Published online May 15, 2017. doi: 10.4251/wjgo.v9.i5.218
Peer-review started: December 9, 2016
First decision: January 10, 2017
Revised: February 2, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 15, 2017
Processing time: 154 Days and 6.4 Hours
To critically appraise short-term outcomes in patients treated in a new Pelvic Exenteration (PE) Unit.
This retrospective observational study was conducted by analysing prospectively collected data for the first 25 patients (16 males, 9 females) who underwent PE for advanced pelvic tumours in our PE Unit between January 2012 and October 2016. Data evaluated included age, co-morbidities, American Society of Anesthesiologists (ASA) score, Eastern Cooperative Oncology Group (ECOG) status, preoperative adjuvant treatment, intra-operative blood loss, procedural duration, perioperative adverse event, lengths of intensive care unit (ICU) stay and hospital stay, and oncological outcome. Quantitative data were summarized as percentage or median and range, and statistically assessed by the χ2 test or Fisher’s exact test, as applicable.
All 25 patients received comprehensive preoperative assessment via our dedicated multidisciplinary team approach. Long-course neoadjuvant chemoradiotherapy was provided, if indicated. The median age of the patients was 61.9-year-old. The median ASA and ECOG scores were 2 and 0, respectively. The indications for PE were locally invasive rectal adenocarcinoma (n = 13), advanced colonic adenocarcinoma (n = 5), recurrent cervical carcinoma (n = 3) and malignant sacral chordoma (n = 3). The procedures comprised 10 total PEs, 4 anterior PEs, 7 posterior PEs and 4 isolated lateral PEs. The median follow-up period was 17.6 mo. The median operative time was 11.5 h. The median volume of blood loss was 3306 mL, and the median volume of red cell transfusion was 1475 mL. The median lengths of ICU stay and of hospital stay were 1 d and 21 d, respectively. There was no case of mortality related to surgery. There were a total of 20 surgical morbidities, which occurred in 12 patients. The majority of the complications were grade 2 Clavien-Dindo. Only 2 patients experienced grade 3 Clavien-Dindo complications, and both required procedural interventions. One patient experienced grade 4a Clavien-Dindo complication, requiring temporary renal dialysis without long-term disability. The R0 resection rate was 64%. There were 7 post-exenteration recurrences during the follow-up period. No statistically significant relationship was found among histological origin of tumour, microscopic resection margin status and postoperative recurrence (P = 0.67). Four patients died from sequelae of recurrent disease during follow-up.
By utilizing modern assessment and surgical techniques, our PE Unit can manage complex pelvic cancers with acceptable morbidities, zero-rate mortality and equivalent oncologic outcomes.
Core tip: Pelvic exenteration surgery was introduced by Brunswick in 1948 as a palliative treatment for advanced pelvic tumour, which carries high morbidity and mortality rates. However, decades of medical evolution in preoperative imaging, adjuvant therapy, better anatomical knowledge of the pelvis and modernized surgical techniques has made this procedure safe and effective for treating complex pelvic tumours. This study describes and demonstrates how our new Pelvic Exenteration Unit utilises the advantage of modern assessment and contemporary surgical techniques to achieve excellent outcomes.
- Citation: Chew MH, Yeh YT, Toh EL, Sumarli SA, Chew GK, Lee LS, Tan MH, Hennedige TP, Ng SY, Lee SK, Chong TT, Abdullah HR, Goh TLH, Rasheed MZ, Tan KC, Tang CL. Critical evaluation of contemporary management in a new Pelvic Exenteration Unit: The first 25 consecutive cases. World J Gastrointest Oncol 2017; 9(5): 218-227
- URL: https://www.wjgnet.com/1948-5204/full/v9/i5/218.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i5.218
Surgeries for advanced pelvic tumours constitute technical challenges. Despite better understanding of the pelvic anatomy due to superior imaging modalities, the resection of tumours and extirpation of any contiguous organs continue to be associated with considerable morbidity and risks. In addition, tumours originating from the rectum, gynaecological organs or urological organs behave differently and indications of surgery for each require multidisciplinary coordination and evaluation.
Pelvic exenteration (PE) surgery was first introduced by Brunswick[1] in 1948 but was associated with a high morbidity rate, a perioperative mortality rate of 23%, and a poor postoperative quality of life. As such, a non-surgical approach with chemotherapy and radiotherapy has traditionally been offered to the majority of the patients with pelvic tumours. These approaches may provide transient relief of symptoms but as the disease progress, many of the patients suffer from refractory pain, obstruction, bleeding, malodourous fistulating or erosive malignant cutaneous lesions, and pelvic sepsis. Survival may be increased up to 12-14 mo but remains poor, with < 4% of patients surviving beyond 4 years[2-5].
As a result of better patient selection, perioperative adjuvant chemotherapy and irradiation, careful planning and multidisciplinary involvement as well as advances in surgical techniques in the modern era, PE has become accepted as a procedure that can maintain adequate local disease control, prolong survival and achieve potential cure for advanced pelvic tumours. The most significant advances in surgical techniques have allowed for achievement of an R0 resection, as demonstrated by large-scale reviews which predominantly investigated for the locally-advanced and recurrent types of rectal cancers[6-8].
Accomplishing an R0 resection requires complete or partial removal of the pelvic vessels, muscles, ligaments and bony structures-including the ileum, ischium, pubic rami, sacrum or coccyx-as well as pelvic viscera. Experience gained over the years has led to acceptable morbidity risks and a low mortality rate. In a systematic review, Heriot et al[6] reported exenteration-related morbidity and mortality rates of 27% and 0.6% respectively. Similar trends were found in an Australian study of 148 patients who underwent PE, which reported a 0% 30-d mortality rate and good postoperative quality of life[9].
The development of a dedicated PE Surgical Unit in our institution was borne from recognition of the advantages afforded by an aggressive approach to tackling these advanced pelvic tumours. Nonetheless, the initial phase of conceptualization necessitated discussion on the understanding of pelvic cancer biology and pathophysiology among the various subspecialties, as well as of the appropriate surgical indications. The core members of this PE Unit included: A colorectal surgeon, who had received comprehensive training in PE; a gynaecologist, who specialized in gynaecological malignancies; an urologist, who specialized in urological cancers; and a team of experienced anaesthesiologists. Other subspecialty surgeons-including plastic, vascular and orthopaedic surgeons-were referred on an ad hoc basis. While the concept of PE surgery was not new to this Unit at its inception, the latest surgical techniques for achieving R0 margins had only recently been introduced into its practice.
This article reports our systematic evaluation of the short-term oncological outcomes achieved by the newly established PE group using modern techniques.
Definitions of the PE surgeries described herein correspond to those published in a 2013 systematic review from Yang et al[10], and include.
Whereby rectum, distal colon, genitourinary viscera, internal reproductive organs, draining lymph nodes and pelvic peritoneum are removed. If a sacrectomy is performed, it is specified as TPE with sacrectomy.
Whereby upper rectum, reproductive organs and bladder are removed. The lower rectum may be spared or a perineal excision may be performed.
Whereby the rectum and reproductive organs are removed. The bladder may be spared. If a sacrectomy or coccygectomy was performed, it is specified.
Whereby a lateral pelvic node dissection is performed, with en bloc resection of all involved structures, including viscera and vascular structures. If the sciatic nerve can be preserved, its perineural sheath is excised.
After approval was obtained by the Institutional Review Board of our hospital, a retrospective review of patient records was conducted to identify the first consecutive 25 patients who underwent PE through our new PE Unit. No exclusion criteria were applied. These patients had been treated between January 2012 and October 2016, and all had received or were undergoing follow-up consisting of 3-mo outpatient clinic visits for at least 2 years following the surgery. The follow-up routine included monitoring of carcinoembryonic antigen level (each clinic visit) and computed tomography (CT) chest, abdomen and pelvis scans (once annually for the first 2 years). No patient was lost to follow-up.
Data were expressed as median, maximum range and minimum range due to smaller sample size. Statistical analysis was performed by the Microsoft Excel 2010 software, with Fisher’s exact test used to determine significance, indicated by P value.
All 25 patients had been evaluated by the multidisciplinary team of the PE Unit, which included medical and radiation oncologists as well as surgeons. For each case, all findings from imaging modalities had been retrieved and carefully re-evaluated by a dedicated radiologist. The extent of local regional disease, as well as the potential for distant metastatic disease, had been determined, with the plan for multi-visceral resection and its approach being formulated accordingly.
Patients considered for surgical resection were those who had no evidence of metastatic disease, had good performance status, and represented those who the multidisciplinary team deemed that the ability to achieve a R0 resection was possible. Patients who did not meet operative criteria were those with either unresectable metastatic disease (for who surgery was performed with palliative intent) or unresectable large volume disease, or who were deemed physically or psychosocially unfit for extensive surgery.
Typically, in our institute, patients with primary advanced colorectal cancer undergo long-course neoadjuvant chemoradiotherapy. Upfront surgery is planned only in cases with prior chemoradiotherapy treatment for other cancers (e.g., prostate) or with cancers unlikely to benefit from neoadjuvant therapy (e.g., chordomas). A delay of 8-12 wk after neoadjuvant chemoradiation treatment is routinely advocated to achieve maximum down-staging. Repeat imaging is usually performed at 4 wk after completion of the neoadjuvant treatment, in order to determine response. The organs and planes involved before commencing neoadjuvant treatment are resected, as well, in order to ensure negative margin.
The entire team of specialty surgeons and anaesthetists assigned to the case would perform preoperative counselling in their respective area of resection or reconstruction. The counselling process involved appropriate patient-level explanations on the probability of achieving an R0 resection, the survival benefit post-PE, the morbidity and mortality risks associated with organ-specific resection or reconstruction, the anaesthetic risks and the financial implications. Stoma care and potential need of postoperative rehabilitation were also discussed with both the patient and any caregivers. The surgical candidate was also advised of the potential need for 2-4 wk postoperative inpatient hospital stay, including 1-2 d in the intensive care unit (ICU). The usual consultation process takes 4-6 wk. The majority of that time is allotted to allow patients to decide whether they are keen on the procedure and to come to accept the need for stoma; only after these issues are resolved can the patient provide final consent.
PE cases are highly heterogeneous, and the surgery types vary considerably; however, our PE Unit adheres to certain principles for all cases. All patients undergo oral bowel preparation, as well as mechanical thromboprophylaxis, prior to surgery. Chemical thromboprophylaxis is not routinely administrated, with respect to the potential high-risk of bleeding related to the extra-fascial plane dissection requirement.
All of the 25 cases assessed in this study had dedicated anaesthetists and underwent the PE in the Lloyd-Davis position. For those patients requiring a high sacrectomy (S2 and above), a combined anterior and posterior jack-knife approach was used. After laparotomy and adhesiolysis, any suspicious peritoneal nodules were biopsied and sent for frozen section. Positivity for peritoneal disease would have precluded curative resection, triggering abandonment of the procedure; however, none of the cases in our series showed positivity or peritoneal recurrence during the surgical exploration.
In all of the 25 cases, en bloc resection was the surgical aim. The surgical planes had been determined preoperatively by consensus among all involved surgeons. If an organ was abutting the tumour, en bloc resection was performed. There was no attempt in any case of a trial of dissection for organ preservation to prevent tumour spillage. Ureteric stents were not routinely inserted if bladder or ureteric resection was planned.
The standard approach of anterior or posterior PE, in our PE Unit, is to mobilise the central pelvic compartment (i.e., the rectum) immediately after ligating the inferior mesenteric vessels and performing transection of the distal sigmoid colon. The dissection continues along the total mesorectal excision (TME) plane, if feasible, and down to the pelvic floor. The dissection stops at the level of the organ involving the tumour. In pelvises restrained by adhesions or tumour, extra-fascial plane dissections are performed, but only after vascular control is obtained. Many of the 25 cases described herein necessitated cranial-to-caudal anterior compartment mobilization (i.e., urogenital and gynecological organs) and transection, specifically at the urethra or vagina, before the final transection of the rectum.
In our PE Unit, frozen section is utilized to confirm clear histopathology margins in areas associated with perioperative doubt. Advanced energy medical devices are commonly employed for TME mobilization and pelvic wall dissection, in order to reduce blood loss. The appropriate laparoscopic lengths of these devices are determined according to the narrow-width and deep-depth of the pelvis.
The technique for lateral PE utilized in our case series to achieve clear margins was that described by Höckel et al[11] and Austin et al[12]. The anatomic approach of this technique reaches the plane lateral to the internal iliac vessels. Vascular control of common iliac vessels and external iliac vessels is first achieved with vessel loops, and the external iliac vessels are mobilized to allow easy access to the obturator canal. The internal iliac artery is usually ligated first, before the internal iliac vein is accessed and ligated. All subsequent distal branches are suture ligated. These internal iliac vessels are then resected en bloc with the tumour specimen.
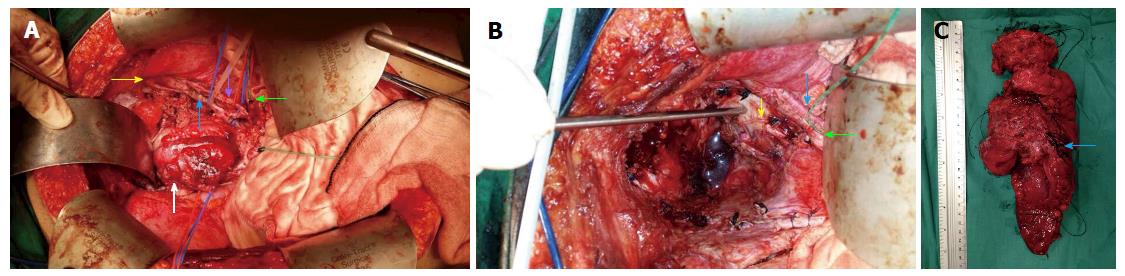
In our case series, the external iliac artery resection was performed only after a graft from the common iliac to the femoral artery was created. In addition, all sciatic nerves were preserved, but the perineural sheath was resected en bloc, if required. Lateral node dissection was also performed if there were suspicious nodes noted preoperatively, or if the tumour extended to the area of the lateral pelvic sidewall. This dissection would commence from the aortoiliac bifurcation, proceed down to the nodes around the common and external iliac vessels, and down to the origin of the internal iliac vessels and the obturator canal (Figure 1).
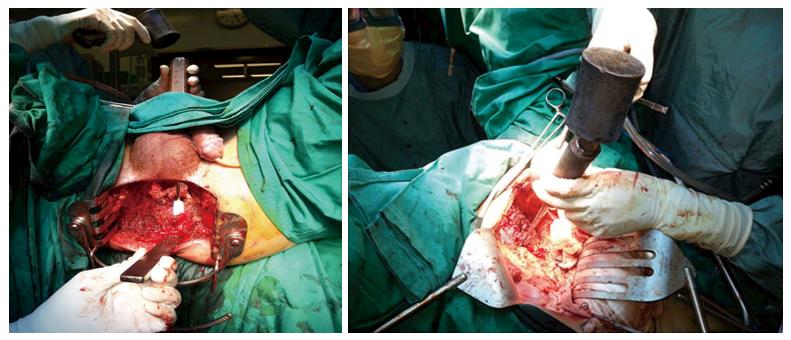
In our PE Unit, for sacrectomies, the abdominal approach is used for low sacrectomy (S3/S4), as described by Solomon et al[13]. For the combined abdominal-perineal approach, the abdominal phase incorporates complete mobilization of the posterior plane, up to 1 cm from the level of the sacrectomy. Ligation of the various internal iliac vessel branches, particularly the sacral, visceral and gluteal veins, is performed. Preservation of the upper sacral nerves is paramount, and all presacral fascia and piriformis muscles are dissected free. The perineal phase begins with an elliptical skin incision, which is followed by dissection below the coccyx and up to the level of the S2/S3 junction posteriorly, with the gluteal muscles and sacrococcygeous ligaments being dissected free. The sacrectomy is then performed by 20-mm osteotome, applied transabdominally, in a medial to lateral manner; this is carried out with a surgical assistant located at the perineum and placing an osteotome below the sacrum to prevent damage or button-holing of the perineal skin (Figure 2). For our cases, the perineal defect was reconstructed by the plastic surgeon using either primary closure and biological mesh reinforcement or myocutaneous pedicle flap.
For high sacrectomy (S1/S2), the orthopaedic team conducts the surgery with the patient in a prone jack-knife position. This procedure is performed only after complete mobilization of all vascular structures and organs off of the sacrum, down to the coccyx. Following ligation of all posterior internal iliac branches and completion of mobilization as described above, a penny towel pack is able to be placed between the sacrum and iliac vessels. The osteotomy site is marked anteriorly, using a drill. A myocutaneous flap is mobilized and tucked deep in the pelvis, a stoma is “matured” if necessary, and finally the abdomen is closed. After turning the patient to prone position, an incision is made down to the level of the sacrectomy and then transected with en bloc resection of the tumour. Reconstruction of the defect is then completed using the flap.
In our PE unit, an ileal conduit is commonly performed as the means of permanent urinary diversion. To avoid urinary complications, it is essential to have technical collaboration between the colorectal surgeons and the urologists. The most important technical step in ileal conduit formation is to ensure delicate handling of the ureters and ileum; the former must be meticulously mobilised with care to preserve ureteric vascularity. Transection of the ureters is performed as distal as possible, without compromising the oncological outcome. Ureteroenteric anastomosis is methodically performed, in order to achieve good tissue vascularity, in a tension-free manner and without malrotation of the ureters. These concepts are crucial to prevent urinary anastomotic leaks, conduit ischaemia and late ureteric strictures, while balancing the need for an adequate resection margin.
Twenty-five consecutive cases were evaluated. The patient demographics and indications for surgeries are summarized in Table 1. The median length of follow-up period was 17.6 mo (range: 6.3-39.0 mo). The most common indications for PE were locally invasive rectal adenocarcinomas (13 cases, including 9 primary and 4 recurrent), followed by advanced colonic adenocarcinomas (5 cases, including 3 primary and 2 recurrent), recurrent cervical carcinomas (3 cases) and malignant sacral chordomas (3 cases). There were 10 TPEs performed, and the majority of these cases were combined with lateral PEs. Three out of those 10 TPE cases also had sacrectomy. Except for 4 isolated lateral PEs, anterior (1 of 4) and posterior PEs (5 of 7) were commonly performed in conjunction with lateral PEs. R0 resection was achieved in 16 cases (64%). These results are summarised in Table 2.
| Variable | |
| Sex, n (%) | |
| Male | 16 (64) |
| Female | 9 (36) |
| Age, n (%) | Median, 61.9 yr (range, 30-72) |
| ASA score, n (%) | I: 11 (44) |
| II: 13 (52) | |
| III: 1 (4) | |
| Median, 2 | |
| ECOG status | 0: 22 (88) |
| 1: 2 (8) | |
| 3: 1 (4) | |
| Median, 0 | |
| Co-morbidities, n (%) | |
| Hypertension | 6 (24) |
| Diabetes mellitus | 5 (20) |
| Hyperlipidaemia | 8 (32) |
| Ischaemic heart disease | 1 (4) |
| Primary cancer type (n = 16) | |
| Colorectal | 12 |
| Chordoma | 3 |
| Gynaecological | 1 |
| Recurrent cancer type (n = 9) | |
| Colorectal | 6 |
| Gynaecological | 3 |
| Incidence of neoadjuvant chemoradiation | |
| Primary cancer | |
| Colorectal | 75% |
| Chordoma | 0% |
| Gynaecological | 0% |
| Recurrent cancer | |
| Colorectal | 67% |
| Gynaecological | 0% |
| Operative procedure, n (%) | |
| Total PE | 1 (4) |
| Total PE with lateral exenteration | 9 (36) |
| Anterior PE | 3 (12) |
| Anterior and Lateral PE | 1 (4) |
| Posterior PE | 2 (8) |
| Posterior and Lateral PE | 5 (20) |
| Lateral PE | 4 (16) |
| Sacrectomy combined with any above PE procedures | 9 (36) |
The median operative time was 11.5 h (range: 6.3-16.8 h). The median volume of blood loss was 3306 mL (range: 650-11000 mL), and the median volume of red cell transfusion was 1475 mL (range: 222-5565 mL). Of note, procedures combined with lateral PEs had higher blood loss (median: 2500 mL, range: 650-11000 mL). The highest blood loss in our series was 11 L, which occurred in a rectal cancer patient with 2nd occurrence of left pelvic wall nodal recurrence, and on who an isolated lateral PE was performed. This surgery was the 3rd procedure after initial ultra-low anterior resection and followed a prior attempt at lateral node dissection. After extensive adhesiolysis, the left pelvic nodal recurrence was resected en bloc with left distal ureter and internal iliac artery and vein. A segment of left external iliac vein was resected for margin, and a prosthetic graft reconstruction was made from common iliac to left femoral vein. The left ureter was reconstructed and re-implanted with a Boari flap.
The median length of ICU stay was 1 d (range: 0-8 d), and the median length of hospital stay was 21 d (range: 8-136 d). There was no perioperative mortality. The postoperative complications are summarized in Table 3. A total of 20 complications occurred in 12 patients. Three patients (12%) experienced major complications, including 2 patients (8%) with grade 3 Clavien-Dindo postoperative complications, which required further invasive interventions. The first patient with high body mass index (BMI) underwent redo-laparotomy for a torn ileal conduit mesentery bleed on postoperative day 1. The second patient, also with high BMI, underwent vertical rectus abdominis myocutaneous flap reconstruction and developed a postoperative large infective seroma in the abdominal wound site, which required percutaneous drainage on postoperative day 24. There was only one patient who required temporary renal dialysis (grade 4A Clavien-Dindo) following TPE with ileal conduit reconstruction, but no revision surgery was needed; the causes of acute renal failure were multifactorial, but did not include the newly-constructed ileal conduit. This patient’s renal function gradually recovered, without long-term disability. The remaining 9 patients had grade 2 complications, which required pharmacological interventions.
| Grade | Feature | n |
| 2 | Wound infection | 6 |
| Urinary tract infection | 4 | |
| Venous access infection | 4 | |
| Prolonged ileus | 1 | |
| Deep vein thrombosis | 1 | |
| Acute myocardial infarction | 1 | |
| 3 | Postoperative bleeding: Re-laparotomy | 1 |
| Donor site-infected seroma percutaneous drainage | 1 | |
| 4 | Temporary renal dialysis | 1 |
| Total adverse events | 20 |
During the study period, 18 out of 25 patients were in remission. There were 7 (30.4%) post-PE recurrences that presented during follow-up, and these included 2 with local regional recurrence, 2 with distant metastasis, and 3 with both regional and distant recurrences. The histopathological origin of cancer and postoperative microscopic margin status for each of these cases are summarized in Table 4. There were no statistically significant relationships among microscopic resection margin status, histopathological origin of tumour and postoperative recurrence (P = 0.67); these results may, however, simply reflect the small size cohort of this study. Among these 7 cases, 4 of the patients died during follow-up. Two of the patients’ deaths were attributed to cardiopulmonary failure from systemic disease burden. The remaining 2 patients’ deaths were related to sepsis secondary to locoregional recurrences, with 1 having developed urosepsis from ileal conduit malignant stricture and the other having developed pelvic sepsis from malignant pelvic floor fistula. The overall median survival from surgery to death was 12 mo (range: 6.1-17.0 mo).
| Pre-PE status | Histology origin | Regional recurrence | Distant metastasis | Regional and distant | R0 | R1 |
| Primary | Colonic | 0 | 0 | 1 | 1 | 0 |
| Primary | Rectal | 0 | 1 | 1 | 0 | 2 |
| Primary | Sacral chordoma | 1 | 0 | 0 | 1 | 0 |
| Recurrent | Rectal | 1 | 0 | 0 | 0 | 1 |
| Recurrent | Cervical | 0 | 1 | 1 | 2 | 0 |
| Total | 2 | 2 | 3 | 4 | 3 |
PE surgery has evolved over the decades. Brunschwig[1] originally developed PE as a palliative intervention, but-as detailed in the Introduction-the procedure had high morbidity and mortality rates and poor long-term outcome. These drawbacks precluded its widespread application by surgeons and acceptance by patients; and, despite its potentially life-saving benefits, this psychological and physical taxing operative procedure was considered with even more caution. However, constant evolution in chemoradiation interventions and surgical techniques, as well as better patient selection, have increased the safely of this procedure when performed by an experienced multidisciplinary team. Now, besides the survival benefits, there are also marked improvements to patients’ quality of life.
Studies have shown that the oncological benefit of PE is best when a negative pathological margin can be achieved[2,10,14-16]. To assess our short experience using a multidisciplinary team approach for PE surgery, the outcomes of a series of 25 consecutive patients were evaluated based on morbidity, mortality and recurrence. A systematic review performed by Young et al[9], which incorporated 23 studies and 1049 patients as a benchmark, noted a 73% R0 resection rate (range: 42%-100%). In that same review, the median perioperative mortality rate was low, at 2.2%, with the majority ranging from 0% to 25%. Our case series demonstrated comparable outcomes, namely 64% R0 resection rate and 0% in-hospital or 30-d perioperative mortality rates. The postoperative complication rate in our case series was 48% but the actual serious morbidity (grades 3 and 4 Clavien-Dindo) was 12%, and two-third of the adverse events in our case series were grade 2 Clavien-Dindo that necessitated pharmacological treatment alone. This finding is comparable to the median rate of 57% that was reported from the systematic review[9]. Short-term follow-up in our case series found a recurrence rate of 28%. There was, however, no statistically significant relationship among pathological resection margin status and post-exenteration recurrence in our study; since this is likely due to a small sample size, we must await our series to expand further before survival benefit can be commented on.
While our case series was large enough to generally assess the learning curve of our PE Unit, our procedures were highly heterogeneous and included complex lateral and posterior PEs that are not commonly performed. The results provide validation that these techniques applied for PE surgery allow for good short-term outcomes; yet, the authors acknowledge that achieving better outcomes would rely also on better decision-making and patient selection. One of the first criteria of patient selection for such extensive surgery is physical fitness and minimal co-morbidities. In our study cohort, the median age of patients was 62-year-old, and the oldest patient was 72-year-old (who underwent surgery for sacral chordoma). In general, our patients were fit; the median ASA score was 2 and the median ECOG score was 0. The one exception was a 42-year-old woman, who underwent the surgery despite being ECOG grade 3 status due to a symptomatic pelvic recurrence that caused significant disability.
The post-surgery social aspects are other important issues that must be considered in the decision-making process. Many patients are reluctant to accept the physical, psychological and financial sacrifices required for the surgery. It is not uncommon that a patient ends up with two permanent ostomies and are then unable to overcome the perceived lack of independence and social stigma. In our case series, multiple consultations were required in order to obtain the appropriate informed consent from the patient and caregiver, with the time frame often being 4-6 wk.
It was crucial in our preoperative planning that attempts were made to obtain histological proof of the tumour before PE, especially for cases of recurrent disease. This was achieved via endoscopic or percutaneous biopsy for accessible tumours. We also had to perform an open biopsy for 1 patient. Yet, this approach was considered especially important to aid in planning of the extra-fascial planes and because dissection is meant to avoid opening up of tumour planes and subsequent spillage of tumour cells. Obtainment of intraoperative biopsies of the tumours and subsequent frozen section histology can take time before proceeding to a PE, creating anxiety and uncertainty in both the patient and relatives, ultimately making the logistic planning of a multidisciplinary surgery difficult and inefficient. A confirmed preoperative diagnosis allows the patient to be convinced of the necessity of such extensive surgery and may avoid any potential medico-legal pitfalls.
Proper preoperative planning is necessary, with adequate time set aside for preanaesthetic assessment, a dedicated operative theatre list, invasive intraoperative haemodynamic monitoring and Level 1 rapid transfuser device set-up, if necessary. Adequate blood and ICU resources must be ensured before the operation commences. This operative planning incurs costs as well. Therefore, success of the programme long-term would also require cost-conscious practices or may negate support from the administrative side for these highly expensive and complex procedures.
For R0 resections, magnetic resonance imaging (MRI) has been shown to be a valuable tool to identify the anatomy of involved organs and to guide the extent of resection and reconstruction options, especially when reviewed by an experienced radiologist. In an expert’s hand, the radiological accuracy of rectal cancer staging improves in sensitivity (from 77% to 96%) and specificity (from 40% to 74%)[17]. We have had the benefit in our team of a dedicated radiologist who specializes in evaluating all images after initial reporting. The key questions asked include the likelihood of involvement of contiguous organs, the presence of undiagnosed peritoneal disease, and, often, the difference between post-radiation fibrosis vs tumour. This is especially pertinent to determine if a low sacrectomy will be required to treat advanced or recurrent rectal cancers. On MRI of a previously irradiated rectal cancer, it can be difficult-even for an expert-to differentiate between viable residual tumour and post-treatment fibrosis[17]. In these instances, as well as when indeterminate loco-regional or systemic organ or nodal disease is encountered on anatomical imaging, the fluorodeoxyglucose positron emission tomography (PET) with CT scan can be utilized. PET CT scan has reported sensitivity of 91% and specificity of 76% for colorectal metastatic lesions, and sensitivity of 91% and specificity of 91% for colorectal recurrence[18]. In addition, PET CT scan can guide the surgical decision for pelvic lymph node dissection to avoid pelvic autonomic nerve injury or late lower limb lymphedema.
MRI PET scan has been introduced for rectal cancer, and shown improved accuracy of T-staging for cases in which standalone MRI and PET CT failed to define the nature of an avid lesion. A small case series study has shown promising results regarding the use of MRI PET as compared to PET CT, with a true positive rate of 86% for the former vs 71% for the latter in overall TNM staging[19]. MRI PET is not readily available in our practice; however, it may represent the next-generation of preoperative imaging for PE planning. In the case of isolated pelvic sidewall or nodal recurrence, where the tumour is not accessible for biopsy and the disease is not apparent, serial imaging and tumour marker surveillance should be conducted after endoscopic re-assessment (if accessible) for anastomosis or luminal recurrence.
For the future of PE surgery, there are proposals to adopt laparoscopic or robotic techniques, especially for colorectal and gynaecological malignancies, due to the potential benefit of the minimally invasive nature of these surgeries. There are some published reports of laparoscopic-assisted anterior PE or TPE in highly selected patients with rectal or gynaecological cancers[20-23]. The preliminary data have shown minimal blood loss, short hospital stays, low morbidity rates, and non-comprising short-term oncological outcome. The first report of robotic PE in advanced rectal cancer patients was published by Shin et al[24] in 2014. The authors reported on 3 consecutive male patients with locally advanced rectal cancer involving prostate and seminal vesicles. The robotic approach was performed with reduced operative time and blood loss. Except for one minor vesical-urethral anastomosis leak requiring temporary suprapubic cystostomy, there were no other major surgical complications. Oncologic outcomes were also favourable in that study. These reports have highlighted the possibilities of minimally invasive surgery in the setting of complex PE. However, the small cohorts on which they are based consist of highly selected patients who have participated in short-term follow-up, and wide adaptation of this novel approach will require larger clinical trials.
Our PE Unit has demonstrated a safe and effective approach to manage complex pelvic cancers, with acceptable morbidity rates, zero-rate mortality and equivalent oncologic outcomes. The success of managing this group of patients was made possible by careful patient selection, detailed preoperative planning, multidisciplinary teamwork and an adaptation of modern operative techniques and technologies.
Advanced pelvic tumour is a debilitating illness, which poses a formidable surgical challenge. Chemotherapy and radiotherapy often improve the symptoms, but the results are transient. As the disease progress to the terminal stage, many patients suffer from refractory pain, bleeding, malodourous fistula or pelvic sepsis. Pelvic exenteration (PE) is a combination of numerous extensive surgical procedures that aims to remove all the diseased organs in order to achieve a negative resection margin. This complex intervention is currently the only curative option for advanced pelvic tumour.
PE has long been associated with high morbidity and mortality rates. However, adaptation of contemporary perioperative medical care approaches and innovative surgical techniques has allowed PE to emerge as the mainstream intervention, offering a good curative rate with low morbidity and mortality rates in selected patients with locally advanced pelvic tumours. Due to the substantial postoperative physiological disturbances associated with PE and the need to attain a negative margin, the focus of recent research has been to identify the suitable patient through comprehensive preoperative screening, detailed radiological staging, and adjuvant downstaging chemoradiotherapy. In addition, the development of methodological lateral PE and abdominal-approach sacrectomy has helped to improve the oncological outcome.
In this study, the authors describe their initial experience and treatment strategy in a newly-established PE Unit that achieves low morbidity, zero-rate mortality and acceptable R0 resection rate. The short-term result is equivalent to other reports in the recent literature. The authors attribute this success to a dedicated multidisciplinary team, state-of-the art perioperative care and modern operative techniques.
This study provides a descriptive patient selection criteria, perioperative non-surgical treatment strategy, and operative techniques that will help to reduce postoperative PE complications and achieve good oncological outcomes.
PE is a generic description of combined surgical procedures that were developed to remove the advanced pelvic tumour. Often, the advanced pelvic tumour has invaded into contiguous organs adjacent to the tumour origin, and therefore multiple surgical procedures are utilised in order to resect all diseased organs and achieve negative pathological resection margin. PE can be subgrouped into four types according to pelvic organs that are resected. Anterior PE involves removal of the upper rectum and genitourinary organs. Posterior PE involves removal of the rectum and reproductive organs, but spares the bladder. Total PE is defined as removal of the rectum, distal colon, genitourinary viscera, internal reproductive organs, draining lymph nodes and pelvic peritoneum. Lateral PE involves removal of the lateral pelvic lymph nodes along with diseased vascular and neural structures. After extensive resection, it is common to combine further procedures, such as permanent faecal or urinary diversion and perineal reconstruction, in order to maintain the physiology and to close the muscular defect.
The newly-established PE Unit reported by the authors offers state-of-the-art exenteration service in Singapore. This study confirms that the modern perioperative treatment strategy and multidisciplinary approach produced excellent short-term outcomes in the first 25 consecutive cases.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ashkenazi I, Han H, Tomazic A S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma. Cancer. 1948;1:77. [PubMed] |
| 2. | Dobrowsky W, Schmid AP. Radiotherapy of presacral recurrence following radical surgery for rectal carcinoma. Dis Colon Rectum. 1985;28:917-919. [PubMed] |
| 3. | Temple WJ, Saettler EB. Locally recurrent rectal cancer: role of composite resection of extensive pelvic tumors with strategies for minimizing risk of recurrence. J Surg Oncol. 2000;73:47-58. [PubMed] |
| 4. | Ito Y, Ohtsu A, Ishikura S, Boku N, Nihei K, Ogino T, Ikeda H. Efficacy of chemoradiotherapy on pain relief in patients with intrapelvic recurrence of rectal cancer. Jpn J Clin Oncol. 2003;33:180-185. [PubMed] |
| 5. | Danjoux CE, Gelber RD, Catton GE, Klaassen DJ. Combination chemo-radiotherapy for residual, recurrent or inoperable carcinoma of the rectum: E.C.O.G. study (EST 3276). Int J Radiat Oncol Biol Phys. 1985;11:765-771. [PubMed] |
| 6. | Heriot AG, Byrne CM, Lee P, Dobbs B, Tilney H, Solomon MJ, Mackay J, Frizelle F. Extended radical resection: the choice for locally recurrent rectal cancer. Dis Colon Rectum. 2008;51:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Harji DP, Griffiths B, McArthur DR, Sagar PM. Surgery for recurrent rectal cancer: higher and wider? Colorectal Dis. 2013;15:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Wells BJ, Stotland P, Ko MA, Al-Sukhni W, Wunder J, Ferguson P, Lipa J, Last L, Smith AJ, Swallow CJ. Results of an aggressive approach to resection of locally recurrent rectal cancer. Ann Surg Oncol. 2007;14:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Young JM, Badgery-Parker T, Masya LM, King M, Koh C, Lynch AC, Heriot AG, Solomon MJ. Quality of life and other patient-reported outcomes following exenteration for pelvic malignancy. Br J Surg. 2014;101:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Yang TX, Morris DL, Chua TC. Pelvic exenteration for rectal cancer: a systematic review. Dis Colon Rectum. 2013;56:519-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Höckel M. Laterally extended endopelvic resection: surgical treatment of infrailiac pelvic wall recurrences of gynecologic malignancies. Am J Obstet Gynecol. 1999;180:306-312. [PubMed] |
| 12. | Austin KK, Solomon MJ. Pelvic exenteration with en bloc iliac vessel resection for lateral pelvic wall involvement. Dis Colon Rectum. 2009;52:1223-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Solomon MJ, Tan KK, Bromilow RG, Al-mozany N, Lee PJ. Sacrectomy via the abdominal approach during pelvic exenteration. Dis Colon Rectum. 2014;57:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Hahnloser D, Nelson H, Gunderson LL, Hassan I, Haddock MG, O’Connell MJ, Cha S, Sargent DJ, Horgan A. Curative potential of multimodality therapy for locally recurrent rectal cancer. Ann Surg. 2003;237:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Akasu T, Yamaguchi T, Fujimoto Y, Ishiguro S, Yamamoto S, Fujita S, Moriya Y. Abdominal sacral resection for posterior pelvic recurrence of rectal carcinoma: analyses of prognostic factors and recurrence patterns. Ann Surg Oncol. 2007;14:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Bouchard P, Efron J. Management of recurrent rectal cancer. Ann Surg Oncol. 2010;17:1343-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Torkzad MR, Påhlman L, Glimelius B. Magnetic resonance imaging (MRI) in rectal cancer: a comprehensive review. Insights Imaging. 2010;1:245-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Brush J, Boyd K, Chappell F, Crawford F, Dozier M, Fenwick E, Glanville J, McIntosh H, Renehan A, Weller D. The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation. Health Technol Assess. 2011;15:1-192, iii-iv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Paspulati RM, Partovi S, Herrmann KA, Krishnamurthi S, Delaney CP, Nguyen NC. Comparison of hybrid FDG PET/MRI compared with PET/CT in colorectal cancer staging and restaging: a pilot study. Abdom Imaging. 2015;40:1415-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Patel H, Joseph JV, Amodeo A, Kothari K. Laparoscopic salvage total pelvic exenteration: Is it possible post-chemo-radiotherapy? J Minim Access Surg. 2009;5:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Ishizaki H, Nakashima S, Hamada T, Nishida T, Maehara N, Ikeda T, Tsukino H, Mukai S, Kamoto T, Kondo K. Laparoscopic anterior pelvic exenteration for locoregionally advanced rectal cancer directly invading the urinary bladder: A case report of low anterior resection with en bloc cystectomy for sphincter preservation. Asian J Endosc Surg. 2015;8:343-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Yang K, Cai L, Yao L, Zhang Z, Zhang C, Wang X, Tang J, Li X, He Z, Zhou L. Laparoscopic total pelvic exenteration for pelvic malignancies: the technique and short-time outcome of 11 cases. World J Surg Oncol. 2015;13:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Uehara K, Nakamura H, Yoshino Y, Arimoto A, Kato T, Yokoyama Y, Ebata T, Nagino M. Initial experience of laparoscopic pelvic exenteration and comparison with conventional open surgery. Surg Endosc. 2016;30:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Shin JW, Kim J, Kwak JM, Hara M, Cheon J, Kang SH, Kang SG, Stevenson AR, Coughlin G, Kim SH. First report: Robotic pelvic exenteration for locally advanced rectal cancer. Colorectal Dis. 2014;16:O9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |