Published online Mar 15, 2017. doi: 10.4251/wjgo.v9.i3.135
Peer-review started: September 7, 2016
First decision: September 29, 2016
Revised: October 19, 2016
Accepted: December 13, 2016
Article in press: December 15, 2016
Published online: March 15, 2017
Processing time: 185 Days and 17.6 Hours
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract, and are characterized by a broad spectrum of clinical, histological and molecular features at presentation. Although focal and scattered calcifications are not uncommon within the primary tumor mass, heavy calcification within a GIST is rarely described in the literature and the clinical-biological meaning of this feature remains unclear. Cases with such an atypical presentation are challenging and may be associated with diagnostic pitfalls. Herein, we report a gastric GIST with the unusual presentation of prominent calcifications that was identified incidentally on imaging during a post-trauma diagnostic work-up. The patient underwent laparoscopic surgery with a radical resection of the mass, which was subsequently characterized by histological analysis as spindle-shaped tumor cells, positive for CD117/c-KIT, CD34 and DOG1, and with calcified areas. Given the intermediate risk of recurrence, no adjuvant therapy was recommended and the patient underwent regular follow-up for 22 mo, with no evidence of relapse. Our case can be considered of interest because of the rarity of clinical presentation and the uniquely large size of the GIST at diagnosis (longest diameter exceeding 9 cm). In closing, we discuss the pathophysiology and clinical implications of calcifications in GISTs by reviewing the most up-to-date relevant literature.
Core tip: Gastrointestinal stromal tumors (GISTs) are heterogeneous neoplasms that may present with a wide range of clinicopathologic characteristics upon diagnosis; among these, massive calcification is an infrequently occurring feature of GIST presentation. By reporting on such a rare clinical case and reviewing the available literature, this manuscript discusses major diagnostic challenges as well as the pathophysiologic and clinical implications related to a heavily calcified mass diagnosed as GIST.
- Citation: Salati M, Orsi G, Reggiani Bonetti L, Di Benedetto F, Longo G, Cascinu S. Heavily calcified gastrointestinal stromal tumors: Pathophysiology and implications of a rare clinicopathologic entity. World J Gastrointest Oncol 2017; 9(3): 135-141
- URL: https://www.wjgnet.com/1948-5204/full/v9/i3/135.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i3.135
Gastrointestinal stromal tumors (GISTs) are the most common type of mesenchymal neoplasm of the gastrointestinal tract (GIT), where they account for roughly 0.1% to 3.0% of all tumors[1]. GISTs were only recently recognized as distinct clinicopathologic entities. Postulated to arise from the interstitial cells of Cajal throughout the entire GIT, the reported GISTs have mainly involved the stomach (60%-70%), with involvement of the small intestine (20%-30%), colon and rectum (5%) and esophagus (< 1%) in fewer cases[2].
The discovery that virtually all GISTs stain positively for c-KIT and/or DOG1 has facilitated their differential diagnosis from other mesenchymal tumors (i.e., leiomyoma, leiomyosarcoma)[3,4]. Due to their slow-growing behavior, GISTs remain asymptomatic for a long period, having in some cases reached a huge size by the time of diagnosis. The most frequently reported symptoms are vague, depending on tumor size and location, and include nausea, vomiting, abdominal discomfort and early satiety; however, an acute abdomen caused by tumor rupture into the peritoneal cavity or gastrointestinal obstruction may represent the first dramatic presentation of a GIST[5]. When studied by imaging, a GIST typically appears as a large, well-defined soft tissue mass with heterogeneous enhancement, reflecting the frequent occurrence of hemorrhagic necrosis and cystic degeneration within.
Although the majority of GISTs present as localized masses amenable to radical surgical resection, at least 50% of them recur locally or spread diffusely throughout the peritoneum and/or the liver, and the malignant potential of GIST varies greatly. Hence, determining the risk of disease recurrence, as assessed by available risk-stratification tools, is helpful in identifying patients most likely to benefit from an adjuvant treatment[6-8]. Size, mitotic activity and primary tumor size are the most reliable predictive factors for risk of relapse after complete surgery.
Discovery of the crucial role played by the oncogenic activating mutations of c-KIT (80%) and PDGFA-R (5%-10%) in the molecular pathogenesis of GISTs[9,10], along with the clinical availability of multitarget tyrosine kinase inhibitors such as imatinib, sunitinib and regorafenib[11], led to dramatic improvement in the outcome of a tumor which, until recently, was regarded as resistant to conventional chemotherapies. Current reports put the disease control rate at up to 85% and the median overall survival of nearly 5 years for patients with unresectable or metastatic GIST receiving frontline imatinib[12]. Moreover, studies of imatinib in the adjuvant setting have shown that it improves the rate of relapse-free survival, as compared with placebo, and that 3 years of therapy gives better rates of relapse-free survival and overall survival than 1 year of therapy in high-risk patients[13,14].
An otherwise healthy 60-year-old woman was admitted to the emergency department with complaint of right-sided chest pain following a car accident in September 2014. The patient was hemodynamically stable and, with the exception of tenderness on the right hemithorax, had unremarkable findings upon physical examination. A total body contrast-enhanced computed tomography (CT) scan was promptly performed and revealed a 7th and 10th right rib fracture, without signs of visceral injury.
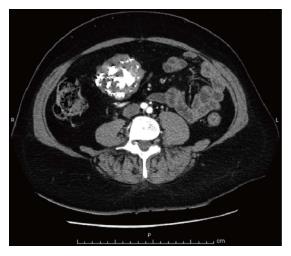
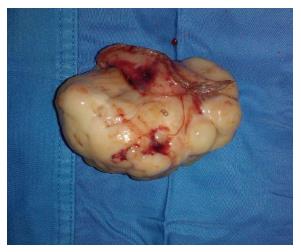
Surprisingly, a large mass, measuring 6.5 cm, arising from the external wall of the gastric antrum and projecting into the abdominal cavity, was incidentally identified on imaging (Figure 1). The mass appeared hyperdense, well-circumscribed, heterogeneously enhanced and with prominent calcifications within; there was neither evidence nor loco-regional nor of distant spread. Hence, laparoscopic surgery was ordered to perform a radical resection of the mass. Gross pathology examination of the resected specimen showed it to be exophytic, lobulated, yellowish-gray and extensively calcified, with necrotic-cystic zones measuring 9.3 cm × 5.5 cm (Figure 2).
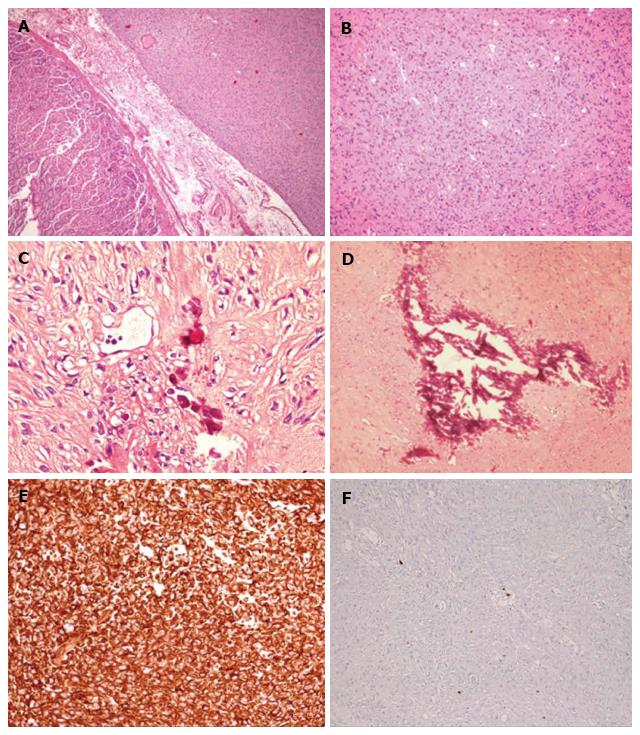
Microscopic pathology examination revealed a population of spindle-shaped tumor cells (Figure 3A and B) and calcified areas (Figure 3C and D), with positive staining for CD117/c-KIT, CD34 and DOG1 (Figure 3E). In addition, Ki67-MIB1 immunostaining indicated a low proliferative rate (count rate < 1%) (Figure 3E). Molecular testing identified the c-KIT gene as wild-type but the PDGFRA gene as mutated (an exon 18 point mutation). The mass was diagnosed accordingly as GIST of gastric origin.
Recovery from the surgical resection was uneventful and prompt. Based on the estimated intermediate risk of recurrence, made according to the modified National Institutes of Health Criteria[15] (gastric site, size of 5-10 cm, mitosis count of < 5 per 50 high power fields, no tumor rupture), only regular follow-up was recommended. To date, the patient has been followed-up for 22 mo and shown no evidence of relapse.
Circumscribed patchy calcifications may occur within the primary mass of large GISTs, and the reported series have indicated a wide variability among these (10% to 50%)[16]. Contrariwise, coarse and extensive calcifications represent an uncommon radiological finding, and only a few cases of these have been reported in the literature[17-23]. Indeed, our search of the MEDLINE/PubMed database identified only 8 cases of extensively calcified GISTs (Table 1).
| Ref. | Year | Age, sex | Site | Size (mm) | Mitotic index(HPF) | IHC pattern | Calcification pattern | Clinical presentation | Risk1 |
| Izawa et al[20] | 2012 | 61 yr, female | Stomach | 32 × 23 × 23 | < 5/50 | KIT+, CD34+, S-100-, desmin-, SMA- | Peripheral ring | Epigastric discomfort | Low |
| Kim et al[17] | 2012 | 53 yr, male | Stomach | 80 × 75 × 50 | < 5/50 | KIT+, CD34+, S-100-, desmin-, SMA- KIT+, CD34+, S-100-, desmin-, SMA- | Extensively calcified | Epigastric pain | Intermediate |
| 62 × 55 × 42 | |||||||||
| 69 yr, female | Stomach | < 5/50 | Extensively calcified | Epigastric pain | Intermediate | ||||
| Yu et al[21] | 2011 | 81 yr, female | Stomach | 60 | < 5/50 | KIT+, CD34+, S-100-, desmin- | Semicircular plate | Abdominal pain, hematemesis | Intermediate |
| Ong et al[18] | 2006 | 94 yr, female | Rectum | 25 × 15 × 15 | < 5/50 | KIT+, CD34+, S-100-, desmin- | Entirely | Rectal bleeding | Low |
| Yoshida et al[22] | 2005 | 77 yr, male | Stomach | 28 × 26 × 26 | < 5/50 | KIT+, CD34+, vimentin+, S-100-, desmin-, SMA- | Patchy | Chest discomfort, loss of consciousness | Low |
| Testroote et al[23] | 2007 | 53 yr, male | Rectum | 31 × 23 × 18 | < 5/50 | KIT+, CD34+, SMA focally positive | Entirely | Abdominal pain, constipation | Low |
| Rana et al[19] | 2006 | 62 yr, female | Colon | 39 × 56 | NA | KIT+, CD34+ | Multiple shadowing | Abdominal pain | NA |
From a clinical standpoint, when facing a calcified intra-abdominal juxta-gastric mass, various differential diagnoses should be ruled out. For example, diffuse and punctate calcifications may accompany mucin-producing adenocarcinoma of the stomach[24,25]. In the pancreas, solid pseudopapillary tumors and mucinous cystic neoplasms may develop peripheral curvilinear wall calcification, whereas serous cystadenomas may have central calcification within the central fibrous scar[26]. Finally, an ingested foreign body in the stomach may mimic gastric calcification.
From a pathogenetic standpoint, several mechanisms have been implicated in calcific deposition. The most acknowledged process is dystrophic calcification, which typically involves degenerated tissues that are characterized by necrosis, inflammation and hemorrhage, as frequently occurs in GISTs. In such a relatively alkaline background, the binding of denatured proteins to both phosphate and calcium ions is ultimately responsible for the formation of calcium phosphate precipitates. On the other hand, the diffuse punctate calcification pattern that is typical of mucin-forming gastric adenocarcinomas seems to be linked instead to calcium salt precipitates with glycoprotein in an alkaline matrix[27]. Metastatic calcifications occurring in the context of malignant hypercalcemia, as well as granulomatous tissue engulfed by the tumor, represent rarer causes of calcification. More recently, a unique case of osseous metaplasia with mature bone formation was described in a newly diagnosed gastric GIST[28].
Among the proposed theories, however, the production of multiple cell mediators-such as PDGF, the receptor of which is frequently mutated in GISTs, and the bone morphogenic protein, which is produced by pluripotent stromal cells committed towards the osteoblastic linage-are thought to play a crucial role in the regulation of ectopic bone formation[28]. Intriguingly, several clinical reports have demonstrated the appearance of calcification in metastatic sites of GIST following treatment with imatinib[29]. The underlying mechanism, which is still far from being fully elucidated, is likely to be different from that occurring in the primary mass, as is suggested by the absence of inflammation or necrosis in the specimen obtained from liver metastasis[30]. It is also interesting that this morphologic change has been hypothesized as predictive of tumor response. Finally, examples of osseous differentiation have also been observed in cases of treated GIST, suggesting that under imatinib suppression, GIST cells may become able to mediate the cell cycle and to express a gene signature associated with other differentiated cell phenotypes, thereby providing a mechanism of acquired drug resistance[31].
Following early evidence that indicated solitary calcification could be an aggressive feature[32], researchers continue to investigate the potential relevance of calcification in predicting the malignant potential of GISTs. However, a study by Kim et al[33] found no CT features, other than size, to be correlated with biological behavior and prognosis of GIST. This finding was confirmed very recently by Chen et al[34]; specifically, that retrospective analysis of 110 patients who underwent endoscopic ultrasonography identified tumor size as an independent risk factor for malignancy (P≤ 0.0001) but not for calcification (P = 0.667).
Although no conclusions can be drawn regarding the predictive value of calcification in GIST because of the limited cases reported and the retrospective nature of the analyses, heavily calcified tumors appear to have a less aggressive course, as shown by their low mitotic rate (Table 1). With regard to our case, a relatively indolent behavior and the alleged long history of disease probably contributed to the development of calcification.
In conclusion, to the best of our knowledge, we have presented herein the largest (> 9 cm) heavily calcified GIST ever described in the literature. Our case is noteworthy because of the rarity of clinical presentation in general and the unique size at diagnosis for this patient in particular. Finally, our case and review of the literature indicates that a diagnosis of GIST should be considered when large calcified masses are identified in the GIT, and even more so when in the stomach.
An otherwise healthy 60-year-old woman was admitted to the emergency department with complaint of right-sided chest pain following a car accident.
Upon admission, the patient was hemodynamically stable and only showed tenderness on the right hemithorax upon physical examination, the findings of which were otherwise unremarkable.
Mucin-producing adenocarcinoma of the stomach; pancreatic solid pseudopapillary tumor and mucinous cystic neoplasm; pancreatic cystadenoma; ingested foreign body in the stomach.
All laboratory findings were within normal limits.
In addition to a 7th and 10th right rib fracture, computed tomography (CT) scan revealed a large mass, measuring 6.5 cm, arising from the external wall of gastric antrum and projecting into the abdominal cavity.
Gastrointestinal stromal tumor (GIST) of gastric origin.
Complete laparoscopic surgical excision of the mass.
Circumscribed patchy calcifications may occur within the primary mass of large GISTs, and the reported series have indicated a wide variability among these (10% to 50%). Contrariwise, coarse and extensive calcifications represent an uncommon radiological finding, and only a few cases of these have been reported in the literature.
Gastrointestinal stromal tumors are the most common mesenchymal neoplasms of the gastrointestinal tract (GIT), where they account for roughly 0.1% to 3.0% of all tumors. GISTs were only recently recognized as distinct clinicopathologic entities. Postulated to arise from the interstitial cells of Cajal throughout the entire GIT, the reported GISTs have mainly involved the stomach (60%-70%), with involvement of the small intestine (20%-30%), colon and rectum (5%) and esophagus (< 1%) in fewer cases.
The authors’ case is noteworthy because of the rarity of clinical presentation of calcified GISTs and the unique large size of the tumor at diagnosis. A diagnosis of GIST should be considered when large calcified masses are identified in the GIT, and even more so when in the stomach.
This article offers an interesting overview of calcified GISTs, discussing the pathophysiology and clinical implication of calcifications in GISTs by reviewing the most up-to-date relevant literature. While the case reported is not the first that describes a massively calcified GIST, the incidental diagnosis and unique size of the mass are peculiar and noteworthy.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aoyagi K, de Bree E, Lee HC, Li CF, Marin JJG S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Goettsch WG, Bos SD, Breekveldt-Postma N, Casparie M, Herings RM, Hogendoorn PC. Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer. 2005;41:2868-2872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol. 2003;54:3-24. [PubMed] |
| 3. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 4. | West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, Zhu S, Ball CA, Nielsen TO, Patel R. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 457] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 5. | Misawa S, Takeda M, Sakamoto H, Kirii Y, Ota H, Takagi H. Spontaneous rupture of a giant gastrointestinal stromal tumor of the jejunum: a case report and literature review. World J Surg Oncol. 2014;12:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 865] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 7. | Miettinen M, Makhlouf H, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol. 2006;30:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 437] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 8. | Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 280] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3215] [Cited by in RCA: 3113] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 10. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1721] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 11. | Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1817] [Cited by in RCA: 1646] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 12. | Gastrointestinal Stromal Tumor Meta-Analysis Group (Meta GIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 366] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 13. | Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 968] [Article Influence: 60.5] [Reference Citation Analysis (1)] |
| 14. | Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 681] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 15. | Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 864] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 16. | Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283-304, 456; quiz 532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | Kim HS, Sung JY, Park WS, Kim YW. Gastrointestinal stromal tumors of the stomach with extensive calcification: report of two cases. Intern Med. 2012;51:2555-2558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Ong K, Singaporewalla RM, Tan KB. Extensive calcification within a gastrointestinal stromal tumour: a potential diagnostic pitfall. Pathology. 2006;38:451-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Rana R, Nikolaidis P, Miller F. Calcified gastrointestinal stromal tumor of the sigmoid colon mimicking inspissated barium on CT. AJR Am J Roentgenol. 2006;187:W322-W323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Izawa N, Sawada T, Abiko R, Kumon D, Hirakawa M, Kobayashi M, Obinata N, Nomoto M, Maehata T, Yamauchi S. Gastrointestinal stromal tumor presenting with prominent calcification. World J Gastroenterol. 2012;18:5645-5648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Yu CC, Wu CC, Hwang JI, Wang J, Chang CS. Thick calcification from a GIST of the stomach penetrating into pericolic soft tissue--report of a case. World J Surg Oncol. 2011;9:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Yoshida H, Mamada Y, Taniai N, Mizuguchi Y, Nakamura Y, Nomura T, Okuda T, Uchida E, Fukuda Y, Watanabe M. Spurt bleeding from a calcificated gastrointestinal stromal tumor in the stomach. J Nippon Med Sch. 2005;72:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Testroote M, Hoornweg M, Rhemrev S. Rectal GIST presenting as a submucosal calculus. Dig Dis Sci. 2007;52:1047-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Dickson AM, Schuss A, Goyal A, Katz DS. Radiology-Pathology Conference: Calcified untreated gastric cancer. Clin Imaging. 2004;28:418-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Balestreri L, Canzonieri V, Morassut S. Calcified gastric cancer--CT findings before and after chemotherapy. Case report and discussion of the pathogenesis of this type of calcification. Clin Imaging. 1997;21:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Choi JY, Kim MJ, Kim JH, Kim SH, Lim JS, Oh YT, Chung JJ, Yoo HS, Lee JT, Kim KW. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2006;187:W178-W186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Ichiishi E, Kogawa T, Takeda S, Yanagida K, Yoshikawa T, Kondo M. Eight cases of gastric tumors with calcification. Intern Med. 1995;34:1038-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Giorlandino A, Caltabiano R, Gurrera A, Lanzafame S. Gastrointestinal stromal tumour of the stomach with osseous differentiation: a case report. Pathologica. 2014;106:345-347. [PubMed] |
| 29. | Warakaulle DR, Gleeson F. MDCT appearance of gastrointestinal stromal tumors after therapy with imatinib mesylate. AJR Am J Roentgenol. 2006;186:510-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1324] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 31. | Agaram NP, Besmer P, Wong GC, Guo T, Socci ND, Maki RG, DeSantis D, Brennan MF, Singer S, DeMatteo RP. Pathologic and molecular heterogeneity in imatinib-stable or imatinib-responsive gastrointestinal stromal tumors. Clin Cancer Res. 2007;13:170-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Ghanem N, Altehoefer C, Furtwängler A, Winterer J, Schäfer O, Springer O, Kotter E, Langer M. Computed tomography in gastrointestinal stromal tumors. Eur Radiol. 2003;13:1669-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Kim HC, Lee JM, Kim KW, Park SH, Kim SH, Lee JY, Han JK, Choi BI. Gastrointestinal stromal tumors of the stomach: CT findings and prediction of malignancy. AJR Am J Roentgenol. 2004;183:893-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Chen TH, Hsu CM, Chu YY, Wu CH, Chen TC, Hsu JT, Yeh TS, Lin CJ, Chiu CT. Association of endoscopic ultrasonographic parameters and gastrointestinal stromal tumors (GISTs): can endoscopic ultrasonography be used to screen gastric GISTs for potential malignancy? Scand J Gastroenterol. 2016;51:374-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |