1655-1673
Published online Oct 15, 2021. doi: 10.4239/wjd.v12.i10.1655
Published online Oct 15, 2021. doi: 10.4239/wjd.v12.i10.1655
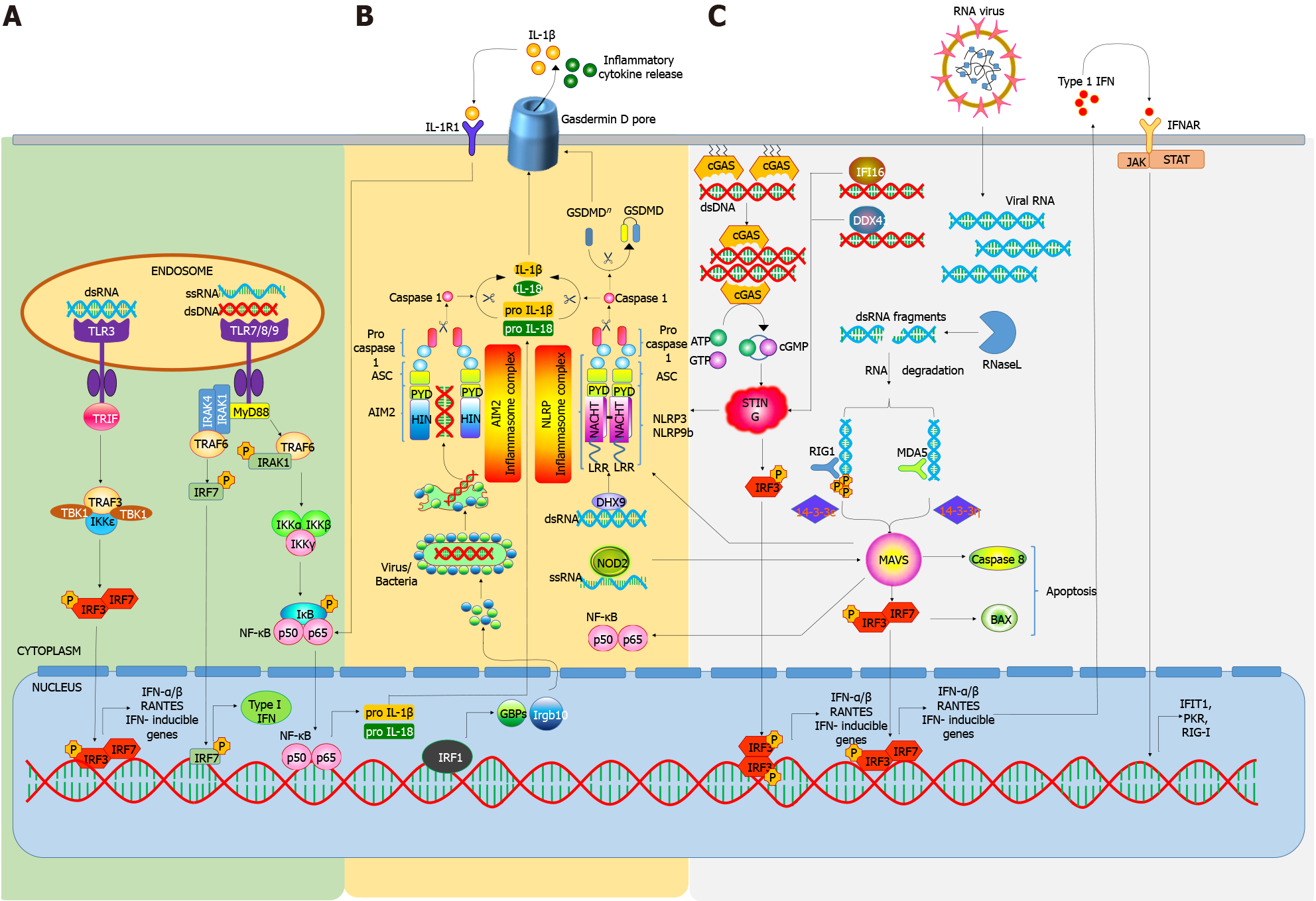
Figure 1 Nucleic acid sensors and their signaling pathways involved in autoimmune diseases including type 1 diabetes.
A: Toll-like receptor (TLR) signaling: Priming of nucleic acid sensing is mediated by the activation of several TLRs, which are located in endosomes. For e.g., TLR3 recognizes double stranded RNA initiating downstream TIR-domain-containing adapter-inducing interferon (IFN)-β dependent signaling cascade via activation of IRF3 and IRF7, resulting in the induction of IFN-stimulated genes (ISGs). On the other hand, TLR7, TLR8 and TLR9 recognize ssRNA and dsDNA to trigger downstream signaling via Myd88, resulting in higher expression of either type1 IFNs or NF-κB via IRF7 and IκB phosphorylation, respectively. NF-κB activation further stimulates the production of pro interleukin (IL)-1β and pro IL-18, which get cleaved by caspase 1 into mature IL-1β and IL-18, respectively; B: Inflammasome complexes: Following recognition of nucleic acids, recruitment of various adaptor proteins occurs to form mature inflammasome complexes, which further cleave pro-caspase 1 and gasdermin D (GSDMD) into active caspase 1 and GSDMDn (GSDMD n-terminal), respectively. GSDMD gets inserted into the plasma membrane and helps in the release of inflammatory cytokines; C: Cytosolic Receptors: cGAS is another DNA sensor localized close to the plasma membrane. It recognizes and forms complexes with dsDNA. cGAS-dsDNA binding induces the catalytic synthesis of cGAMP from ATP and GTP, which further culminates in the stimulation of STING. Other DNA binding proteins (or sensors) like IFI16 and DDX41 also recognize DNA and activate STING, which further facilitates NLRP inflammasome activation. STING also activates the battery of IFN genes via IRF phosphorylation. Different forms of RNA originating from wide sources, like viral RNA, degraded self-RNA, etc. are recognized by RLRs, including RIG-1 and MDA5, following which they are imported to mitochondrial antiviral signaling (MAVS). MAVS further activates ISGs via IRF3-IRF7 activation. IFNs also work in an autocrine fashion and stimulate more production of different nucleic acid sensors and other ISGs. AIM2: Absent in melanoma; ASC: Apoptosis-associated speck-like protein containing a CARD (Caspase activation and recruitment domain) Domain; BAX: Bcl-2-associated X protein; cGAS: Cyclic GMP-AMP synthase; DDX41: DEAD-Box helicase 41; DHX: DEXH-box helicase; GBP: Guanylate-binding proteins; GSDMD: Gasdermin D; GSDMDn: Gasdermin D (N-Terminal); HIN: Hematopoietic IFN-inducible nuclear protein; IFI16: Interferon gamma inducible 16; IFIT1: Interferon induced protein with tetratricopeptide repeats 1; IFN: Interferon; IFNR: IFN receptor; IGRB10: Immunity-related GTPase family member B10; IKK: Iκb (Inhibitor of Nuclear Factor Kappa B) Kinase; IL: Interleukin; IL-1R1: IL-1 receptor 1; IRAK: Interleukin-1 receptor associated kinase; IRF: Interferon-regulatory factors; ISG: Interferon stimulated genes; JAK: Janus kinase; MAVS: Mitochondrial antiviral-signaling protein; MDA5: Melanoma differentiation-associated protein 5; Myd88: Myeloid differentiation primary response 88; NLRP: NLR (NOD-like receptor) family pyrin domain; NOD: Nucleotide binding and oligomerization domain; PKR: Protein kinase R; PYD: PYRIN Domain; RIG1: Retinoic acid-inducible gene I; STAT: Signal transducer and activator of transcription; STING: Stimulator of interferon genes; TBK1: TANK (TRAF family member-associated NF-kappa-B activator)-binding kinase 1, TLR: Toll-like receptor; TRAF: TNF (Tumor necrosis factor) receptor associated factors; TRIF: TIR [toll/interleukin-1 (IL-1) receptor] domain containing adapter inducing interferon-β.
- Citation: Badal D, Sachdeva N, Maheshwari D, Basak P. Role of nucleic acid sensing in the pathogenesis of type 1 diabetes. World J Diabetes 2021; 12(10): 1655-1673
- URL: https://www.wjgnet.com/1948-9358/full/v12/i10/1655.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i10.1655