Published online May 15, 2017. doi: 10.4251/wjgo.v9.i5.209
Peer-review started: July 30, 2016
First decision: September 29, 2016
Revised: December 20, 2016
Accepted: March 23, 2017
Article in press: March 24, 2017
Published online: May 15, 2017
Processing time: 288 Days and 21.5 Hours
To identify whether CpG island methylator phenotype (CIMP) is predictive of response to neoadjuvant chemoradiotherapy (NACRT) and outcomes in rectal cancer.
Patients undergoing NACRT and surgical resection for rectal cancer in a tertiary referral centre between 2002-2011 were identified. Pre-treatment tumour biopsies were analysed for CIMP status (high, intermediate or low) using methylation specific PCR. KRAS and BRAF status were also determined using pyrosequencing analysis. Clinical information was extracted from case records and cancer services databases. Response to radiotherapy was measured by tumour regression scores determined upon histological examination of the resected specimen. The relationship between these molecular features, response to NACRT and oncological outcomes were analysed.
There were 160 patients analysed with a median follow-up time of 46.4 mo. Twenty-one (13%) patients demonstrated high levels of CIMP methylation (CIMP-H) and this was significantly associated with increased risk of extramural vascular invasion (EMVI) compared with CIMP-L [8/21 (38%) vs 15/99 (15%), P = 0.028]. CIMP status was not related to tumour regression after radiotherapy or survival, however EMVI was significantly associated with adverse survival (P < 0.001). Intermediate CIMP status was significantly associated with KRAS mutation (P = 0.01). There were 14 (9%) patients with a pathological complete response (pCR) compared to 116 (73%) patients having no or minimal regression after neoadjuvant chemoradiotherapy. Those patients with pCR had median survival of 106 mo compared to 65.8 mo with minimal regression, although this was not statistically significant (P = 0.26). Binary logistic regression analysis of the relationship between EMVI and other prognostic features revealed, EMVI positivity was associated with poor overall survival, advanced “T” stage and CIMP-H but not nodal status, age, sex, KRAS mutation status and presence of local or systemic recurrence.
We report a novel association of pre-treatment characterisation of CIMP-H with EMVI status which has prognostic implications and is not readily detectable on pre-treatment histological examination.
Core tip: There is wide and unpredictable response of rectal cancer to neoadjuvant therapy which carries significant side effects and relies on limited pre-treatment risk stratification. Methylation specific PCR was used to determine CpG island Methylator phenotype (CIMP) status in 160 rectal cancers and compared with response to therapy, clinical and pathological outcomes. CIMP status was not directly related to tumour regression but was related to extramural vascular invasion which confers an adverse survival risk.
- Citation: Williamson JS, Jones HG, Williams N, Griffiths AP, Jenkins G, Beynon J, Harris DA. Extramural vascular invasion and response to neoadjuvant chemoradiotherapy in rectal cancer: Influence of the CpG island methylator phenotype. World J Gastrointest Oncol 2017; 9(5): 209-217
- URL: https://www.wjgnet.com/1948-5204/full/v9/i5/209.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i5.209
Locally advanced rectal cancer is usually treated with neoadjuvant chemoradiotherapy to downstage and/or downsize the tumour prior to surgery[1,2]. The response of rectal cancer to neoadjuvant therapy varies significantly between patients. The most successful outcome is a pathological complete response (pCR) in which no viable tumour cells are seen upon subsequent histological examination of the resected bowel. In this scenario patients have a significantly improved 5-year survival of up to 85%-100%, although any residual lymph-nodal involvement is associated with a significantly worse survival despite complete local tumour regression[3]. This compares favourably with those showing minimal response to radiotherapy who may expect 5-year survival of between 55%-66%[4].
pCR occurs in between 10%-20% of patients undergoing neoadjuvant chemoradiation therapy[4-6], however up to 30% of patients do not show any response[7]. Furthermore, those patients not responding to neoadjuvant treatment risk progression of their disease with either local progression or distant metastases during preoperative treatment. The use of imaging technology including magnetic resonance imaging (MRI) and endorectal ultrasound are not sufficiently reliable[8,9] to be implemented as a sole means of discriminating between those with pCR and those without.
The adverse prognostic value of extramural vascular invasion (EMVI) is well established and is known to be associated with poor survival[10], increased risk of local recurrence[11] and death[11-14]. Furthermore, the presence of EMVI has a relative risk of 3.7 for the development of systemic recurrence when detectable on preoperative MRI scanning[15]. The role of EMVI in directing treatment is relatively new and not well established. In particular, National Institute of Health and Care Excellence recommend that EMVI may confer a higher risk of recurrence in stage II rectal cancers and suggest adjuvant chemotherapy may be considered in those patients with EMVI where the relative benefits of this treatment are not otherwise clear[16]. EMVI status may also influence the decision to offer neoadjuvant radiotherapy, as it has been demonstrated that chemoradiation (CRT) can cause vessel fibrosis in EMVI-positive tumours, which may influence survival outcomes[17].
EMVI is detectable in rectal cancer patients on MRI, however, sensitivity and specificity are relatively low at 62% and 88% respectively[17]. It is therefore important that not only is EMVI accurately characterised but should be available early to inform decisions regarding neoadjuvant chemoradiotherapy and influence overall treatment outcomes.
Developments in genetics and epigenetics lend support to the notion that tumours display characteristic clinicopathological and morphological features depending on the nature of specific combinations of molecular patterns[18]. In particular, the CpG island methylator phenotype (CIMP), which may account for up to 20% of all colorectal cancers[19,20], is associated with differences in tumour location, patient gender and association with characteristic gene mutations including KRAS, BRAF and p53[18], although this relationship has not been explored in EMVI. CpG islands are typically short (300-3000 base pairs) Cytosine-Guanine phosphodiester bonded sequences found in or around the promoter region of a gene where they are usually unmethylated if the genes are expressed. The CIMP phenotype is characterised by epigenetic DNA hyper-methylation and consequent suppression of key genes important in controlling cell growth and survival, which is associated with poor survival in rectal cancer[21,22]. It is becoming increasingly clear that epigenetic factors affecting specific gene promoter regions (CpG islands) can be equally as important as genetic alterations in all disease processes, as these can affect every component of gene regulation. Previous work has demonstrated that genetic factors such as KRAS mutation has an inverse relationship with EMVI[23] but little is known of the influence of epigenetic factors in the development of EMVI. The purpose of this study was to explore the relationship between CIMP and response to chemoradiotherapy and EMVI in rectal cancer.
Patients undergoing neoadjuvant chemoradiotherapy and subsequent surgical resection for rectal adenocarcinoma with curative intent were identified from a prospectively maintained pathology database of all colorectal cancers between the years 2002 and 2011. All patients underwent endoscopic diagnostic biopsy in order to confirm histological evidence of rectal adenocarcinoma prior to treatment. After pre-treatment staging with thoracic-abdominal-pelvic computed tomography (CT), pelvic MRI, clinical examination under anaesthesia (EUA) and in some cases endorectal ultrasound (ERUS), patients were discussed by the multidisciplinary team and offered neoadjuvant chemoradiotherapy according to the local protocol. Local indications for neoadjuvant CRT were extensive mesorectal or pelvic sidewall nodal disease, predicted mesorectal fascia involvement by tumour and/or lymph nodes based on MRI imaging, or clinical fixity of tumour to surrounding structures. After a 6 to 8 wk period following completion of chemoradiotherapy patients underwent restaging investigations (MRI, CT, ERUS and/or EUA) to assess response to treatment and to plan surgical resection. Standardised surgical techniques to maximise complete excision were used including total mesorectal excision and extralevator pelvic floor excision. In some cases multivisceral resection was required for tumours beyond conventional planes. Neoadjuvant radiotherapy in all cases was administered at South West Wales Oncology Centre (Singleton Hospital, Swansea, United Kingdom) and delivered with concurrent 5-fluoroUracil (Capecitebine) according to local protocol.
Pre-treatment biopsy specimens stained with Haematoxylin and Eosin were examined by a consultant histopathologist to ensure they contained at least 60% adenocarcinoma tissue. Post treatment resection specimens were examined by two consultant histopathologists who were blinded to patient details and recorded their reports conforming to the Royal College of Pathologists colorectal cancer data set (2nd edition 2007) on separate sheets which were stored in a locked cabinet and not seen by other investigators until the data analysis stage. If the reports given by pathologists differed, a third pathologist would be asked to give an opinion and the final report reflected the consensus. When examining tumour regression scores, to ensure there was agreement between the two pathologists scoring the regression, Cohen’s kappa statistic was utilised to measure agreement between both raters. For the Royal College of Pathologists tumour regression score there was almost perfect agreement (k = 0.856 P < 0.001). Patients not completing a full course of neoadjuvant CRT or those not proceeding to surgery were excluded from this study. Patients with rectosigmoid junction tumours, history of inflammatory bowel disease or known high risk genetic predisposition to colorectal cancer (familial adenomatous polyposis or Lynch syndrome) and those undergoing treatment for recurrent cancer were also excluded.
Demographic and clinical outcome data for patients in this study were gathered from patients’ case notes, clinic letters and computerised patient hospital records. Patients with local and systemic recurrence were also identified in this way. To identify patients who had died following their treatment, the NHS Wales Informatics Service (Myrddin) database was utilised which records the date of death for each patient if this has occurred. Overall survival, local and systemic recurrence free survival were calculated from the date of surgical resection until either the date of death or the date that recurrence was confirmed clinically, radiologically or histopathologically. If no death or recurrence had occurred, the reference date of last known follow-up was used to calculate survival. These data were also cross referenced against the Cancer Information Network System Cymru database which records data for all patients undergoing cancer treatment in South Wales to ensure its accuracy. Ethical approval for this study was granted by South West Wales REC (Project Ref No.:11/WA/0256). Consent was not required in accordance with the Human Tissue Act 2004 (chapter 30).
Formalin fixed paraffin embedded pre-treatment biopsy specimens were utilised for this study. Several representative 5 μm sections of the biopsy were cut and mounted unstained onto glass slides and DNA from these tissues was obtained using the MasterPure Complete DNA and RNA purification kit (Epicentre, Illumina, WI, United States).
The quantity and quality of DNA was measured at absorbance between 230 nm and 320 nm using spectrophotometry (Nanodrop ND-1000, Software v3.1.2, Thermoscientific, DE, United States). DNA quantity was calculated by multiplying the measured concentration following spectrophotometry at 260 nm with the dilution factor. DNA was diluted to a working concentration of 20 ng/μL. Purity was further analysed by calculating the absorbance at 260 nm to absorbance at 280 nm ratio.
Methylation specific PCR is accomplished by performing bisulfite conversion of genomic DNA (Imprint DNA Modification Kit, Sigma Aldrich, United States). The PCR products were resolved using gel electrophoresis on a 30% polyacrylamide gel. Depending on the methylation status of each CpG island, each patient could be classified as one of three epigenotypes; CIMP-High, Intermediate or Low using a two panel approach[24,25]. The first panel consists of SOCS1, MINT-1 and hMLH, which are associated strongly with CIMP-H. The second panel consist of NEUROG1, THBD, HAND1, ADAMTS1, IGFBP3. CIMP status could then be determined using the following system: (1) CIMP-High if ≥ 2/3 group 1 markers methylated; (2) CIMP-Intermediate if < 2/3 group 1 but ≥ 3/5 group 2 methylated; and (3) CIMP-Low if < 2/3 group 1 and < 3/5 group 2 methylated.
Pyrosequencing analysis was performed in collaboration with the Leeds Cancer Research United Kingdom Centre, (Leeds Institute of Cancer Studies and Pathology, Clinical Sciences Building, level 6, St. James’s University Hospital, Leeds, LS9 7TF). Pyrosequencing conditions used were as previously published by this group[26]. Substitution and insertion/deletion mutations in KRAS codon 12, 13 and 61 and BRAF-600 were examined for all specimens using this method.
Tumours were defined as low (0-5 cm from anal verge), mid (5-10 cm) or high (10-15 cm) rectal based on preoperative rigid sigmoidoscopy and according to where the majority of the tumour was located. Predicted circumferential resection margin (CRM) involvement was defined by the presence of tumour foci (primary, nodal or extranodal deposit) within 1 mm of the mesorectal fascia or cylindrical resection margin for low tumours. An involved CRM was defined pathologically as tumour within 1 mm of the CRM. The original definition of EMVI describes “a rounded mass of tumour in an endothelium-lined space either surrounded by a rim of smooth muscle or containing red blood cells[27]”. More recent definitions suggest venous invasion may also be suspected when a rounded or elongated tumour profile is identified adjacent to an artery, especially when no separate accompanying vein can be identified (the “orphan” artery sign), or where smooth tongues of tumour extend into pericolic/perirectal fat (“protruding tongue’’ sign)[28].
Statistical analysis was performed using SPSS v.18 Chicago: SPSS Inc. Data was tested for normality using a Kolomogorov-Smirnov test, and a Student’s t-test was for analysis of normally distributed continuous data. Categorical variables were compared using χ2 or Fishers exact test where expected frequencies were less than 10. Relationship between independent variables and time to event was compared using Kaplan-Meier methodology using the Log Rank test to determine significance. Multivariable analysis was performed using bivariate logistical regression and Cox Proportional Hazards modelling. Statistical significance was assumed at the 5% level.
There were 160 patients included in this study. There were 113 (71%) males and 47 (29%) females and the average age by the time of surgery was 65.4 years. By the time of this analysis, 53 (33%) patients had died and the median time from surgery to death was 26.2 mo (IQR 11.9-48.5).
Of the surviving patients, the median follow-up time from surgery was 46.4 mo (IQR 33.8-56.0). Local recurrence data were available for 152 patients and of these, 8 (5%) had evidence of local recurrence a median of 19.7 mo after surgery. Systemic recurrence data were available for 151 patients and of these, 37 (25%) had evidence of systemic recurrence at median 16.3 mo after surgery. Overall survival for all patients was estimated using Kaplan-Meier analysis at 73.3 mo (95%CI: 63.3-83.2). 4 (3%) patients had an involved CRM which was related to worse overall survival (74.1 mo vs 37.2 mo, P = 0.047).
There were 14 (9%) patients with a pCR compared to 116 (73%) patients having no or minimal regression after neoadjuvant chemoradiotherapy. Of those undergoing pCR, 8 were male, 6 were female and had a mean age of 66 years. None of the pCR patients demonstrated CIMP-H, whereas 2 were CIMP-I and 12 were CIMP-L. Those patients with pCR had median survival of 106 mo compared to 65.8 mo with minimal regression, although this was not statistically significant (P = 0.26). There were 52 patients (33%) with demonstrable KRAS mutation, but only a single BRAF mutation was detected in the study sample.
CIMP status was determined in all patients, 21 (13%) were CIMP-H, 40 (25%) were CIMP-I and 99 (62%) were CIMP-L. Comparison of patient characteristics by CIMP status revealed no differences in mean age, gender, “T” or “N” stage, presence of systemic or local recurrence, CRM involvement, survival or tumour regression scores. Sub-analysis of individual CIMP markers with tumour regression scores revealed no significant differences. However, CIMP-H was significantly related to EMVI positivity with 8/21 (38%) CIMP-H patients demonstrating EMVI compared with 15/99 (15%) who were CIMP-L. (CIMP-H/ EMVI+ 38% vs CIMP-L/EMVI+ 15%, Fishers exact, P = 0.028). Furthermore, a higher proportion of CIMP-I patients demonstrated KRAS mutation than other CIMP groups [CIMP-I + KRAS mutation 20/40 (50%) vs CIMP-H/L + KRAS mutation 32/120 (27%), Fishers exact, P = 0.01] (Table 1).
| CIMP-H | CIMP-I | CIMP-L | P value | |
| Mean age | 66 | 69.2 | 63.9 | |
| Sex | ||||
| Female | 5 | 14 | 28 | |
| Male | 16 | 26 | 71 | |
| ypT stage | ||||
| 0 or pCR | 2 | 2 | 16 | |
| 1 | 3 | 1 | 7 | |
| 2 | 2 | 10 | 20 | |
| 3 | 11 | 24 | 48 | |
| 4 | 3 | 3 | 8 | |
| ypN stage | ||||
| 0 | 11 | 27 | 65 | |
| 1 | 6 | 8 | 21 | |
| 2 | 4 | 5 | 13 | |
| Systemic recurrence | ||||
| Absent | 14 | 30 | 66 | |
| Present | 5 | 2 | 22 | |
| Local recurrence | ||||
| Absent | 20 | 37 | 87 | |
| Present | 0 | 2 | 6 | |
| EMVI | ||||
| Negative | 13 | 33 | 84 | |
| Positive | 8 | 7 | 15 | CIMP-L vs CIMP-H P = 0.028 |
| KRAS status | ||||
| Wildtype | 15 | 20 | 73 | |
| Mutant | 6 | 20 | 26 | KRAS Mut + CIMP-I P = 0.01 |
| CRM | ||||
| Not involved | 21 | 39 | 95 | |
| Involved | 0 | 0 | 4 | |
| RC path score | ||||
| 1 (pCR) | 0 | 2 | 12 | |
| 2 | 6 | 9 | 14 | |
| 3 | 15 | 29 | 73 | |
| Total | 21 | 40 | 99 |
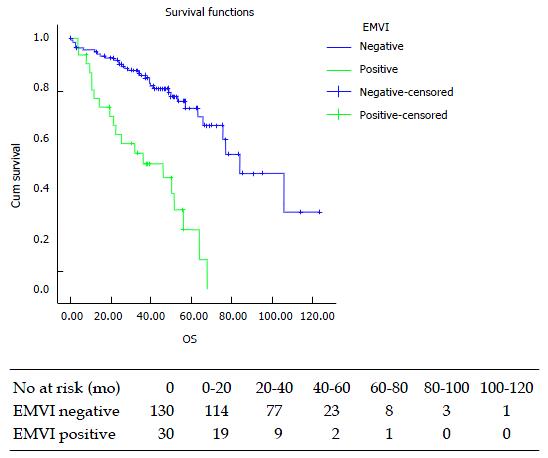
None of 21 (0%) patients with CIMP-H tumours experienced a pCR compared with 12/99 (12%) CIMP L patients, however this was not statistically significant (Fishers exact = 0.12). There were 30 (19%) patients with EMVI-positivity on histopathological examination of the specimen. This was associated with a significant reduction in median overall survival (83.8 mo vs 43.9 mo, P < 0.001, Figure 1).
No patient with pCR displayed EMVI, whereas 29 (25%) with RC Path score of 3 (minimal regression) displayed EMVI (P = 0.039, Table 2).
| EMVI+ | EMVI- | P value | |
| RC Path 1 (pCR) | 0 | 14 | 0.039 |
| RC Path 2 | 1 | 28 | |
| RC Path 3 | 29 | 88 |
Cox hazard regression analysis revealed that EMVI-positivity was the only factor that was significantly related to adverse survival (Table 3).
| Wald statistic | OR | 95%CI (lower) | 95%CI (upper) | P value | |
| T stage | 1.735 | 1.392 | 0.851 | 2.279 | 0.188 |
| N stage | 0.268 | 0.857 | 0.479 | 1.535 | 0.605 |
| EMVI | 9.422 | 4.041 | 1.657 | 9.857 | 0.002 |
| CIMP status | 0.982 | 0.791 | 0.498 | 1.257 | 0.322 |
| KRAS status | 2.162 | 1.740 | 0.832 | 3.640 | 0.141 |
| Sex | 0.439 | 0.764 | 0.344 | 1.695 | 0.508 |
| Local recurrence | 0.861 | 1.763 | 0.532 | 5.839 | 0.353 |
| Systemic recurrence | 2.165 | 1.729 | 0.834 | 3.584 | 0.141 |
| Tumour regression (pCR) | 0.052 | 0.793 | 0.109 | 5.785 | 0.819 |
| Involved CRM | 0.146 | 1.339 | 0.299 | 6.002 | 0.703 |
Binary logistic regression analysis of the relationship between EMVI and other prognostic features revealed, EMVI positivity was associated with poor overall survival, advanced “T” stage and CIMP-H but not nodal status, age, sex, KRAS mutation status and presence of local or systemic recurrence (Table 4).
| OR | 95%CI (lower) | 95%CI (upper) | P value | |
| Overall survival | 0.936 | 0.893 | 0.981 | 0.006 |
| T stage | 7.764 | 1.749 | 34.463 | 0.007 |
| N stage | 2.552 | 0.851 | 7.651 | 0.095 |
| Age | 1.024 | 0.969 | 1.081 | 0.405 |
| Systemic recurrence | 0.865 | 0.200 | 3.749 | 0.846 |
| Sex | 0.564 | 0.119 | 2.668 | 0.470 |
| Local recurrence | 1.841 | 0.193 | 17.562 | 0.596 |
| Involved CRM | 0.276 | 0.009 | 8.376 | 0.459 |
| KRAS mutation | 1.577 | 0.389 | 6.391 | 0.524 |
| CIMP-H | 6.368 | 1.091 | 37.162 | 0.040 |
CIMP-positivity has been implicated as an adverse survival predictor in patients with colorectal cancer[29-31], however, the majority of studies investigating survival outcomes in relation to methylation status regard colon and rectal cancers as one entity. Most investigators identify CIMP as an adverse prognostic feature, particularly in colorectal cancer taken as a whole and this was also corroborated by a recent meta-analysis including all colorectal sub sites, which found shorter survival in CIMP positive patients[32,33].
The current understanding of the role of CIMP in colorectal cancer is that tumours with a greater level of CpG island methylation (CIMP-High or CIMP +) have distinct molecular and clinical characteristics compared to low levels of CpG methylation (CIMP-Low or CIMP -)[34]. There is some evidence that CIMP-Positivity is related to shorter overall survival[35] and disease free survival[36], however the populations in these studies generally lack homogeneity of factors such as KRAS and BRAF mutation status, MSI status and tumour stage[34].
The present study did not demonstrate any relationship between CIMP status and survival. CIMP status was however significantly associated with EMVI positivity which itself was associated with worse survival. Therefore it is likely that the relative contribution of these phenomena to prognosis is more complex than previously understood and should be studied in more detail and with particular distinction of rectal cancers from colon cancers.
Relatively few studies have studied the role of CIMP as a predictive marker of rectal cancer response to neoadjuvant chemoradiotherapy. A factor that complicates the evidence is that there is no agreed definition on CIMP classification, and therefore widely ranging and contradicting results are found in the literature. Our research did not find that CIMP status was a predictor of response to chemoradiotherapy, although others have found that detecting the methylation status of individual gene promoter-regions affected the response to neoadjuvant treatment.
Ebert et al[37] examined a total of 294 patients with colorectal cancer undergoing neoadjuvant chemotherapy (5-fluorouracil, oxaliplatin and irinotecan), and analysed the expression, methylation and function of the TFAP2E gene. They demonstrated that hypermethylation of the promoter regions of TFAP2E was associated with down-regulation of the gene, and the subsequent up-regulation of a down-stream target. Furthermore, TFAP2E hypermethylation was a marker of 5-fluorouracil resistance in CRC in this study, but there was no effect on response to treatment with oxaliplatin or irinotecan.
Ogino et al[38] examined methylation in 840 colorectal cancers led to the proposal that a further subset of intermediate methylation associated tumours exist but which do not fulfil the criteria for CIMP-High. These tumours (termed CIMP-intermediate) were independently associated with male gender and KRAS mutation. The three epigenotype model was further supported by Yagi et al[24], who used a large scale mass spectrometry analysis and hierarchical clustering to identify two panels of markers, the first to identify CIMP-High tumours and then a second panel to distinguish between CIMP-intermediate and low tumours. In our research, CIMP-I had a significant association with KRAS-mutation compared to CIMP-H or CIMP-L tumours (P = 0.01), confirming this association in our patients, although no difference with regards to survival was demonstrated.
The adverse prognostic value of EMVI is well established and is known to be associated with poor survival[10] and has a relative risk of 3.7 for the development of systemic recurrence when detectable on preoperative MRI scanning[15]. This is supported by data from the present study, which revealed significantly decreased survival with EMVI.
EMVI was also associated with a lack of response to neoadjuvant chemoradiotherapy. If EMVI is present before treatment and is absent after treatment, then this would indicate a response, whereas failure of EMVI to regress would indicate a lack of response. However, the presence of EMVI is not currently detectable on histological analysis of pre-treatment biopsy specimens. In the present study, a novel association between EMVI and CIMP-H status was identified. This finding does provide a novel insight into potential mechanisms for the association of poor survival with CIMP-H seen in other studies.
There are several mechanisms which may explain the link between CpG island hypermethylation and EMVI. For example, angiogenesis and subsequent local invasion of colorectal tumours has previously been linked to hypermethylation and silencing of micro-RNA-126 (miRNA-126), which is associated with up-regulation of vascular endothelial growth factor and subsequent increased likelihood tumour invasion[39]. Other research has suggested that silencing the gene that codes for E-Cadherin (a molecule that forms the adherens junctions between normal cells, preventing spread of tumour cells across the epithelial basement membrane)[40] is associated with increased risk of EMVI and reduced response to neoadjuvant chemoradiotherapy and worse survival in rectal cancers[41]. Finally, the invasion of cancer cells into the surrounding extracellular matrix depends on the function of matrix metalloproteinases (MMPS), which are themselves regulated by tissue inhibitors of matrix metalloproteinases (TIMPS). In vitro and animal studies have demonstrated that aberrant epigenotypes affecting the MMP/TIMPS axis can lead to increased tumour invasion and migration in vitro and increased tumourigenesis and therapeutic reversal of this aberrant methylation can suppress these tumourigenic phenomenon[42,43].
Given that CIMP is deemed to represent a phenotypic hypermethylated state, it is likely that the presence of the CIMP-H state explains the association of EMVI-positivity and poor survival seen in rectal cancer patients. The detection of a hypermethylated state in individual gene promoter regions may well further our understanding of the response to chemoradiotherapy in the future.
The authors wish to acknowledge the support of Mr. Phil Chambers of the Cancer Research United Kingdom centre, Leeds for his assistance with pyrosequencing of KRAS and BRAF mutations in these specimens. We also wish to thank Dr. Owen Bodger statistician at Swansea University School of Medicine for his assistance with statistical analysis, Dr. Mau Don Phan clinical oncologist at Singleton Hospital for her assistance with outcome data collection as well as consultant colorectal surgeons Mr. Umesh Khot, Mr. TV Chandrasekaran, Mr. Mark Davies and Mr. Martyn Evans for their collaboration with patient data collection.
There is wide variation in response to neoadjuvant chemoradiotherapy (NACRT) in rectal cancer, which has a significant impact on survival. There is currently no reliable means to predict response to NACRT, which carries significant side effects. The CpG island methylator phenotype (CIMP) is characterised by epigenetic DNA hyper-methylation and suppression of key genes controlling cell growth and survival and occurs in approximately 20% of colorectal cancers. The role of CIMP status in the prognosis and response of rectal cancer to neoadjuvant therapy is not well understood but evidence is emerging that it may be an adverse prognostic indicator.
Previous studies have demonstrated an association of high levels of CIMP associated methylation with adverse survival and differential responses to neoadjuvant treatment where methylation is seen in specific genes in rectal cancer, however, the mechanism and exact nature of this association is not clear.
This study reports a novel association of CIMP related methylation with extra mural vascular invasion which represents an adverse prognostic indicator and provides a novel insight into potential mechanisms for the association of poor survival with CIMP H which may be related to epigenetic silencing of the normal inhibitory mechanisms which prevent cell migration, proliferation and vascular invasion.
Extramural vascular invasion (EMVI) has recently been associated with adverse survival and risk of metastasis and although it features in the National Institute of Health and Care Excellence United Kingdom guidelines for the treatment of rectal cancer, suggesting that short course neoadjuvant therapy should be considered in these patients on this basis, the current guidelines concede that the risks and benefits in this group are unclear and further research is needed. Indeed the prediction of EMVI on preoperative imaging is notoriously difficult and non-reproducible. EMVI is detectable in rectal cancer patients on magnetic resonance imaging, however, sensitivity and specificity are relatively low at 62% and 88% respectively and it is possible that in future, CIMP status could be used to enhance preoperative EMVI detection and subsequent risk stratification.
CpG islands are typically short (300-3000 base pairs) Cytosine-Guanine phosphodiester bonded sequences found in or around the promoter region of a gene where they are usually unmethylated if the genes are expressed. The CIMP phenotype is characterised by epigenetic DNA hyper-methylation and consequent suppression of key genes important in controlling cell growth and survival. High levels of CIMP associated methylation (deemed CIMP-High), are associated with poor survival in rectal cancer. Extramural vascular invasion of a tumour is defined as “a rounded mass of tumour in an endothelium-lined space either surrounded by a rim of smooth muscle or containing red blood cells”. Venous invasion may also be suspected when a rounded or elongated tumour profile is identified adjacent to an artery, especially when no separate accompanying vein can be identified or where smooth tongues of tumour extend into pericolic/perirectal fat.
The authors aimed to identify whether CIMP status is predictive of response to neoadjuvant chemoradiotherapy and outcomes in rectal cancer. They found that a novel association of CIMP status with extramural vascular invasion which represents an adverse prognostic indicator and provides a novel insight into potential mechanisms for the association of poor survival with CIMP-H rectal cancers. The study is well-designed and presented. The results are all clear and understandable, the descriptions of methods and materials are also clear.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee HC, Mayol J, Sipos F, Zouiten-Mekki L S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4460] [Article Influence: 212.4] [Reference Citation Analysis (1)] |
| 2. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2040] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 3. | Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, Choi DH, Nam H, Kim JS, Cho MJ. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg. 2010;252:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Park SY, Chang HJ, Kim DY, Jung KH, Kim SY, Park JW, Oh JH, Lim SB, Choi HS, Jeong SY. Is step section necessary for determination of complete pathological response in rectal cancer patients treated with preoperative chemoradiotherapy? Histopathology. 2011;59:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Beddy D, Hyland JM, Winter DC, Lim C, White A, Moriarty M, Armstrong J, Fennelly D, Gibbons D, Sheahan K. A simplified tumor regression grade correlates with survival in locally advanced rectal carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2008;15:3471-3477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Kuo LJ, Chiou JF, Tai CJ, Chang CC, Kung CH, Lin SE, Hung CS, Wang W, Tam KW, Lee HC. Can we predict pathologic complete response before surgery for locally advanced rectal cancer treated with preoperative chemoradiation therapy? Int J Colorectal Dis. 2012;27:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Topova L, Hellmich G, Puffer E, Schubert C, Christen N, Boldt T, Wiedemann B, Witzigmann H, Stelzner S. Prognostic value of tumor response to neoadjuvant therapy in rectal carcinoma. Dis Colon Rectum. 2011;54:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Tytherleigh MG, Ng VV, Pittathankal AA, Wilson MJ, Farouk R. Preoperative staging of rectal cancer by magnetic resonance imaging remains an imprecise tool. ANZ J Surg. 2008;78:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Pastor C, Subtil JC, Sola J, Baixauli J, Beorlegui C, Arbea L, Aristu J, Hernandez-Lizoain JL. Accuracy of endoscopic ultrasound to assess tumor response after neoadjuvant treatment in rectal cancer: can we trust the findings? Dis Colon Rectum. 2011;54:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Bokey EL, Chapuis PH, Dent OF, Newland RC, Koorey SG, Zelas PJ, Stewart PJ. Factors affecting survival after excision of the rectum for cancer: a multivariate analysis. Dis Colon Rectum. 1997;40:3-10. [PubMed] |
| 11. | Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317-1329. [PubMed] |
| 12. | Krasna MJ, Flancbaum L, Cody RP, Shneibaum S, Ben Ari G. Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance. Cancer. 1988;61:1018-1023. [PubMed] |
| 13. | Ouchi K, Sugawara T, Ono H, Fujiya T, Kamiyama Y, Kakugawa Y, Mikuni J, Tateno H. Histologic features and clinical significance of venous invasion in colorectal carcinoma with hepatic metastasis. Cancer. 1996;78:2313-2317. [PubMed] |
| 14. | Taylor F, Mangat N, Swift IR, Brown G. Proforma-based reporting in rectal cancer. Cancer Imaging. 2010;10 Spec no A:S142-S150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol. 2014;69:619-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Poston GJ, Tait D, O’Connell S, Bennett A, Berendse S. Diagnosis and management of colorectal cancer: summary of NICE guidance. BMJ. 2011;343:d6751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 18. | Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1004] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 19. | Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 1498] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 20. | Boland CR, Shin SK, Goel A. Promoter methylation in the genesis of gastrointestinal cancer. Yonsei Med J. 2009;50:309-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Bae JM, Kim JH, Cho NY, Kim TY, Kang GH. Prognostic implication of the CpG island methylator phenotype in colorectal cancers depends on tumour location. Br J Cancer. 2013;109:1004-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Jo P, Jung K, Grade M, Conradi LC, Wolff HA, Kitz J, Becker H, Rüschoff J, Hartmann A, Beissbarth T. CpG island methylator phenotype infers a poor disease-free survival in locally advanced rectal cancer. Surgery. 2012;151:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Gunal A, Hui P, Kilic S, Xu R, Jain D, Mitchell K, Robert M, Kenney B. KRAS mutations are associated with specific morphologic features in colon cancer. J Clin Gastroenterol. 2013;47:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Yagi K, Akagi K, Hayashi H, Nagae G, Tsuji S, Isagawa T, Midorikawa Y, Nishimura Y, Sakamoto H, Seto Y. Three DNA methylation epigenotypes in human colorectal cancer. Clin Cancer Res. 2010;16:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 25. | Kaneda A, Yagi K. Two groups of DNA methylation markers to classify colorectal cancer into three epigenotypes. Cancer Sci. 2011;102:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, Taylor G, Barrett JH, Quirke P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931-5937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 464] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 27. | Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67:439-442. [PubMed] |
| 28. | Maguire A, Sheahan K. Controversies in the pathological assessment of colorectal cancer. World J Gastroenterol. 2014;20:9850-9861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Samowitz WS, Curtin K, Wolff RK, Tripp SR, Caan BJ, Slattery ML. Microsatellite instability and survival in rectal cancer. Cancer Causes Control. 2009;20:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Colella S, Shen L, Baggerly KA, Issa JP, Krahe R. Sensitive and quantitative universal Pyrosequencing methylation analysis of CpG sites. Biotechniques. 2003;35:146-150. [PubMed] |
| 31. | Dahlin AM, Palmqvist R, Henriksson ML, Jacobsson M, Eklöf V, Rutegård J, Oberg A, Van Guelpen BR. The role of the CpG island methylator phenotype in colorectal cancer prognosis depends on microsatellite instability screening status. Clin Cancer Res. 2010;16:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Juo YY, Johnston FM, Zhang DY, Juo HH, Wang H, Pappou EP, Yu T, Easwaran H, Baylin S, van Engeland M. Prognostic value of CpG island methylator phenotype among colorectal cancer patients: a systematic review and meta-analysis. Ann Oncol. 2014;25:2314-2327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 33. | Phipps AI, Limburg PJ, Baron JA, Burnett-Hartman AN, Weisenberger DJ, Laird PW, Sinicrope FA, Rosty C, Buchanan DD, Potter JD. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148:77-87.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 34. | Gallois C, Laurent-Puig P, Taieb J. Methylator phenotype in colorectal cancer: A prognostic factor or not? Crit Rev Oncol Hematol. 2016;99:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Van Rijnsoever M, Elsaleh H, Joseph D, McCaul K, Iacopetta B. CpG island methylator phenotype is an independent predictor of survival benefit from 5-fluorouracil in stage III colorectal cancer. Clin Cancer Res. 2003;9:2898-2903. [PubMed] |
| 36. | Ahn JB, Chung WB, Maeda O, Shin SJ, Kim HS, Chung HC, Kim NK, Issa JP. DNA methylation predicts recurrence from resected stage III proximal colon cancer. Cancer. 2011;117:1847-1854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Ebert MP, Tänzer M, Balluff B, Burgermeister E, Kretzschmar AK, Hughes DJ, Tetzner R, Lofton-Day C, Rosenberg R, Reinacher-Schick AC. TFAP2E-DKK4 and chemoresistance in colorectal cancer. N Engl J Med. 2012;366:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Ogino S, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagn. 2006;8:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 39. | Zhang Y, Wang X, Xu B, Wang B, Wang Z, Liang Y, Zhou J, Hu J, Jiang B. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncol Rep. 2013;30:1976-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 711] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 41. | Bhangu A, Wood G, Brown G, Darzi A, Tekkis P, Goldin R. The role of epithelial mesenchymal transition and resistance to neoadjuvant therapy in locally advanced rectal cancer. Colorectal Dis. 2014;16:O133-O143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Zhang S, Zhong B, Chen M, Yang L, Yang G, Li Y, Wang H, Wang G, Li W, Cui J. Epigenetic reprogramming reverses the malignant epigenotype of the MMP/TIMP axis genes in tumor cells. Int J Cancer. 2014;134:1583-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Cao B, Yang Y, Pan Y, Jia Y, Brock MV, Herman JG, Guo M. Epigenetic silencing of CXCL14 induced colorectal cancer migration and invasion. Discov Med. 2013;16:137-147. [PubMed] |