Published online Sep 15, 2017. doi: 10.4251/wjgo.v9.i9.363
Peer-review started: December 28, 2016
First decision: February 4, 2017
Revised: March 16, 2017
Accepted: July 7, 2017
Article in press: July 10, 2017
Published online: September 15, 2017
Processing time: 260 Days and 23.2 Hours
To characterise venous thromboembolism (VTE) in gastrointestinal cancer and assess the clinical utility of risk stratification scoring.
We performed a retrospective analysis using electronic patient records of 910 gastro-oesophageal (GO) cancer and 1299 colorectal cancer (CRC) patients referred to a tertiary cancer centre to identify the incidence of VTE, its relationship to chemotherapy and impact on survival. VTE risk scores were calculated using the Khorana index. Patients were classified as low risk (0 points), intermediate risk (1 to 2 points) or high risk (3 points). Data was analysed to determine the sensitivity of the Khorana score to predict VTE.
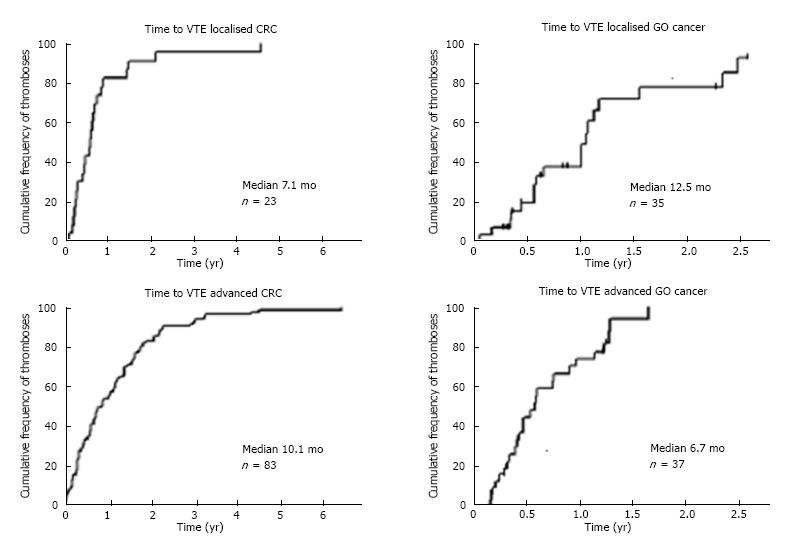
The incidence of VTE was 8.9% for CRC patients and 9.7% for GO cancer patients. Pulmonary emboli (PE) were more common in advanced than in localised CRC (50% vs 21% of events respectively) and lower limb deep vein thrombosis (DVT) were more common in localised than in advanced CRC (62% vs 39% of events respectively). The median time to VTE from cancer diagnosis was 8.3 mo for CRC patients compared to 6.7 mo in GO cancer. In localised CRC median time to VTE was 7.1 mo compared with 10.1 mo in advanced CRC. In contrast in GO cancer, the median time to VTE was 12.5 mo in localised disease and 6.8 mo in advanced disease. No survival difference was seen between patients with and without VTE in this cohort. The majority of patients with CRC in whom VTE was diagnosed had low or intermediate Khorana risk score (94% for localised and 97% in advanced CRC). In GO cancer, all patients scored either intermediate or high risk due to the primary site demonstrating a limitation of the risk assessment score in discriminating high and low risk patients with GO cancers. Additional risk factors were identified in this cohort including surgery, chemotherapy or hospital admission. Overall, 81% of patients with CRC and 77% of patients with GO cancer had one or more of these factors within 4 wk prior to diagnosis VTE. These should be factored into clinical risk assessment scores.
The Khorana score has low sensitivity for thrombotic events in CRC and cannot discriminate low risk patients in high risk cancer sites such as GO cancer.
Core tip: Analysis of clinical outcomes in 2209 patients with gastrointestinal cancers demonstrated that the Khorana score to assess venous thromboembolism (VTE) risk may have inadequate sensitivity to be clinically useful beyond short term VTE risk.
- Citation: Metcalf RL, Al-Hadithi E, Hopley N, Henry T, Hodgson C, McGurk A, Mansoor W, Hasan J. Characterisation and risk assessment of venous thromboembolism in gastrointestinal cancers. World J Gastrointest Oncol 2017; 9(9): 363-371
- URL: https://www.wjgnet.com/1948-5204/full/v9/i9/363.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i9.363
Approximately 20% of cases of venous thromboembolism (VTE) occur in cancer patients[1]. The cumulative 2-year incidence rate of VTE in cancer patients is approximately 1.6%[2], with an elevated risk with gastro-intestinal cancers[3].
In addition to the classical Virchow’s triad of venous stasis, thrombophilia and endothelial injury, increased incidence of VTE in cancer patients has been linked to platelet activation, direct factor X activation, decreased hepatic anticoagulant synthesis, reduced hepatic clearance of coagulation factors, and the development of antiphospholipid antibodies[4].
Symptomatic VTE has a detrimental effect on cancer survival, in addition to causing substantial morbidity[5,6]. To minimise the occurrence and clinical impact of VTE in patients with cancer, current guidelines recommend the prophylactic anticoagulation of patients during medical admissions and following surgery[7,8] which are recognised as periods of high risk of VTE[9,10]. However, the majority of cancer patients receive their treatment in the outpatient setting, and VTE is still a frequent occurrence in this group[4]. Current ASCO and ESMO guidance[8,11] does not recommend routine anticoagulation in ambulatory patients, but that it may be considered in high risk patients. There is some debate, however, on how such high risk patients are identified.
Clinical trials randomising ambulatory patients with cancer to receive placebo or prophylactic anticoagulation have shown inconsistent results[12-24]. Some have shown beneficial use of thromboprophylaxis in ambulatory cancer patients[12,13]. A meta-analysis[25] of 7622 patients found a reduction in the incidence of symptomatic VTE (RR = 0.56, 95%CI: 0.40-0.74) at the expense of an increased risk of minor bleeding (RR = 1.32, 95%CI: 1.02-1.71). Overall, however, these studies have been unable to convincingly demonstrate significantly reduced rates of VTE. This may be related to the fact that these studies have included a large number of patients who have low VTE risk, and the benefit of anticoagulation has been weakened as a result.
Two studies[20,26] have shown the use of higher doses of anticoagulation in high risk ambulatory pancreatic cancer patients. The FRAGEM trial[20] used therapeutic doses of dalteparin, whilst the CONKO-004[26] trial used half therapeutic doses of enoxaparin. They demonstrated significant reduction in VTE incidence (RR = 0.145 and 0.12 respectively) with an increased bleeding risk and no overall effect on survival.
A risk model has been developed to identify patients at higher risk of developing VTE based upon blood results (elevated leukocytes, elevated platelets, and reduced haemoglobin), elevated BMI and the primary cancer site[27]. This assessment categorises patients into groups with a VTE incidence of 6.7% for high risk, 2% for intermediate risk and 0.3% for low risk patients over a median of 2.5 mo follow up, and the use of this risk assessment score has been advocated within ASCO and ESMO guidance[8,11] for clinical decision making in the anticoagulation of selected ambulatory outpatients receiving chemotherapy. The AVERT clinical trial (NCT02048865) is currently evaluating the benefit of thrombo-prophylaxis in ambulatory outpatients who have high and intermediate VTE risk scores.
There is also scope for further development of the Khorana scoring system as has been shown with the Vienna prediction score[28] which combines the Khorana score with additional lab parameters such as D-dimer; and the Protecht prediction score[29] which takes into consideration whether patients have been given chemotherapy associated with additional VTE risk.
In order to determine the applicability of the VTE risk score to the “real world setting”, we sought to characterise the incidence and risk factors for VTE in patients with gastrointestinal cancers treated at a tertiary cancer centre. In the colorectal cancer (CRC) population, we evaluated the sensitivity of a high VTE risk score to detect patients with VTE in their lifetime. Patients with gastro-oesophageal (GO) cancers were not included within this analysis as all GO cancer patients score as very high risk as this is classified as a high risk cancer site in the risk assessment tool[3].
The electronic patient records of 2209 patients [1299 with colorectal cancer (CRC) and 910 with gastro-oesophageal (GO) cancers] that were referred to The Christie NHS Foundation Trust between 2006 and 2012 were screened as previously described[30] to identify patients diagnosed with VTE. Briefly, the electronic patient record was searched using the search text function for the terms “thromb”, “embol”, “DVT” (deep vein thrombosis) and “PE” (pulmonary embolism) recorded anywhere within the patient notes to identify patients with VTE (cases). Demographic and clinical data including details of previous chemotherapy, surgery, hospital admissions and patient survival was extracted from the records of patients with VTE for analysis. From the patients without VTE, a control cohort was generated matching by site and stage of disease and performance status. Survival data was collected on the matched cohort to evaluate the prognostic effect of VTE in these patients. A VTE risk score[27] was calculated for the patients with CRC diagnosed with VTE as previously reported. One point each was allocated for platelets > 350 × 109/L, haemoglobin < 10 g/dL, leukocytes > 11 × 109/L and body mass index > 35 kg/m2 and one point for a high risk cancer site (lung, lymphoma, gynaecologic, bladder, and testicular) and two points for a very high risk site (stomach and pancreas). Patients were classified as low risk (0 points), intermediate risk (1 to 2 points) or high risk (3 points). In view of the fact that GO cancers automatically score 2 points as a very high risk site, this risk score was only calculated for CRC patients in order to evaluate the sensitivity of this approach to identify patients at risk of VTE with a low risk primary site.
Data was exported to Microsoft Excel and analysed using SPSS for Windows (Chicago, IL, United States). To evaluate for the impact of VTE on patient survival, a control cohort matched for primary site, stage of disease and performance status was identified in whom no VTE was diagnosed in the patient lifetime. This data was tested by univariate survival analysis using the Kaplan-Meier method and log-rank test and P ≤ 0.05 was considered significant. Survival analysis was performed on all patients and separate analyses performed on patients with localised and advanced disease. Time to event analysis was performed using cumulative incidence plot and median time to thrombosis was calculated. Statistical review of the study was conducted by Clare Hodgson, department of biostatistics, the Christie NHS Foundation Trust. Clinical records were also reviewed to identify additional risk factors for VTE in this cohort in order to establish whether the Khorana score could be modified or used alongside the Khorana score to more accurately identify high risk patients.
Of the 2209 patients with GI cancers, VTE was diagnosed in 203 patients giving a cumulative incidence rate of 9.2%. The incidence of VTE was 8.9% (115/1299) for CRC patients and 9.7% (88/822) for upper GI cancer patients.
Table 1 shows the site of thromboses in patients with CRC. As a proportion of all events, pulmonary emboli (PE) were more common in advanced than in localised CRC (50% vs 21% of events respectively) and lower limb deep vein thrombosis (DVT) were more common in localised than in advanced CRC (62% vs 39% of events respectively). This difference was not seen in patients with GO cancers. In localised GO cancers, DVT and PE made up 48% and 44% of VTE and in advanced GO cancers they made up 40% and 51% of VTE respectively. This difference may in part be attributed to the increased patient survival in advanced CRC compared to GO cancer. Patients with longer survival tend to have an increased number of CT scans to evaluate their disease and consequently may have a greater number of PEs detected as incidental findings on routine imaging. To evaluate this further, the proportion of VTE identified as incidental findings on routine clinical imaging was recorded. Consistent with this hypothesis, VTE were more frequently detected as incidental findings on diagnostic imaging in CRC, accounting for 40% of events, compared with 26% in GO cancer.
| Colorectal cancer | Gastro-oesophageal cancer | |
| All | ||
| Lower limb thrombosis | 51 (44) | 44 (50) |
| Upper limb thrombosis | 9 (8) | 4 (4) |
| Visceral thrombosis | 6 (5) | 2 (2) |
| Pulmonary embolism | 49 (43) | 39 (44) |
| Localised | ||
| Lower limb thrombosis | 18 (62) | 19 (43) |
| Upper limb thrombosis | 1 (3) | 2 (5) |
| Visceral thrombosis | 4 (14) | 2 (4) |
| Pulmonary embolism | 6 (21) | 21 (48) |
| Advanced | ||
| Lower limb thrombosis | 33 (39) | 25 (56) |
| Upper limb thrombosis | 8 (9) | 2 (4) |
| Visceral thrombosis | 2 (2) | 0 (0) |
| Pulmonary embolism | 43 (50) | 18 (40) |
To determine the relationship between the time of cancer diagnosis and the occurrence of VTE, the cumulative incidence of VTE was plotted over time (Figure 1). For patients with CRC, the median time to VTE from cancer diagnosis was 8.3 mo for all patients. This compares with a median time to VTE for patients with GO cancers of 6.7 mo. Analysing patients with localised and advanced disease separately identified that for patients with localised CRC median time to VTE was 7.1 mo and in advanced CRC, median time to VTE was 10.1 mo. In contrast in GO cancer, the median time to VTE was 12.5 mo in localised disease and 6.8 mo in advanced disease, which may reflect the increased frequency of recurrent disease and the shorter time to progression following first line therapy seen in patients with GO cancers.
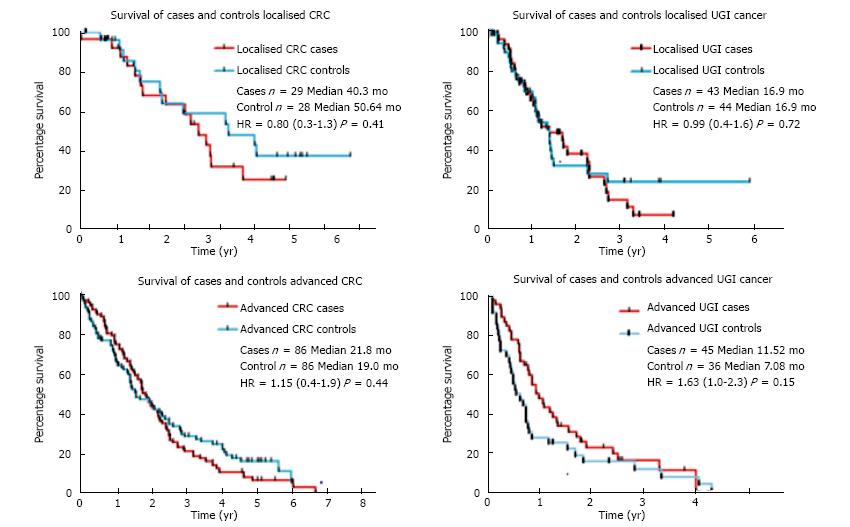
Consistent with previous approaches[31] to evaluate for the impact of VTE on patient survival, a control cohort matched for primary site, stage of disease and performance status was identified in whom no VTE was diagnosed in the patient lifetime. Kaplan-Meier survival analyses of cases and controls are summarised in Figure 2. For patients with CRC, median survival was 23.8 mo for cases and 24.7 mo for controls (P = 0.20) and for upper gastrointestinal cancer, median survival was 13.9 mo for cases and 10.2 mo for controls (P = 0.59). Sub-group analysis of the patients with localised disease for each cancer type showed that although there was no significant difference in median survival between cases and controls (40.3 mo vs 50.7 mo for CRC cases and controls, P = 0.41; and 16.9 and 17.0 mo for GO cancer cases and controls, P = 0.72), the curves began to separate after the median. A limitation of this approach is an inherent underestimation of the association between VTE and reduced survival. This plateau in the survival curves seen in the control cohorts in both CRC and GO cancer was not evident in the cases with VTE. This observation suggests that a subset of patients with long term survival is present in the control cohort which is not seen in the VTE cohort.
The Khorana VTE risk score[27] identifies patients at higher short term risk of VTE and is being incorporated into clinical trials of prophylactic anticoagulation in the ambulatory out-patient setting. To evaluate the broader clinical utility of Khorana VTE risk score in patients without a “high risk” primary site, we determined the sensitivity of the risk score for predicting subsequent risk of VTE in CRC patients. The risk score was calculated in the patients with CRC in whom VTE had been diagnosed. Sufficient clinical data to complete this assessment were available for 72/115 (63%) patients. Using the cut-off score of > 2 points to classify patients as high risk for VTE, the sensitivity of the risk score was low for prediction of subsequent VTE. The majority of patients with CRC in whom VTE was diagnosed had low or intermediate Khorana risk score (94% for localised and 97% in advanced CRC). This finding demonstrates that although the Khorana risk score has been validated to identify patients at high short term risk of VTE whilst receiving chemotherapy, it fails to identify the majority of VTE which occur in patients with cancer beyond this short term window.
Clinical records were reviewed to identify additional risk factors of surgery, chemotherapy or hospital admission within 4 wk prior to the event for all patients identified to have VTE. Overall, 81% of patients with CRC and 77% of patients with GO cancer had one or more of these factors within 4 wk prior to the diagnosis of VTE. These characteristics, which are well described as risk factors for the occurrence of VTE, may be utilised alongside characteristics included in the Khorana risk score to identify patients at highest risk of developing VTE.
This study has characterised VTE in patients with gastrointestinal cancers treated at a tertiary cancer centre, reporting a cumulative incidence of VTE approaching 1 in 10 in both CRC and GO cancer. The incidence of 9.7% we report in GO cancer is consistent with previous reports identifying a cumulative incidence of 9.4% in advanced disease[32] and 12.5% to 13% in patients with localised disease undergoing dual modality treatment[6,33]. However, the identification of VTE in 8.9% of patients with CRC is higher than previous reports. Retrospective analyses of cancer registry databases identified VTE incidence of 5.4% in CRC patients in England[34] and 3.1% in the Californian Cancer Registry, using data from the 1990s[35]. However, the risk of VTE is higher for patients with Stage IV disease than Stages I-III disease (HR = 3.08, 95%CI: 1.95-4.84) and for patients receiving chemotherapy (HR = 1.39, 95%CI: 1.14-1.69)[34], both of which would be over-represented in the patients treated at the tertiary cancer centre included in the current study. In addition, VTE are becoming more common, and more recent United States cancer registry database studies found a VTE incidence of 10.6%[36] consistent with the current study.
All the studies described above report a higher incidence of VTE in GI cancers than the 2.2% incidence reported in the development and validation of the VTE risk assessment model[27]. This can be explained by the short follow up (median follow up of 2.5 mo following initiation of chemotherapy) in the development of this model. The current study found that the median time to VTE was 8.3 mo for CRC and 6.7 mo for GO cancers.
Although no statistically significant difference in survival was seen in the VTE cohort compared with the control cohort, an intriguing difference was seen in the long term survivorship in patients with localised disease experiencing VTE. The survival curves separate beyond three years suggesting a sub-group of long term survivors in the control cohort and this was not seen in the VTE cases. This finding suggests that the occurrence of VTE in localised disease is a surrogate for occult micro-metastatic disease, as metastatic relapse is responsible for the majority of the cases of death from cancer. Previous studies of the prognostic impact of VTE in CRC have also found this selective effect in localised disease[35]. This hypothesis may also explain why an impact of VTE on survival is not seen in advanced disease.
This work has identified that as a proportion of all thrombotic events, PE are more frequent in advanced than in localised CRC however, representing 50% and 21% of VTE respectively. In contrast, in GO cancer, PE comprises 44% and 51% of VTE in localised and advanced disease respectively. Although incidental PE were a more frequent occurrence in CRC than GO cancer in this series which may be due to the longer survival time in CRC and an increased number of CTC scans performed in the patient lifetime, another explanation for this finding may be that PE are a surrogate for aggressive tumour biology seen in localised GO cancer.
Although no statistically significant difference in survival was detected between cases and controls in this analysis, this may be influenced by a bias in selection of the control cohort. Within the control cohort, some of the patients would be predicted to have died of a non-VTE related cause with short follow up, who may have gone on to develop VTE if they had a longer survival time. Although this could be mitigated by including events which occurred within a short follow up period, for example 3 mo from diagnosis, this approach would miss a large proportion of all clinically meaningful VTE events. However, this study demonstrates that this approach would fail to capture most VTE events occurring. The consequences of this are two-fold. Firstly, the total number of events detected and analysed would be reduced, reducing both the utility of both descriptive and statistical analysis. Secondly, as the majority of VTE events would fall outside this follow up window, the clinical relevance of such an analysis would be reduced.
Retrospective data collection permits a larger number of events to be captured from cancer databases; however it is not without limitations. As VTE may occur after cancer diagnosis, patients with shorter survival are less likely to develop a VTE. This represents an inherent bias underestimating the association between VTE and worse prognosis. In the subjects who did not experience VTE, the time to event analysis can only include the patients who experienced a VTE, and this approach does not account for censored subjects who died without having an event. This limitation applies to all retrospective analyses of this nature, however, the current study is more powerful as it is able to identify a larger number of events and characterise those in more detail.
Another limitation of the approach to retrospective data collection undertaken in this study and other studies of this type is that we cannot guarantee that all thrombotic events were identified and the actual number may be underestimated. However, the approach to evaluation of both the clinical records and imaging assessments will mitigate this effect. However, the clinical heterogeneity between symptomatic and incidental VTE may impact on the relationship between VTE and survival. Future studies may require the use of an analytical strategy to account for this variation.
A high Khorana risk score has been validated as a predictor of short term risk of VTE in patients receiving chemotherapy, and the sensitivity of the risk score, describing the probability of patients experiencing VTE being attributed a high risk score was reported as 40%[27]. We sought to test the sensitivity of this model in a larger cohort of patients with CRC, finding that when looking at a patient’s lifetime risk of VTE, almost no patients who sustained VTE had a high risk score. This was performed in patients with CRC as opposed to GO cancer as the latter is considered a very high risk primary site which automatically scores 2 points on the Khorana risk score placing all patients in the upper intermediate risk category as a minimum.
The observation that almost no patients with CRC who sustained a VTE in their lifetime were classified as high risk using the Khorana assessment is significant as the findings of clinical studies using this risk score to enrich for patients with a high risk of thrombosis occurring shortly after diagnosis will not be generally applicable to the majority of thrombotic events which neither occurs in high risk patients nor within a short follow up. The current study suggests that these other events in patients not classified as high risk make up the majority of thrombotic events in the real world setting. This study does not bring into question the validity of the Khorana risk score for the identification of patients at higher risk of developing VTE whilst receiving chemotherapy. However, it does question the applicability of this risk score alone to enrich for patients who may benefit from prophylactic anti-coagulation.
This study also highlights the difficulty of taking a score made for general application and using it in a particular patient group. The Khorana score was not developed to be applied to specific cancer sites but rather cancer patients as a whole. The fact that the Khorana score did not perform as well in colorectal cancer patients specifically suggests that further validation studies in site-specific cancer groups may be needed.
Upper limb thromboses have been included in our results, something which differs from the original Khorana study. Although upper limb thromboses are strongly associated with indwelling catheters in general, the pro-thrombotic nature of cancer patients may be expected to make these events more frequent in this cohort of patients. Including these patients in the data analysis has allowed us to obtain a complete picture of the clinical burden of VTE in relation to cancer. Overall, the indwelling catheter-associated thromboses represent a small proportion of the overall events in this cohort. However, looking into the incidence of indwelling-catheter associated thromboses in colorectal cancer patients as compared to the general population would present an interesting future study.
Although the classification of patients as high risk failed to identify patients with VTE, the majority of VTE occurred in patients with established risk factors such as surgery, chemotherapy and hospitalisation within 4 wk prior to the event in this study. This finding suggests that an alternative approach to prophylactic anticoagulation is to study extended anticoagulation following these high risk periods. Studies have already begun looking to develop a modified risk score to increase the sensitivity of this approach and reduce the number needed to treat to prevent VTE. For example, data from the Protecht study[13], a prospective placebo controlled randomised trial evaluating anticoagulation with nadroparin in patients receiving chemotherapy was used to generate a modified risk score[29] which was more effective at identifying patients at high risk of VTE compared with the Khorana risk score. However, this approach has yet to be validated in a prospective cohort.
Finally, the current clinical guidance[8,11] does not recommend the routine prophylactic anticoagulation of ambulatory cancer patients. In addition to efforts to optimise the risk stratification of patients with cancer using datasets such as those evaluated in the current study, developments of novel oral anticoagulant (NOAC) therapies may alter the risk to benefit ratio if these drugs have an improved therapeutic index and do not require daily sub-cutaneous administration.
In summary, we have shown that VTE affects up to 1 in 10 patients with GI cancers and the peak incidence is in the first 6 mo following diagnosis. The prognostic impact of VTE in CRC is more evident in patients with localised disease than with advanced disease, suggesting that VTE may be a surrogate for occult metastasis. Finally, we demonstrate that the VTE risk score may have inadequate sensitivity to be clinically useful for the majority of thrombotic events occurring in a patient’s lifetime. The findings of the current study warrant validation in a larger cohort, and this work is on-going. However, the implications of these findings are significant, as for studies enriching patients based upon VTE risk score to be applicable to clinical practice; the score requires a clinically meaningful sensitivity. If the majority of patients who experience VTE have low risk scores, and these events do not occur in the immediate few months following diagnosis, then most VTE events would remain unaffected by the strategy of treating high and intermediate risk patients being employed in the AVERT clinical trial. This study raises the possibility that risk stratification using the Khorana VTE risk score, whilst an appealing approach to the incorporation in clinical trial design, may not be applicable to reducing the lifetime burden of VTE risk in clinical practice.
Venous thromboembolic disease is a frequent even in patients with cancer and there is a need for predictors of these events to identify patients at high risk occurrence to target prophylactic interventions.
This study characterised venous thromboembolism in a large cohort of patients with gastrointestinal cancer and evaluated clinical and biochemical predictors of events in these patients.
This study identified that existing risk scores using clinical and biochemical parameters have a low sensitivity for thrombotic events in colorectal cancer; the majority of thrombotic events occur in patients who are classified as low or intermediate risk. This study identifies the need for refined risk scores to identify groups of patients with cancer at high risk of thrombosis.
The identification of high risk groups will enable prospective studies to be planned to formally compare prophylactic anticoagulation in patients with cancer.
Metcalf and coworkers present the results of a very interesting manuscript assessing the performance of the Khorana VTE risk score on a cohort of patients with gastrointestinal tumors. This study highlighted the limitations of said score. Overall the paper is well written and the analysis and discussion are sound and balanced.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lazo-Langner A, Smith RC S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer. 2011;117:1334-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 903] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 3. | Riess H, Habbel P, Jühling A, Sinn M, Pelzer U. Primary prevention and treatment of venous thromboembolic events in patients with gastrointestinal cancers - Review. World J Gastrointest Oncol. 2016;8:258-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Piazza G. Venous thromboembolism and cancer. Circulation. 2013;128:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1183] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 6. | Khanna A, Reece-Smith AM, Cunnell M, Madhusudan S, Thomas A, Bowrey DJ, Parsons SL. Venous thromboembolism in patients receiving perioperative chemotherapy for esophagogastric cancer. Dis Esophagus. 2014;27:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Khorana AA, Streiff MB, Farge D, Mandala M, Debourdeau P, Cajfinger F, Marty M, Falanga A, Lyman GH. Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol. 2009;27:4919-4926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 589] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 9. | Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 333] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 10. | Agnelli G, Bolis G, Capussotti L, Scarpa RM, Tonelli F, Bonizzoni E, Moia M, Parazzini F, Rossi R, Sonaglia F. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 511] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 11. | Mandalà M, Falanga A, Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22 Suppl 6:vi85-vi92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 371] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 12. | Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P, Mouret P, Chaudhari U, Lawson F, Turpie AG. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 401] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 13. | Agnelli G, Gussoni G, Bianchini C, Verso M, Mandalà M, Cavanna L, Barni S, Labianca R, Buzzi F, Scambia G. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 452] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 14. | Altinbas M, Coskun HS, Er O, Ozkan M, Eser B, Unal A, Cetin M, Soyuer S. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 333] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 15. | Haas SK, Freund M, Heigener D, Heilmann L, Kemkes-Matthes B, von Tempelhoff GF, Melzer N, Kakkar AK. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost. 2012;18:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Kakkar AK, Levine MN, Kadziola Z, Lemoine NR, Low V, Patel HK, Rustin G, Thomas M, Quigley M, Williamson RC. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the fragmin advanced malignancy outcome study (FAMOUS). J Clin Oncol. 2004;22:1944-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 476] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 17. | Klerk CP, Smorenburg SM, Otten HM, Lensing AW, Prins MH, Piovella F, Prandoni P, Bos MM, Richel DJ, van Tienhoven G. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 439] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 18. | Lebeau B, Chastang C, Brechot JM, Capron F, Dautzenberg B, Delaisements C, Mornet M, Brun J, Hurdebourcq JP, Lemarie E. Subcutaneous heparin treatment increases survival in small cell lung cancer. “Petites Cellules” Group. Cancer. 1994;74:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Lecumberri R, López Vivanco G, Font A, González Billalabeitia E, Gúrpide A, Gómez Codina J, Isla D, Galán A, Bover I, Domine M. Adjuvant therapy with bemiparin in patients with limited-stage small cell lung cancer: results from the ABEL study. Thromb Res. 2013;132:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Maraveyas A, Waters J, Roy R, Fyfe D, Propper D, Lofts F, Sgouros J, Gardiner E, Wedgwood K, Ettelaie C. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012;48:1283-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 21. | Perry JR, Julian JA, Laperriere NJ, Geerts W, Agnelli G, Rogers LR, Malkin MG, Sawaya R, Baker R, Falanga A. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010;8:1959-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 22. | Riess H, Pelzer U, Hilbig A, Stieler J, Opitz B, Scholten T, Kauschat-Brüning D, Bramlage P, Dörken B, Oettle H. Rationale and design of PROSPECT-CONKO 004: a prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy). BMC Cancer. 2008;8:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | van Doormaal FF, Di Nisio M, Otten HM, Richel DJ, Prins M, Buller HR. Randomized trial of the effect of the low molecular weight heparin nadroparin on survival in patients with cancer. J Clin Oncol. 2011;29:2071-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Weber C, Merminod T, Herrmann FR, Zulian GB. Prophylactic anti-coagulation in cancer palliative care: a prospective randomised study. Support Care Cancer. 2008;16:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Akl EA, van Doormaal FF, Barba M, Kamath G, Kim SY, Kuipers S, Middeldorp S, Yosuico V, Dickinson HO, Schünemann HJ. Parenteral anticoagulation for prolonging survival in patients with cancer who have no other indication for anticoagulation. Cochrane Database Syst Rev. 2007;CD006652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Pelzer U, Opitz B, Deutschinoff G, Stauch M, Reitzig PC, Hahnfeld S, Müller L, Grunewald M, Stieler JM, Sinn M. Efficacy of Prophylactic Low-Molecular Weight Heparin for Ambulatory Patients With Advanced Pancreatic Cancer: Outcomes From the CONKO-004 Trial. J Clin Oncol. 2015;33:2028-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 27. | Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1530] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 28. | Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122:2011-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 29. | Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7:291-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 284] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 30. | Metcalf RL, Fry DJ, Swindell R, McGurk A, Clamp AR, Jayson GC, Hasan J. Thrombosis in ovarian cancer: a case control study. Br J Cancer. 2014;110:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 32. | Starling N, Rao S, Cunningham D, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Thromboembolism in patients with advanced gastroesophageal cancer treated with anthracycline, platinum, and fluoropyrimidine combination chemotherapy: a report from the UK National Cancer Research Institute Upper Gastrointestinal Clinical Studies Group. J Clin Oncol. 2009;27:3786-3793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Rollins KE, Peters CJ, Safranek PM, Ford H, Baglin TP, Hardwick RH. Venous thromboembolism in oesophago-gastric carcinoma: incidence of symptomatic and asymptomatic events following chemotherapy and surgery. Eur J Surg Oncol. 2011;37:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Walker AJ, West J, Card TR, Humes DJ, Grainge MJ. Variation in the risk of venous thromboembolism in people with colorectal cancer: a population-based cohort study from England. J Thromb Haemost. 2014;12:641-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, Zhou H, White RH. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24:1112-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 36. | Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 324] [Article Influence: 24.9] [Reference Citation Analysis (0)] |