Published online Apr 15, 2017. doi: 10.4251/wjgo.v9.i4.153
Peer-review started: July 21, 2016
First decision: September 13, 2016
Revised: December 11, 2016
Accepted: January 16, 2017
Article in press: January 18, 2017
Published online: April 15, 2017
Processing time: 263 Days and 17 Hours
To investigate the effects of Clostridium perfringens enterotoxin (CPE) on gastric cancer cells which highly expressed claudin-4 (CL4) protein.
In this study, we detected expression of CL4 protein in different gastric cancer cell lines. Then, we investigated the effects of CPE on SGC7901 cells which highly expressed CL4 protein and the effects of CPE on subcutaneous tumor in nude mice models.
CL4 are highly expressed in SGC7901 cells. CPE expressed significant cytotoxicity in SGC7901 cells. Suppression of CL4 expression significantly decreased CPE-mediated cytotoxicity. CPE also inhibited tumor growth in subcutaneous tumor xenograft models.
CPE showed CL4 mediated cytotoxicity on gastric cancer cells SGC7901 and inhibited tumor growth in nude mice models.
Core tip: This study firstly investigated the the effects of Clostridium perfringens enterotoxin (CPE) on gastric cancer cells SGC7901, and indicated CPE’s potential effect in gastric cancer therapy.
- Citation: Liang ZY, Kang X, Chen H, Wang M, Guan WX. Effect of Clostridium perfringens enterotoxin on gastric cancer cells SGC7901 which highly expressed claudin-4 protein. World J Gastrointest Oncol 2017; 9(4): 153-159
- URL: https://www.wjgnet.com/1948-5204/full/v9/i4/153.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i4.153
Gastric cancer is the second leading cause of cancer-related death through the world[1]. System therapy including radical surgery, adjuvant chemotherapy, biology therapy and so on. However, 5 year survival rate in advanced gastric cancer patients was still low[2,3].
Tight junction is the important structure between epithelial cells maintaining the cell polarity and membrane integrity[4]. Tight junction are formed by some tight junction proteins including occludin, claudins, ZO-1 and so on[5]. Recently, some studies show claudin-4 (CL4) protein plays a crucial role in tumor’s proliferation, transformation, and metastasis[6,7]. CL4 protein are highly expressed in many kinds of malignant tumors, such as ovarian cancer[8]. In 2015, Liu et al[9] reported that overexpression of CL4 protein was associated with progress of gastric cancer and poor prognosis of gastric cancer patients. More and more researches indicated that CL4 may be an emerging target for cancer therapy.
Clostridium perfringens enterotoxin (CPE), a 35-kDa single polypeptide comprised of 319 amino acids, could bind with CL4 and formed CPE-CL4 complex. CPE-CL4 complex induced resultant pore formation on cell membranes of epithelial cells, and caused cell apoptosis via the influx of Ca2+ into the cell[10]. In pancreatic cancer cell lines HPAC cells, CPE showed a dose-dependent cytotoxic effect[11]. In ovarian tumors, CPE also showed a dose-dependent cytotoxic effect in vitro. CPE significantly inhibited tumor growth and progression in SCID mouse xenografts of human ovarian cancer[12]. However, little was known about the effect of CPE on gastric cancer cells.
In this study, we assessed the expression of CL4 protein in different gastric cancer cell lines. Then we investigated the effects of CPE on SGC7901 cells which highly expressed CL4. In addition, we observed CPE effects on subcutaneous tumor growth of gastric cancer cell SGC7901 in nude mice model.
Goat polyclonal antibodies against CL4 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, United States). Rabbit polyclonal antibodies were obtained from Abcam (Cambridge, MA, United States). The secondary antibodies were horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-goat immunoglobulin (Ig) G (ZSGB-BIO, Beijing, China), Alexia Flour 488 (green)-labeled donkey anti-goat IgG (abcam, Cambridge, CA, United States).
The human gastric cancer cell line SGC7901, MKN45, AGS, MGC803, BGC823 and HGC27 were used to assess the expression of CL4 protein. Colon cancer cell line Caco-2 was considered as CL4 positive control. Normal gastric epithelium cell line GES-1 was considered as negative control. All these cell lines were obtained from Cell Bank, Shanghai Institutes for Biological Sciences. All cells were maintained in RPMI-1640 with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 mg/mL streptomycin and cultured at 37 °C in a humidified 5% CO2 atmosphere.
Cells were cultured in 25-mm2 Tissue Culture Flasks. Total protein was extracted by the Protein extraction kit (KeyGEN BioTECH, Shanghai, China). Twelve percent SDS-PAGE was used for electrophore. After completely separated, the target protein was transferred onto a polyvinylidene difluoride membrane (Immobilon; Millipore). The membrane was saturated with PBS containing 4% skim milk, and then incubated for one night at 4 °C with primary antibodies (diluted 1:1000) in PBS. After rinsing in PBS containing 0.1% Tween 20, the membrane was incubated for 2 h at room temperature with HRP-conjugated anti-rabbit or anti-goat IgG (diluted 1:10000) in PBS. It was then rinsed again, and finally reacted using an immobilon western chemiluminescent HRP substrate (Immobilon; Millipore). Signals in immunoblots were quantified using Quantity One-4.4.0 (Bio-Rad).
The DNA sequence of CPE was synthesized and amplified by polymerase chain reaction (PCR) and subcloned into vector pET28a, and the sequence was transfected to Escherichia coli. The CPE protein expression was induced by isopropy-β-D-thiogalactoside (IPTG) and porified by Ni-IDA. This process was made by the company of Biogot technology (Nanjing, China).
Cells were grown on 96-well plates to reach confluent density and incubated for 24 h with either the vehicle or CPE. Then 20 μL 5 mg/mL methylthiazolyldiphenyl-tetrazolium bromide (MTT) was added to each well and incubated for 4 h. One hundred and fifty microlitre dimethyl sulfoxide was added to every well after the supernatant was wiped off. Then the plates were wobbled for 10 min. The optical density at the wavelengh of 490 nm (OD490) was detected with a microplate reader ELX800 (BioTeK, VT). The inhibition ratio was calculated by formula [OD490 (CPE group)/OD490 (control group)] × 100%. The final results were the average of 3 times and the image was made by OriginPro 9.2 (OriginLab).
Stealth siRNA duplex oligonucleotides against human CL4 were synthesized by Invitrogen. The sequences were as follows: Sense (UCUGUUUUGUAAUUUAAGATT) and antisense (UCUUAAAUUACAAAACAGAAA). SGC7901 cells were transfected with siRNAs (final concentration was 10 nmol/L) or a Stealth RNAi negative control by using Lipofectamine RNAiMAX Reagent (Invitrogen) according to the manufacturer’s protocols.
Cells grown on coverslips were fixed in paraformaldehyde for 15 min at room temperature. After being covered with 10% BSA for 1 h, they were watched three times with PBS and incubated for a night at -4 °C with primary antibodies and rinsed again with PBS, followed by reaction for 2 h at room temperature with appropriate secondary antibodies. All samples were examined using a laser scanning confocal microscope (LSM710, Carl Zeiss, Jena, Germany). Photographs were recorded using a computer (Fujitsu) and ZEN 2009 (Carl Zeiss) and processed with Zeiss LSM Image Browser (Carl Zeiss) and Photoshop CS6 (Adobe).
SGC7901 cells (2 × 106 cells in 100 μL of medium RPMI-1640) were subcutaneously injected into the inguinal region of 6-wk-old nude male mice (BALB/c, nu/nu, SLRC Laboratory Animal Technology Co, Shanghai, China). The mice were killed to get the tumor mass at 2 wk after injection. The tumor mass was cut into pieces with a diameter of 2 mm and planted into the subcutaneous of inguinal region of 6-wk-old nude male mice. After 2 wk the mice were divided into two groups (+CPE and -CPE), and 2 μg of CPE in 100 μL of saline, or 100 μL of PBS was injected around the tumor every day for 10 d. The tumor volume (mm3) was calculated by the formula 0.5 × long diameter (mm)2 × short diameter (mm). On day 10, the tumors were removed and the diameters of tumor were measured. All aspects of the study were approved by the Animal Use and Care Committee of Nanjing Drum Tower hospital (Nanjing, China).
All measured values are presented as the mean ± SD. Statistical significance of differences was evaluated using One-Way ANOVA analysis and LSD test. Repeated measures analysis of variance was used for evaluating the animal studies.
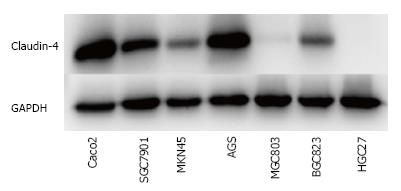
We firstly evaluated the expression level of CL4 protein in different gastric cancer cells (SGC7901, MKN45, AGS, MGC803, BGC823 and HGC27 cells). Colon cancer cell line Caco-2 which highly expressed CL4 protein was considered as positive control cell. We found CL4 protein was highly expressed in two types of cell lines: SGC7901 and AGS cells (Figure 1). Because AGS cells can not be used in nude mice models, we select SGC7901 cells in further study to investigate CPE effects.
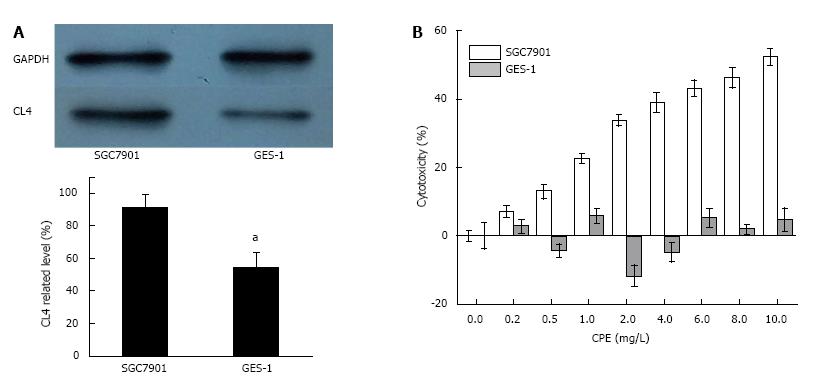
We confirmed the CL4 level of gastric cancer cells SGC7901 and normal gastric epithelium cells GES-1 by western blot. The relative level of CL4 on GES-1 cells was significantly lower than SGC7901cells (Figure 2A). Cytotoxicity of up to 56% was observed 24 h in SGC7901 cells after CPE treatment and the cytotoxicity of CPE (2, 4, 6, 8, 10 mg/L) showed significantly differences (P < 0.05) compared with CPE (0.2 mg/L). However, CPE had no significant cytotoxic effects on GES-1 cells under the same conditions (Figure 2B).
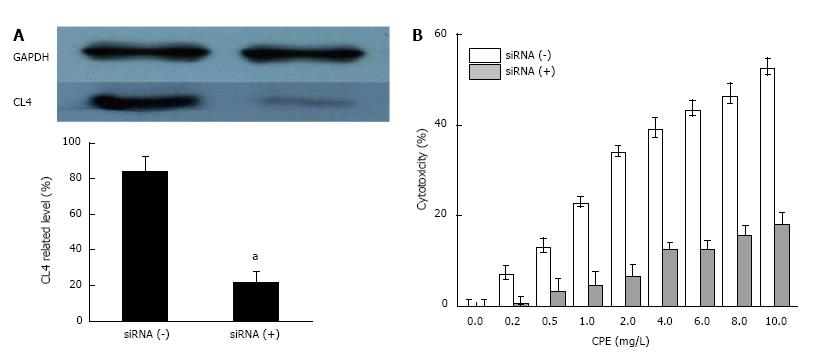
We used an RNAi approach to knock down the CL4 protein in SGC7901 cells. Cells were transfected with the siRNA against human CL4 and incubated for 48 h. Western blot analysis showed that the siRNA significantly reduced CL4 protein expression in SGC7901 cells (Figure 3A). When the expression of CL4 was suppressed, CPE-mediated cytotoxicity was significantly decreased in SGC7901 cells according to MTT assay (Figure 3B).
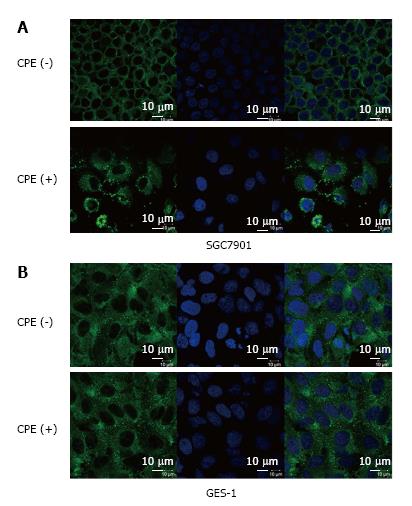
Cells were performed immunostaining and observed under a laser-scanning confocal microscope. The results showed that CL4 mainly expressed on the cell membrane in SGC7901 cells. After treatment with CPE (10 mg/L) for 24 h, CL4 protein expression in cell membrane were suppressed and partly translocated to cytoplasm (Figure 4A). However, CL4 expressed both on cell membrane and in cytoplasm in GES-1 cells. Cell membrane damage was not observed in GES-1 after CPE treatment (10 mg/L) for 24 h (Figure 4B).
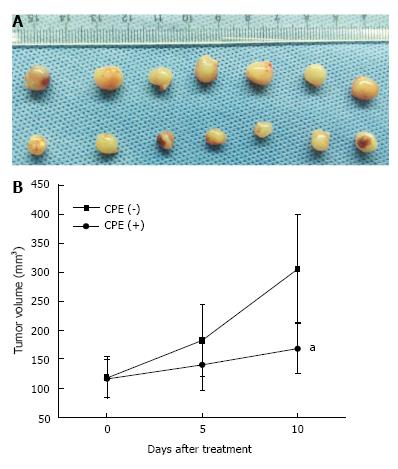
To evaluate the cytotoxic effect of CPE in vivo, SGC7901 cells were used to establish the xenograft models. When the subcutaneous tumor diameter reached about 5-7 mm, the nude mice were randomly divided into two groups [CPE (+) group, n = 7; CPE (-) group, n = 7]. CPE (+) group was injected with CPE (2 mg in 100 μL of PBS) around the tumors once a day for 10 d, while CPE (-) group was injected with PBS (100 μL) around the tumors once a day for 10 d. Tumor volumes were measured on days 0, 5, and 10 after treatment. Mice were killed and the tumors were removed after 10 d. The tumor tissues were shown in Figure 5A, CPE significantly suppressed tumor growth, and obvious reduction of tumor volume was observed in CPE (+) group compared with CPE (-) group (Figure 5B). However, in CPE (+) group, injection site skin necrosis and enteritis were also observed in 3 mice.
The receptors of CPE were mainly considered as CL3 and CL4 protein. CL4 has been found highly expressed in some gastric cancer tissues[13-16]. Hung Jung found the expression rate of CL4 was 44.4% in gastric cancer tissues, and expressions of CL4 was significantly lower in cases with positive lymphatic invasion[13]. Liang et al[14] found the expression of CL4 in normal stomach samples was only 15.9%. Maeda’s study found that inhibiting the expression of CL4 significantly reduced the CPE toxicity, but inhibiting the expression of CL4 slightly increased the toxicity of CPE in prostate cancer[17]. In this study, we also observed inhibiting CL4 expression significantly reduced CPE-mediated toxicity in gastric cancer cells. These results revealed CL4 protein could be a potential target agent in gastric cancer therapy.
Our study found that CPE almost had no significant toxicity on normal gastric epithelium cells GES-1 and the laser confocal microscopy confirmed that CPE had little effects on membrane morphology in GES-1 cells. According to the former study, the toxicity of CPE was associated not only with CL4 expression, but also the subcellular localization of CL4. As the target of CPE, CL4 mainly distributed in the cell membrane in SGC7901 cells, but distributed both in cell membrane and cytoplasm in GES-1 cells. While, the overall expression of CL4 in GES-1 was significantly lower than SGC7901 cells according to the Western blot test. We speculated that little CL4 protein distributed in membranes in GES-1 cells. In addition, recent study found the formation of intact tight junction could alleviate the cytotoxicity of CPE[17]. Studies also found solid tight junctions could be formed between GES-1 cells[18]. These findings maybe explained the different effects of CPE on GES-1 cells and SGC7901 cells.
Tight junction plays a very important role in the proliferation, differentiation and cell polarity of epithelial cells[19]. In our experiment, SGC7901 cell membrane was integrity and the size was substantially uniform. After CPE treatment, part of the cell membrane was not complete. Some nucleus split into smaller pieces after CPE treatment (10 mg/L). This phenomenon agreed with Smedley’s study, which found CPE caused apoptosis at low concentrations while oncosis at high concentrations[10].
Although CPE showed potential therapeutic effects on some malignant tumors, there were still no clinical data or trials available. CPE’s side effect limited its clinical application in tumor therapy. In this study, CPE injection site skin necrosis and enteritis were observed in 3/7 mice. Garcia et al[20] also found the rabbit’s small intestine and colon were damaged after as little as a 1-h treatment with 50 μg/mL of CPE. These studies indicated that serious adverse effects should be considered in CPE-based cancer therapy. To overcome these disadvantages, some researchers cut off the N-terminal region of CPE which mainly cause cell death and then obtain C terminal CPE (C-CPE) which mainly target to the cells. C-CPE is a smaller molecule without cytotoxicity but also combined with CL4 protein. C-CPE can disrupt the tight junction and increase paracellular permeability, enhance chemotherapy drugs to get into the cells[21]. Li et al[22] observed the safety of the C-terminal of CPE and confirmed that injection of CL-targeted toxin injured the liver but not the kidney. To alleviate the side effect of C-CPE is highlight in future research.
In summary, this study investigated the effects of CPE on gastric cancer cells SGC7901. CPE showed CL4 mediated cytotoxicity on gastric cancer cells,and inhibited tumor growth in nude mice models. These results provide CPE may be a novel potential tool for gastric cancer’s therapy. More studies need to be performed to overcome the limitation of CPE before its clinical application.
Clostridium perfringens enterotoxin (CPE) showed therapeutic effects on malignant tumors which highly expressed claudin-4 (CL4) protein. However, little was known about the effects of CPE on gastric cancer cells.
In this study, the authors firstly investigated the effects of CPE on SGC7901 cells which highly expressed CL4 protein. CPE showed CL4 mediated cytotoxicity on gastric cancer cells SGC7901 and inhibited tumor growth in nude mice models.
These results provide CPE may be a novel potential tool for gastric cancer’s therapy.
This is an interesting article reporting the therapeutic effect of CPE on gastric cancer cells (SGC7901 cells) and on a subcutaneous tumor in nude mice model.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Wijarnpreecha K S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Almasi Z, Rafiemanesh H, Salehiniya H. Epidemiology characteristics and trends of incidence and morphology of stomach cancer in Iran. Asian Pac J Cancer Prev. 2015;16:2757-2761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 3. | Badgwell B, Roy-Chowdhuri S, Chiang YJ, Matamoros A, Blum M, Fournier K, Mansfield P, Ajani J. Long-term survival in patients with metastatic gastric and gastroesophageal cancer treated with surgery. J Surg Oncol. 2015;111:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Ding L, Lu Z, Lu Q, Chen YH. The claudin family of proteins in human malignancy: a clinical perspective. Cancer Manag Res. 2013;5:367-375. [PubMed] |
| 5. | Overgaard CE, Daugherty BL, Mitchell LA, Koval M. Claudins: control of barrier function and regulation in response to oxidant stress. Antioxid Redox Signal. 2011;15:1179-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Runkle EA, Mu D. Tight junction proteins: from barrier to tumorigenesis. Cancer Lett. 2013;337:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Tsutsumi K, Sato N, Tanabe R, Mizumoto K, Morimatsu K, Kayashima T, Fujita H, Ohuchida K, Ohtsuka T, Takahata S. Claudin-4 expression predicts survival in pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2012;19 Suppl 3:S491-S499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Agarwal R, D’Souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 2005;65:7378-7385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 284] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 9. | Liu JX, Wei ZY, Chen JS, Lu HC, Hao L, Li WJ. Prognostic and clinical significance of claudin-4 in gastric cancer: a meta-analysis. World J Surg Oncol. 2015;13:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Kitadokoro K, Nishimura K, Kamitani S, Fukui-Miyazaki A, Toshima H, Abe H, Kamata Y, Sugita-Konishi Y, Yamamoto S, Karatani H. Crystal structure of Clostridium perfringens enterotoxin displays features of beta-pore-forming toxins. J Biol Chem. 2011;286:19549-19555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Yamaguchi H, Kojima T, Ito T, Kyuno D, Kimura Y, Imamura M, Hirata K, Sawada N. Effects of Clostridium perfringens enterotoxin via claudin-4 on normal human pancreatic duct epithelial cells and cancer cells. Cell Mol Biol Lett. 2011;16:385-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Santin AD, Cané S, Bellone S, Palmieri M, Siegel ER, Thomas M, Roman JJ, Burnett A, Cannon MJ, Pecorelli S. Treatment of chemotherapy-resistant human ovarian cancer xenografts in C.B-17/SCID mice by intraperitoneal administration of Clostridium perfringens enterotoxin. Cancer Res. 2005;65:4334-4342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Jung H, Jun KH, Jung JH, Chin HM, Park WB. The expression of claudin-1, claudin-2, claudin-3, and claudin-4 in gastric cancer tissue. J Surg Res. 2011;167:e185-e191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Zhu JL, Gao P, Wang ZN, Song YX, Li AL, Xu YY, Wang MX, Xu HM. Clinicopathological significance of claudin-4 in gastric carcinoma. World J Surg Oncol. 2013;11:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Ma Y, Ma L, Guo Q, Zhang S. Expression of bone morphogenetic protein-2 and its receptors in epithelial ovarian cancer and their influence on the prognosis of ovarian cancer patients. J Exp Clin Cancer Res. 2010;29:85. [PubMed] [DOI] [Full Text] |
| 16. | Wang H, Yang X. The expression patterns of tight junction protein claudin-1, -3, and -4 in human gastric neoplasms and adjacent non-neoplastic tissues. Int J Clin Exp Pathol. 2015;8:881-887. [PubMed] |
| 17. | Maeda T, Murata M, Chiba H, Takasawa A, Tanaka S, Kojima T, Masumori N, Tsukamoto T, Sawada N. Claudin-4-targeted therapy using Clostridium perfringens enterotoxin for prostate cancer. Prostate. 2012;72:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Wu HL, Gao X, Jiang ZD, Duan ZT, Wang SK, He BS, Zhang ZY, Xie HG. Attenuated expression of the tight junction proteins is involved in clopidogrel-induced gastric injury through p38 MAPK activation. Toxicology. 2013;304:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Zihni C, Terry SJ. RhoGTPase signalling at epithelial tight junctions: Bridging the GAP between polarity and cancer. Int J Biochem Cell Biol. 2015;64:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Garcia JP, Li J, Shrestha A, Freedman JC, Beingesser J, McClane BA, Uzal FA. Clostridium perfringens type A enterotoxin damages the rabbit colon. Infect Immun. 2014;82:2211-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Suzuki H, Kondoh M, Li X, Takahashi A, Matsuhisa K, Matsushita K, Kakamu Y, Yamane S, Kodaka M, Isoda K. A toxicological evaluation of a claudin modulator, the C-terminal fragment of Clostridium perfringens enterotoxin, in mice. Pharmazie. 2011;66:543-546. [PubMed] |
| 22. | Li X, Saeki R, Watari A, Yagi K, Kondoh M. Tissue distribution and safety evaluation of a claudin-targeting molecule, the C-terminal fragment of Clostridium perfringens enterotoxin. Eur J Pharm Sci. 2014;52:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |