Published online Apr 15, 2017. doi: 10.4251/wjgo.v9.i4.176
Peer-review started: December 6, 2016
First decision: January 7, 2017
Revised: January 22, 2017
Accepted: February 28, 2017
Article in press: February 28, 2017
Published online: April 15, 2017
Processing time: 125 Days and 19.7 Hours
To investigated the incidence and risk factors of venous thromboembolism (VTE) in patients with advanced gastric cancer (AGC) receiving chemotherapy.
All consecutive chemotherapy-naïve patients with AGC who would receive palliative chemotherapy between November 2009 and April 2012 in our hospital were recruited. Their pretreatment clinical and laboratory variables, including D-dimer, were recorded. The frequency of VTE development and survival rates during each chemotherapy cycle and regularly thereafter were assessed.
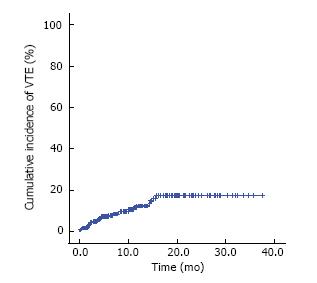
A total of 241 patients enrolled between November 2009 and April 2012 were analyzed. During a median follow-up duration of 10.8 mo (95%CI: 9.9-11.7), 27 patients developed VTE and the incidence of VTE was 17.5% (95%CI: 10.5-24.0, 12.0 events/100 person-years). The 6-mo and 1-year cumulative incidences were 7.8% (95%CI: 4.2%-11.4%) and 12.4% (95%CI: 7.3-17.2), respectively. Thirteen (48.1%) patients were symptomatic and the other 14 (51.9%) patients were asymptomatic. In multivariate analysis, pretreatment D-dimer level was the only marginally significant risk factor associated with VTE development (hazard ratio = 1.32; 95%CI: 1.00-1.75, P = 0.051).
The incidence of VTE is relatively high in patients with AGC receiving chemotherapy, and pretreatment D-dimer level might be a biomarker for risk stratification of VTE.
Core tip: The incidence of venous thromboembolism (VTE) is relatively high in patients with advanced gastric cancer receiving chemotherapy, and pretreatment D-dimer level is a risk factor for VTE. Considering the usefulness of D-dimer as a biomarker given its ease of use and low cost, pretreatment D-dimer might be a risk stratification factor for VTE development and patient selection for thromboprophylaxis.
- Citation: Park K, Ryoo BY, Ryu MH, Park SR, Kang MJ, Kim JH, Han S, Kang YK. Incidence of venous thromboembolism and the role of D-dimer as predictive marker in patients with advanced gastric cancer receiving chemotherapy: A prospective study. World J Gastrointest Oncol 2017; 9(4): 176-183
- URL: https://www.wjgnet.com/1948-5204/full/v9/i4/176.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i4.176
In general, patients with cancer carry a significantly higher risk of venous thromboembolism (VTE) than case-control subjects without cancer[1,2]. Gastrointestinal cancers including gastric cancer were ranked third in incidence of VTE, following hematological malignancies and lung cancer[1]. Lee et al[3] reported that patients with advanced gastric cancer (AGC) had a much higher likelihood of developing VTE (24.4%) than patients with lower-stage gastric cancer (0.5%-3.5%), and Blom et al[1] observed that chemotherapy increased the VTE risk 2.2-fold. These findings suggest that VTE might be more common in patients with AGC receiving chemotherapy, who have several potential risk factors of VTE development including cancer, especially highly vulnerable gastric cancer, advanced stage, and chemotherapy. On the other hand, patients with cancer who develop VTE also have shorter survival durations than those who do not develop VTE[4-6]. Activation of hemostasis, as indicated by development of VTE reflects more aggressive tumor biology[7], additionally, VTE development might hinder the continuation of chemotherapy, resulting in poor outcomes. Considering the relatively high incidence of VTE and its impact on survival, information about the VTE is important in patients with AGC receiving chemotherapy. While, the information about VTE in this cohort is not yet clear. Because most of the previous results were retrospectively analyzed with heterogeneous population and included this cohort as a part of small fraction among heterogeneous various groups[8-11].
It has been challenging for oncologists to conduct a practical use of thromboprophylaxis effectively to prevent VTE. The major problem is that the increased rates of complications such as bleeding outweigh its efficacy[12]. Thus, we must more precisely target thromboprophylaxis, especially in gastric cancer, since it has a high risk of bleeding at the endothelial lesion. Therefore, we need to confirm the exact incidence and identify the predictive factors of future VTE in this cohort. To address these issues, we conducted this prospective cohort study to determine the exact incidence and risk factors of VTE in patients with AGC who are undergoing palliative chemotherapy and assess whether VTE development correlates with survival.
This is a prospective observational single-center study. The cohort consisted of all consecutive patients with histologically confirmed adenocarcinoma of the stomach or esophagogastric junction that was in an advanced state (e.g., initially metastatic, locally inoperable, or recurrent), and who started palliative chemotherapy between November 2009 and April 2012 at Asan Medical Center, South Korea. All patients were chemotherapy-naïve or had undergone adjuvant chemotherapy 6 mo before being included in the study. All patients had adequate bone marrow, renal, hepatic, and cardiac functions that would allow them to withstand chemotherapy. The following patients were excluded: Those who presented initially with brain metastasis, had been treated with warfarin or low-molecular-weight heparin 4 wk before being screened for inclusion in the study, had undergone major surgery or experienced significant traumatic injuries 4 wk before screening, or were lost to follow up during the first two weeks without evidence of disease progression or VTE. All included patients were observed prospectively for at least 1 year after enrollment of the last patient or until death, loss of follow-up, or withdrawal of consent occurred. The study was approved by the institutional review board of Asan Medical Center and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines (ClinicalTrials.gov identifier: NCT01047618). All participants provided written informed consent before enrollment.
The chemotherapy regimens mainly included fluoropyrimidine plus platinum-based for the 1st-line, taxane-based for the 2nd-line, and irinotecan-based for the 3rd-line chemotherapy. Each chemotherapy line was continued until disease progression, intolerable toxicity, patient demand, or the attending physician’s decision. Routine erythropoiesis-stimulating agent was not applied during the study period.
Within one week before starting palliative chemotherapy, we checked the clinicopathologic factors and laboratory tests including D-dimer. Close history taking, a physical examination, CBC, and chemistry analyses were performed at every chemotherapy cycle and regularly during the follow-up period. The response of each patient to the treatment was assessed every 6 wk. When VTE was suspected due to a constellation of new clinical symptoms or signs, imaging analyses were performed. Doppler sonography and/or CT venography were used to detect DVT, while chest CT and/or pulmonary artery CT angiography were employed to detect pulmonary embolism (PE). Incidentally detected VTE was also counted as an event.
The primary endpoint of this study was the incidence of VTE in patients with AGC receiving chemotherapy, and the secondary endpoints were to identify risk factors of VTE and to evaluate the impact of VTE on survival in this cohort. For risk factor analysis of VTE, we used time to VTE to discriminate early vs delayed development considering the different clinical significance. The baseline characteristics such as baseline biomarkers levels, clinical parameter, cancer diagnosis information, medication and therapy are expressed as the median value with a range (continuous variables) or frequency with proportion (%) (categorical variables). The incidence of VTE was calculated as both cumulative incidence and person-time (events/100 person-years). The statistical significance of the difference in characteristic between symptomatic and incidental VTE was assessed using the χ2 test or Fisher’s exact test. The time to VTE or overall survival (OS) were measured from the date of first chemotherapy to the date of diagnosis of VTE or death or were censored at the last follow-up date. The time to event data were summarized using the Kaplan-Meier method. Uni- and multivariate Cox regression models were used to detect the association between clinical or pretreatment factors and time to VTE or OS. In the multivariate regression model, all potential factors with a P value ≤ 0.15 on univariate analyses were employed to detect adjustment factors. The final models were determined by backward elimination. In the regression modeling, log-transformation for severely skewed variables was used to obtain more stable regression coefficients. All statistical analyses were performed by using SPSS software, version 21.0 (SPSS Inc., Chicago, IL, United States) and R software 2.10.1. All reported P values are two-sided and P values < 0.05 are considered to be statistically significant.
Between November 2009 and April 2012, a total of 261 consecutive patients were enrolled in this study. Of these patients, 20 (7.7%) did not receive chemotherapy or were lost to follow-up during the first 2 wk without evidence of disease progression or DVT, so they were excluded from the analysis. The remaining 241 patients had a median age of 56 years (range, 24-83 years) and 169 patients (70.1%) were male. The pretreatment clinicopathological and laboratory characteristics of all patients and those who developed VTE during palliative chemotherapy are summarized in Table 1. All patients received one or more cycles of chemotherapy; the median numbers of 9 cycles (range, 1-42) and 2 lines (range, 1-4).
| Variable | Total patients | Patients with VTE | Risk of time to VTE | ||
| HR | 95%CI | P value | |||
| Total, n (%) | 241 (100.0) | 27 (11.2) | |||
| Age (yr) | |||||
| < 65 | 178 (73.9) | 18 (10.1) | |||
| ≥ 65 | 63 (26.1) | 9 (14.3) | 1.632 | 0.732-3.641 | 0.231 |
| Gender | |||||
| Male | 169 (70.1) | 18 (10.7) | |||
| Female | 72 (29.9) | 9 (12.5) | 1.052 | 0.472-2.345 | 0.9 |
| ECOG PS | |||||
| 0-1 | 229 (95.0%) | 27 (11.8) | |||
| 2 | 12 (5.0%) | 0 | 0.046 | 0.000-99.991 | 0.432 |
| BMI | |||||
| < 25 | 202 (83.8) | 23 (11.4) | |||
| ≥ 25 | 39 (16.2) | 4 (10.3) | 0.793 | 0.274-2.296 | 0.669 |
| Previous CVC | |||||
| No | 233 (96.7) | 25 (10.7) | |||
| Yes | 8 (3.3) | 2 (25.0) | 3.13 | 0.738-13.266 | 0.122 |
| Previous gastrectomy | |||||
| No | 209 (86.7) | 26 (12.4) | |||
| Yes | 32 (13.3) | 1 (3.1) | 0.196 | 0.027-1.448 | 0.11 |
| Primary tumor site | |||||
| Antrum/pylorus | 94 (39.0) | 10 (10.6) | |||
| Body | 32 (13.3) | 9 (11.0) | 1.131 | 0.458-2.791 | 0.79 |
| Cardia/fundus | 28 (11.6) | 3 (10.7) | 1.138 | 0.313-4.144 | 0.844 |
| Diffuse | 25 (10.4) | 5 (20.0) | 2.413 | 0.823-7.073 | 0.108 |
| Histology | |||||
| W/D or M/D | 84 (34.9) | 6 (7.1) | |||
| P/D or SRC | 154 (63.9) | 21 (13.6) | 2.084 | 0.840-5.166 | 0.113 |
| Unclassified | 3 (1.2) | 0 | NA | ||
| Peritoneal seeding | |||||
| No | 137 (56.8) | 11 (8.0) | |||
| Yes | 104 (43.2) | 16 (15.4) | 1.945 | 0.902-4.191 | 0.09 |
| Liver metastasis | |||||
| No | 156 (64.7) | 20 (12.8) | |||
| Yes | 85 (35.3) | 7 (8.2) | 0.741 | 0.313-1.754 | 0.495 |
| Lung metastasis | |||||
| No | 220 (91.3) | 26 (11.8) | |||
| Yes | 21 (8.7) | 1 (4.8) | 0.509 | 0.069-3.764 | 0.509 |
| Bone metastasis | |||||
| No | 225 (93.4) | 24 (10.7) | |||
| Yes | 16 (6.6) | 3 (18.8) | 2.344 | 0.701-7.835 | 0.167 |
| Number of metastatic sites | |||||
| 0-1 | 109 (45.2) | 9 (8.3) | |||
| ≥ 2 | 132 (54.8) | 18 (13.6) | 1.898 | 0.851-1.898 | 0.118 |
| CEA (log) median (range, ng/mL) | 2.5 (0.3-8070) | 1.133 | 0.947-1.355 | 0.173 | |
| CA19-9 (log) median (range, U/mL) | 19.3 (1.4-30800) | 0.882 | 0.726-1.073 | 0.209 | |
| CA72-4 (log) median (range, U/mL) | 5.1 (1.7-6490) | 1.099 | 0.885-1.364 | 0.395 | |
| Hb median (range, g/dL) | 12.0 (7.0-17.6) | 0.956 | 0.786-1.163 | 0.653 | |
| WBC median (range, × 109/L) | 7100 (2600-19300) | 1 | 1.000-1.000 | 0.026 | |
| Platelet median (range, × 109/L) | 274 (107-731) | 1.001 | 0.998-1.005 | 0.508 | |
| CRP median (range, mg/dL) | 2.01 (0.10-19.22) | 1.056 | 0.952-1.053 | 0.302 | |
| Fibrinogen (log) median (range, × 109/L) | 360 (66-897) | 1.132 | 0.337-3.796 | 0.841 | |
| PAI-1 (log) median (range, × 109/L) | 35.0 (2.0-112.0) | 1.197 | 0.662-2.165 | 0.551 | |
| D-dimer (log) median (range, × 109/L) | 1.02 (0.06-82.3) | 1.401 | 1.069 1.836 | 0.015 | |
During a median follow-up duration of 10.8 mo (95%CI: 9.9-11.7), 27 patients developed VTE and the VTE incidence was 17.5% (95%CI: 10.5%-24.0%; 12.0 events/100 person-years). The 6-mo and 1-year cumulative incidences of VTE were 7.8% (95%CI: 4.2%-11.4%) and 12.4% (95%CI: 7.3-17.2%), respectively (Figure 1). Regarding VTE type, 19 of 27 patients (70.4%) developed deep vein thrombosis (DVT) only, 4 patients (14.8%) developed PE only, and 4 patients (14.8%) had both DVT and PE. The median time to VTE in these patients who developed VTE was 3.9 mo (95%CI: 2.8-5.1). VTE was detected within 3 mo in 10 patients (37.0%), from 3 to 6 mo in 6 patients (22.2%), from 6 mo to 1 year in 7 patients (25.9%), and after 1 year in the remaining 4 patients (14.8%). Thirteen patients (48.1%) had symptomatic VTE and the other 14 (51.9%) had incidentally detected VTE. A total of 25 patients received treatments for VTE, 22 took warfarin or low-molecular-weight heparin, and the other three were treated with an inferior vena cava filter. The remaining two patients were treated conservatively. VTE-associated delay or discontinuation of chemotherapy occurred in 10 patients and was significantly more common in patients with symptomatic VTE than in patients with incidental VTE (P = 0.018). There were no cases of VTE-induced death (Table 2).
| Total (n = 27) | Symptomatic VTE (n = 13) | Incidental VTE (n = 14) | P value | |
| Time to VTE duration (median, mo) | 6.1 | 7.5 | 4.7 | 0.16 |
| VTE incidence | ||||
| < 3 | 10 (37.0) | 4 (30.8) | 6 (42.9) | |
| 3-6 | 6 (22.2) | 3 (23.1) | 3 (21.5) | |
| 6-12 | 7 (25.9) | 3 (23.1) | 4 (28.6) | |
| > 12 | 4 (14.8) | 3 (23.1) | 1 (7.2) | 0.68 |
| Types of VTE | ||||
| DVT | 19 (70.4) | 8 (61.5) | 11 (78.6) | |
| PTE | 4 (14.8) | 3 (23.1) | 1 (7.2) | |
| PTE + DVT | 4 (14.8) | 2 (15.4) | 2 (14.3) | 0.608 |
| Treatment of VTE | ||||
| Medication (anticoagulation) | 22 (81.4) | 10 (76.9) | 12 (75.7) | |
| IVC filter | 3 (11.1) | 3 (23.1) | 0 | |
| No treatment | 2 (7.5) | 0 | 2 (14.3) | 0.031 |
| Delay of chemotherapy | ||||
| None | 17 (62.9) | 5 (38.5) | 12 (85.7) | |
| Yes | 10 (37.1) | 8 (61.5) | 2 (14.3) | 0.018 |
The pretreatment characteristics and laboratory data were assessed to determine the association with time to VTE development. In univariate analyses, log-transformed D-dimer levels and WBC count had a statistically significant association with time to VTE (Table 1). Prior history of central venous catheter, differentiation, prior history of gastrectomy, peritoneal seeding, and number of metastatic sites trended toward a potential risk factor for VTE (P ≤ 0.15). In multivariate analysis, any prognostic factors were not statistically significant, but the log transformed D-dimer level was only a marginally significant risk factor in the final model (HR = 1.32, 95%CI: 1.00-1.75, P = 0.051) (Table 3).
| Variable | HR | 95%CI | P value |
| Prior gastrectomy (no vs yes) | 0.25 | 0.03-1.89 | 0.178 |
| History of CVC (no vs yes) | 2.21 | 0.51-9.50 | 0.286 |
| D-dimer (log) | 1.32 | 1.00-1.75 | 0.051 |
In case of patients with VTE, the mean levels of D-dimer were 4.19 × 109/L (baseline) and 11.18 × 109/L (at the time of VTE development), respectively, with a significant increase of D-dimer levels at the time of VTE development (P = 0.004). Additionally, according to symptom development of VTE, D-dimer levels were increased significantly in patients with symptomatic VTE (P = 0.004), on the other hand, those in patients with incidental VTE showed only numerical increase (P = 0.198) (Table 4).
| Total(n = 27) | Symptomatic VTE(n = 13) | Incidental VTE(n = 14) | P value | |
| Baseline D-dimer | 4.19 | 3.62 | 4.72 | 0.835 |
| D-dimer at the time of VTE development | 11.18 | 14.11 | 8.45 | 0.436 |
| P value | P value | P value | ||
| 0.004 | 0.01 | 0.198 |
There was no significant difference in the time to VTE according to fluoropyrimidine or different platinum use as the 1st-line treatment or the addition of targeted agents or angiogenesis inhibitors (Table 5).
| Variable | Patients (n) | Patients with VTE n (%) | Risk of time to VTE | ||
| HR | 95%CI | P value | |||
| Fluoropyrimidine | |||||
| SP | 96 | 11 (11.5) | |||
| XP | 22 | 3 (13.6) | 1.67 | 0.47-6.18 | 0.422 |
| Platinum | |||||
| XP | 22 | 3 (13.6) | |||
| XELOX | 27 | 4 (14.8) | 0.94 | 0.21-4.25 | 0.94 |
| Addition of targeted agents | |||||
| Conventional chemotherapy | 158 | 17 (10.8) | |||
| + targeted agents | 83 | 10 (12.0) | 1.21 | 0.56-2.65 | 0.627 |
| Addition of VEGFR inhibitors | |||||
| Conventional chemotherapy | 217 | 27 (12.4) | |||
| + VEGFR inhibitors | 24 | 0 | 0.04 | 0.00-12.13 | 0.227 |
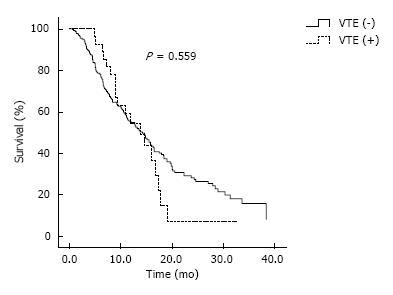
During a median observational duration of 13.8 mo (95%CI: 11.6-14.8), the median OS was 14.2 mo (95%CI: 11.8-16.6). There was no significant difference in OS between patients with and those without VTE (13.8 mo, 95%CI: 9.3-18.3; 14.2 mo, 95%CI: 11.7-16.7, P = 0.559) (Figure 2). According to symptom development of VTE, patients with symptomatic VTE was not also noted statistically significant difference in OS compared with patients without VTE (P = 0.337). According to VTE type, there was no significant difference in OS between patients with DVT alone and with PE alone or PE/DVT (P = 0.597). In the analysis that excluded four patients who developed VTE after 12 mo, there was no significant difference in OS between patients with or without VTE (P = 0.609). On the contrary, in the analysis that excluded 10 patients who developed VTE before 3 mo, there was no significant difference in OS between patients with or without VTE (P = 0.337).
The current study assessed the incidence and risk factors of VTE as well as the influence of VTE on survival in AGC patients undergoing palliative chemotherapy. To our knowledge, this is the first prospective study to evaluate VTE in a homogeneous cohort. This study showed a relatively high cumulative incidence of VTE and the role of pretreatment D-dimer as a risk factor for development of VTE. Although the development of VTE was not correlated with poor survival, patients with symptomatic VTE had more interruptions or delays of chemotherapy than those with incidental VTE.
The 6-mo, 1-year, and 2-year cumulative incidences of VTE were 7.8%, 12.3%, and 17.3%, respectively. Our results showed a relatively high incidence of VTE despite AGC being are presentative frequent site in cancer-associated VTE[13]. In our previous report of retrospective analysis of a similar patient population, the 1- and 2-year cumulative incidences of venous VTE were 3.5% and 4.9%, respectively[8]. Other prior studies also reported VTE incidences of 5.3%-11.4%[14-16], which was somewhat lower than our results. The reason for this discrepancy should be preferentially presumed that this study was conducted for homogeneous patients who were high tumor burden of advanced state and receiving palliative chemotherapy. Regarding tumor burden in cancer-associated VTE, Lee et al[3] reported that the VTE incidence increased with stage in gastric cancer patients. Considering that stage is correlated with tumor burden in cancer patients, we can give careful consideration to our results that a history of previous gastrectomy and multiple metastases were slightly related with the development of VTE, but the correlation was not statistically significant. Regarding chemotherapy of cancer-associated VTE, prior studies reported that chemotherapy is another important risk factor for VTE development in cancer patients[1,17]. In the current study, 13 (46.4%) and 20 (71.4%) patients developed VTE within 3 and 6 mo, respectively, which suggests that starting chemotherapy is an important risk factor for VTE development. Blom et al[1] also reported that the risk of thrombosis was the highest in the first 3 mo after the diagnosis of cancer and declined thereafter, which supports the finding of the current study.
In multivariate analysis, pretreatment D-dimer level was the only marginally significant risk factor for VTE development. D-dimer, a general biomarker that globally indicates hemostasis and fibrinolysis activation, is a well-studied biomarker in the diagnosis and management of VTE in non-cancer patients[18]. Khorana et al[17] proposed a predictive model for chemotherapy-associated VTE that included the primary cancer site, pretreatment platelet count, hemoglobin, leukocyte count, and body mass index. However, D-dimer level was not included in this model, which might be because the pretreatment D-dimer level was not routinely checked in the target population. In the subsequent studies, Ay et al[19] reported that a high D-dimer level predicted occurrence of VTE in a variety of cancer patients, and Arpaia et al[20] demonstrated that pretreatment D-dimer was correlated with chemotherapy-associated VTE. However, these studies only included a small number of gastric cancer patients, 35 of 821 (4.3%) and 10 of 124 (8.1%). Moreover, subcohorts of these patients were heterogeneous since the patients were treated variably with surgery or radiotherapy (or even untreated). Thus, the role of D-dimer as a risk factor of VTE in AGC patients receiving chemotherapy remains to be clarified. We demonstrated here that pretreatment D-dimer was indeed an independent risk factor of VTE in this homogeneous cohort. This means that pretreatment D-dimer might be used to more precisely target patients for thromboprophylaxis and lead to enhanced use of thromboprophylaxis in AGC patients treated with palliative chemotherapy. On the other hand, prior established risk factors including the aforementioned Khorana score were not associated with VTE in this study (data not shown).A possible explanation might be the small sample size; however, the unique characteristics of stomach cancer or even ethical characteristics might also contribute.
The occurrence of VTE has been reported to have a significant adverse effect on survival in many studies[3,5,6]. However, we found no statistical difference in survival according to VTE development. The results did not change after adjustment for the presence of symptoms and signs, VTE type, or detection time. The reason for the negative result in the current study may be too small a sample size to detect a difference. On the other hand, due to the short survival of patients with advanced-stage disease, VTE may have a greatly reduced impact on survival. Previous studies reported that an impact of VTE on survival might be somewhat different from that in our study because they mainly included localized stage of cancer[3] or showed a prominent impact of VTE on survival in subgroups of patients with localized cancer[5] or in those with breast cancer, which has a characteristic longer survival duration[6]. Our previous retrospective study with a larger cohort of similar patients also reported that VTE was not a statistically significant factor for survival[8]. As such, whether VTE really has adverse effect on survival in this cohort should be further clarified. Meanwhile, more patients with symptomatic VTE than asymptomatic VTE had chemotherapy interruptions or delays. Although symptomatic VTE did not show poor survival in the subgroup analysis, we should make an effort to detect VTE before symptoms develop.
This study has several limitations. First, we did not evaluate serial measurements of D-dimer; thus, we could not identify the usefulness of the serial changes as a predictive marker for VTE. Considering that the current study showed that D-dimer level at the time of VTE development is significantly increased compared with that at baseline in patients with VTE, serial measurements of D-dimer might detect early changes and predict the development of VTE. Another issue is that the current study did not calculate the proper number of patients, so it might not have been adequately powered to detect statistically significant differences in other characteristics such as risk factors or survival. For these reasons, it is obvious that the present study might not be a confirmative, rather preliminary study for hypothesis generation.Despite these limitations, the current study showed the incidence of VTE and role of pretreatment D-dimer as risk factors in a homogeneous group of AGC patients receiving palliative chemotherapy. Considering the usefulness of D-dimer as a biomarker such as its ease of use and low cost, pretreatment D-dimer might be used as a risk stratification factor for VTE and in patient selection for thromboprophylaxis.
In conclusion, the incidence of VTE is relatively high in patients with AGC receiving chemotherapy, and pretreatment D-dimer level is a risk factor for VTE. Considering the usefulness of D-dimer as a biomarker given its ease of use and low cost, pretreatment D-dimer might be a risk stratification factor for VTE development and patient selection for thromboprophylaxis.
Patients with cancer, especially gastric cancer, are higher risk of venous thromboembolism (VTE). Among cancer, advanced status such as recurrent, metastatic, or locally advanced, is high risk factor and treatment with chemotherapy is also risk factor of VTE. So, information about the VTE in patients with advanced gastric cancer (AGC) receiving chemotherapy is important. However, there has been no report about this homogeneous group, so they planned this study.
This study is the first prospective study focused to evaluate VTE and risk factor of VTE in this homogeneous cohort. They reported an incidence of VTE and role of pretreatment D-dimer as risk factor.
The incidence of VTE is relatively high in patients with AGC receiving chemotherapy17.5% (95%CI: 10.5%-24.0%; 12.0 events/100 person-years), and pretreatment D-dimer level is a risk factor for VTE.
Considering the usefulness of D-dimer as a biomarker given its ease of use and low cost, pretreatment D-dimer might be a risk stratification factor for VTE development and patient selection for thromboprophylaxis.
AGC: Gastric cancer of advanced status such as initially metastatic, locally inoperable, or recurrent.
Naturally an increased level of circulating fibrin d-dimer may be a good parameter to control the evolution of gastric cancer venous originated metastasis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Amedei A, Ammendola M, Baeg MK, Doll D, Ferreira Caboclo JL, Uno K S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1460] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 2. | Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1563] [Cited by in RCA: 1604] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 3. | Lee KW, Bang SM, Kim S, Lee HJ, Shin DY, Koh Y, Lee YG, Cha Y, Kim YJ, Kim JH. The incidence, risk factors and prognostic implications of venous thromboembolism in patients with gastric cancer. J Thromb Haemost. 2010;8:540-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 904] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 5. | Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1184] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 6. | Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 244] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | Kang MJ, Ryoo BY, Ryu MH, Koo DH, Chang HM, Lee JL, Kim TW, Kang YK. Venous thromboembolism (VTE) in patients with advanced gastric cancer: an Asian experience. Eur J Cancer. 2012;48:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Tetzlaff ED, Correa AM, Baker J, Ensor J, Ajani JA. The impact on survival of thromboembolic phenomena occurring before and during protocol chemotherapy in patients with advanced gastroesophageal adenocarcinoma. Cancer. 2007;109:1989-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822-2829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 412] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 11. | Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 324] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 12. | Trujillo-Santos J, Nieto JA, Tiberio G, Piccioli A, Di Micco P, Prandoni P, Monreal M. Predicting recurrences or major bleeding in cancer patients with venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost. 2002;87:575-579. [PubMed] |
| 14. | Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2010;362:858-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, Wenczl M, Goker E, Cisar L, Wang K. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1458] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 17. | Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1531] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 18. | Di Nisio M, Squizzato A, Rutjes AW, Büller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5:296-304. [PubMed] [DOI] [Full Text] |
| 19. | Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2009;27:4124-4129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 309] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 20. | Arpaia G, Carpenedo M, Verga M, Mastrogiacomo O, Fagnani D, Lanfredini M, Milani M, Cimminiello C. D-dimer before chemotherapy might predict venous thromboembolism. Blood Coagul Fibrinolysis. 2009;20:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |