Published online Jan 15, 2017. doi: 10.4251/wjgo.v9.i1.21
Peer-review started: August 16, 2016
First decision: September 28, 2016
Revised: October 8, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: January 15, 2017
Processing time: 149 Days and 23.5 Hours
L-type amino acid transporters (LATs) mainly assist the uptake of neutral amino acids into cells. Four LATs (LAT1, LAT2, LAT3 and LAT4) have so far been identified. LAT1 (SLC7A5) has been attracting much attention in the field of cancer research since it is commonly up-regulated in various cancers. Basic research has made it increasingly clear that LAT1 plays a predominant role in malignancy. The functional significance of LAT1 in cancer and the potential therapeutic application of the features of LAT1 to cancer management are described in this review.
Core tip: The discovery of molecules preferentially expressed in cancer cells is extremely valuable for the development of molecular target drugs in cancer therapy. Amino acid transporters have been receiving a great amount of attention as a candidate of such molecular targets. This review summarizes new initiatives for clinical applications of the basic research relative to L-type amino acid transporters, which are commonly expressed in cancers.
- Citation: Hayashi K, Anzai N. Novel therapeutic approaches targeting L-type amino acid transporters for cancer treatment. World J Gastrointest Oncol 2017; 9(1): 21-29
- URL: https://www.wjgnet.com/1948-5204/full/v9/i1/21.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i1.21
Cancers consume a huge amount of materials for biochemical reactions, and a continuous supply of sufficient nutrients is essential for their survival. Hydrophilic nutrients are delivered into cells by transporters. Recent studies have revealed several transporters preferentially expressed in cancers. Inhibition of cancer specific-nutrient transporters would be a good strategy for cancer management with minimal side effects. Indeed, a therapeutic approach using transporter inhibitors for cancer prevention has been proven to be efficacious in cell lines and animal experiments and is now under evaluation in a clinical trial.
Many cells take advantage of transporters to incorporate what is necessary at the time of need. Transporters fall into two broad categories based on ATP dependency for their transport form[1]. ATP-dependent transporters, known as ATP-binding cassette, hydrolyze ATP to obtain the energy for translocation of their substrates across the membrane (active transport). Transporters with no ATPase, called solute carriers (SLCs), facilitate diffusive transport. Each SLC transporter is named in combination with the family numeral based on the sequence similarity and individual number with letter A between them (e.g., SLC3A2), with a few exceptions. Most of the amino acid transporters were formerly categorized into several groups (“System”) on the basis of their substrates and sodium dependency (e.g., System L, which incorporates neutral amino acids without sodium), but they are currently classified into SLCs according to their protein homology.
L-type amino acid transporters (LATs) are categorized as system L transporters. LATs mainly deliver neutral amino acids into cells in a sodium-independent manner. So far, four LATs have been identified.
LAT1 (SLC7A5) was identified as the first LAT by two groups in 1998[2,3]. The major substrate of LAT1 is large neutral amino acids as typified by leucine. The expression of LAT1 in normal adults is detected in proliferative zones of gastrointestinal mucosa, testicular sertoli cells, ovarian follicular cells, pancreatic islet cells, and some endothelial cells that serve as a barrier between tissues (blood-brain, blood-retinal and blood-follicle barrier)[4]. Recent studies revealed a crucial role of LAT1 in activated T cells[5,6]. As described below, LAT1 expression is commonly up-regulated in various cancers.
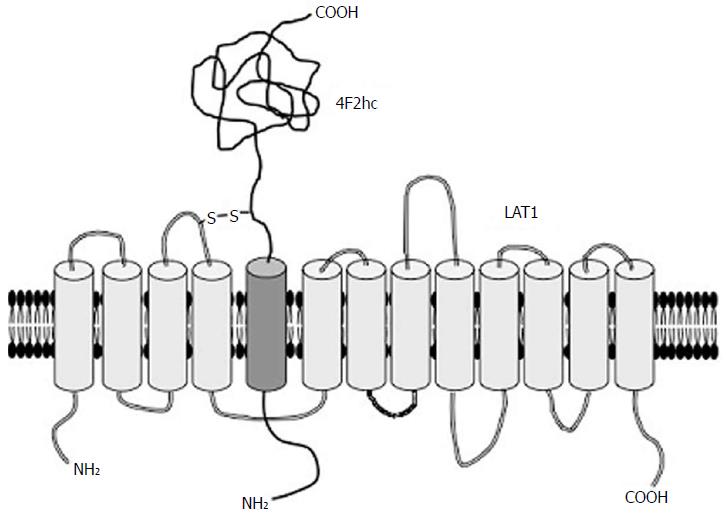
LAT2 (SLC7A8) was subsequently isolated on the basis of sequence similarity to LAT1[7-9]. LAT2 has broader specificity of its substrates including polar uncharged and small neutral amino acids than that of LAT1[8]. LAT2 is ubiquitously expressed in normal body[4], though LAT2 knockout mice show a mild phenotype and almost no visible symptoms except aminoaciduria[10]. Both LAT1 and LAT2 are composed of 12 transmembrane domains that form the pathway of their substrates[11] (Figure 1). They associate with the heavy glycoprotein subunit 4F2hc (SLC3A2) by sulfur bond[11]. Although 4F2hc does not seem to have a function to directly transfer the substrates, it makes the localization of its partner LATs more stable at the plasma membrane[12].
LAT3 (SLC43A1) was isolated by expression cloning from hepatocarcinoma cells[13]. Sequence analysis revealed that LAT3 was identical to POV1, which was originally identified as a cancer-up-regulated gene[14,15]. The substrate selectivity of LAT3 was similar to that of LAT1. LAT3 mRNA is expressed in the liver, skeletal muscle, and pancreas[16]. The physiological role of LAT3 in normal individuals of mammals remains unknown, but it was shown that LAT3 functions for podocyte development in zebrafish[17].
LAT3 appears to behave as a critical transporter in several cancers. LAT3 is up-regulated in response to androgen and knockdown of LAT3 expression by RNA interference (RNAi) significantly inhibits the leucine uptake and cell proliferation in human prostate cancer cell lines in vitro[18]. Furthermore, high expression of LAT3 is detected in prostate cancer patients, and stably knockdown of LAT3 by RNAi in human prostate cancer cell lines results in decrease of their growth and metastatic potential with alteration of cell cycle gene expression after xenografts into mice[19].
LAT4 (SLC43A2) was identified by searching for sequence homology to LAT3[20]. LAT4 is expressed in the basolateral membrane of the small intestine, kidney proximal tubule and thick ascending limb epithelial cells. LAT4 knockout mice are smaller than their controls and die within 9 d, presumably because of defective amino acid absorption[21]. Unlike LAT1 and LAT2, LAT3 as well as LAT4 functions independently of heavy chain.
LAT1 is the most extensively studied transporter among LATs. The interest in LAT1 is because of its extremely high expression in diverse human cancers. LAT1 was originally cloned from mRNA of C6 glioma cells[2]. Subsequent studies have shown that LAT1 is highly expressed in many cancer cell lines. Histological analysis with qualitatively enhanced antibodies confirmed potent expression of LAT1 in human cancers in a broad range of tissues. The number of cancer types that were reported to express a high level of LAT1 is well above twenty (Table 1). LAT1 is thus a commonly up-regulated amino acid transporter in multiple human cancers. Furthermore, LAT1 expression level appears to be associated with prognosis of cancer patients. For example, elevated expression of LAT1 correlates with an adverse prognosis in prostate[22], gastric[23], and pancreatic cancers[24], suggesting that higher-grade tumors are more dependent on LAT1. Not only the expression of LAT1 but also the functional significance of LAT1 in cancers has been verified by use of its inhibitors, by knockdown with RNAi and by gene disruption. 2-Aminobicyclo (2,2,1) heptane-2-carboxylic acid (BCH) is an inhibitor of system L transporters. BCH inhibits leucine uptake and strongly suppresses the proliferation of many cancer cells (Table 1). Genetic manipulation confirmed the functional significance of LAT1 in cancer cells. Knockdown of LAT1 with RNAi[25-29] as well as genetic disruption of LAT1 by zinc fingers nucleases-mediated gene knockout[12] in cancer cells reduces leucine uptake and cell proliferation, indicating that LAT1 is a predominant transporter that is essential for growth of cancers. The reason that so many cancers use LAT1 despite the presence of many other amino acid transporters might be that LAT1 has a prominent capability for substrate transport. Indeed, the affinity of LAT1 for leucine is much higher than that of LAT2[30], although LAT2 is ubiquitously expressed in the normal body[4]. Cancers may therefore be more dependent on LAT1 for rapid uptake of sufficient amino acids, whereas normal cells need less amino acid delivery that can be supported by LAT2.
| Cancer | Expression (method of detection) | Inhibition of amino acid uptake by | Growth inhibition by | Ref. |
| Biliary tract | Immunohistochemistry | BCH | BCH | [71] |
| Bladder | Northernblot (cell line) | BCH | [72] | |
| Bone | Immunohistochemistry | [73] | ||
| Brain | Immunohistochemistry, RT-PCR (cell line), Western blot (tissue, cell line) | BCH | BCH | [74,75] |
| Breast | Immunohistochemistry, RT-PCR (cell line) | BCH | RNAi, BCH | [29,76-78] |
| Colon | Western blot (cell line) | Knockout (cell line) | Knockout (cell line) JPH203 | [12] |
| Esophagus | Immunohistochemistry | [79,80] | ||
| Hepatocyte | Immunohistochemistry | [81] | ||
| Gastrointestine | Immunohistochemistry, Western blot (cell line) | RNAi | [23,45] | |
| Laryngeal | Immunohistochemistry | [82] | ||
| Leukemia | RT-PCR (cell line) | BCH, JPH203 | [33] | |
| Lung | Immunohistochemistry | [41,83-85] | ||
| Melanoma | Immunohistochemistry, Microarray (tissue), Western blot (cell line) | BCH | [86,87] | |
| Myeloma | RT-PCR (cell line) | RNAi | [88] | |
| Neuroendocrine | Immunohistochemistry, RT-PCR (tissue), Western blot (tissue) | [89] | ||
| Ovarian | Immunohistochemistry, RT-PCR (cell line), Western blot (tissue, cell line) | BCH | BCH | [47,65,90] |
| Oral | RT-PCR (cell line) | RNAi | RNAi | [25] |
| Pancreas | Immunohistochemistry Western blot (cell line) | RNAi | RNAi | [24,27,91] |
| Pleura | Immunohistochemistry | [92] | ||
| Prostate | Immunohistochemistry, Western blot (cell line) | RNAi, BCH | RNAi, BCH | [18,19,22,28] |
| Tongue | Immunohistochemistry | [93] | ||
| Thymus | Immunohistochemistry, Western blot (cell line) | JPH203 | JPH203 | [94,95] |
| Urinary tract | Immunohistochemistry | [96] |
The definite effect of LAT1 on the growth of various cancer cell lines prompted researchers to apply the LAT1 inhibitor in a clinical setting. However, the concentration of BCH required for suppression of cancer growth is extremely high (usually around 10 mmol/L). Moreover, the unselective effect of BCH that inhibits all LATs is another problem, since LATs other than LAT1 are considered to have functions in the normal body. It has been necessary to develop drugs that act on just LAT1 but not other transporters at a low concentration. In 2010, Endo and colleagues designed a new compound named JPH203 ((S)-2-amino-3-(4-((5-amino-2-phenylbenzo[d]oxazol-7-yl) methoxy)-3,5-dichloropheyl) propanoic acid)[31]. JPH203 has structural analogy to tyrosine, but it inhibits only LAT1 without affecting any other LATs. JPH203 displayed potent suppressive effects on the growth of cancers in vitro[12,32,33]. Moreover, this compound has the ability to powerfully inhibit the proliferation of tumor cell lines of the colon and leukemia injected into nude mice[31,33]. Following improvements in its specificity and pharmacological effect, JPH203 is under evaluation in a phase I clinical trial of cancer patients.
By exploiting the characteristics of LAT1 expression, an approach for the diagnosis of cancers through radiolabeled substrates of LAT1 has been attempted. [18F] or [11C]-labeled compound administered into the body can be visualized by positron emission tomography (PET)[34]. Cancers incorporating an isotopically labeled probe can be located by tracing the body with PET. In the past, 2-18F-fluoro-2-deoxy-d-glucose ([18F]FDG) was one of the most commonly used probe for diagnosis of cancer with PET. This strategy exploits the characteristic of cancers consuming a huge amount of glucose compared to that consumed by normal cells. Although [18F]FDG has been of assistance in the clinical diagnosis of many cancers, it sometimes showed false positive results, especially in brain, because even normal brain cells take up a relatively large amount of glucose. To overcome this problem, amino acids have attracted attention as alternative probes to glucose. Representative amino acids or their analogs developed as probes of PET are L-3-[18F]-fluoro-α-methyl tyrosine ([18F]FAMT), 6-18F-fluoro-L-3,4-dihydroxy-phenylalanine (18F-DOPA), l-[11C-methyl] methionine ([11C]MET) and O-(2-[18F]fluoroethyl)-l-tyrosine ([18F]FET). If the compounds are delivered into cells specifically through LAT1, those cells are likely to be cancers. Indeed, [18F]FAMT images accord well with LAT1 distribution[35]. Moreover, FAMT is incorporated by LAT1 but not by other amino acid transporters[35]. Although there is still room for improvement in its specificity, this method is powerful tool for diagnosis of cancers including microcarcinoma.
LAT1 is an attractive molecular target for boron neutron capture therapy (BNCT). BNCT is an anticancer therapy that utilizes high linear energy transfer alpha particles. Particle radiation is produced by fission reaction when irradiated thermal neutrons collide with boron incorporated by a malignant tumor. The traveling distance of particle radiation is limited (5-9 μm), and it therefore disrupts only cancer cells incorporating boron without damage to other cells around target cells[36,37]. A key component of BNCT success is accumulation of boron specifically in cancer cells. This difficult task could be achieved by the synthesis of a boron compound that is selectively delivered by LAT1. Indeed, p-boronophenylalanine (BPA), a boron compound commonly used in BNCT, is incorporated by LAT1[38-40], suggesting that LAT1 is an optimal mediator for delivery of boron in BNCT. However, since we cannot still completely rule out the possibility of BPA uptake by other transporters, it is necessary to develop compounds that exhibit strict selectivity to LAT1. BNCT has accomplished certain clinical outcomes so far, but the problem in the past was that it required a large-scale nuclear reactor to generate neutrons. However, a compact accelerator has been developed as an alternative to a nuclear reactor and it can be installed in a hospital, making BNCT easier to perform. Such technology will expand the applications of BNCT in the future.
It has been suggested that LAT1 is involved in cancer metastasis. A number of studies have shown a correlation of increase in LAT1 expression with metastasis of multiple cancers. Lymph node metastasis-positive squamous cell carcinomas express LAT1 whereas there is no positive signal of LAT1 in metastasis-negative cells[41]. LAT1 mRNA level was significantly higher in renal cell carcinoma with metastasis[42]. A group of cells with high LAT1 expression showed a larger size of the metastatic lesion of gastric carcinoma[43]. LAT1 expression in neuroendocrine tumors was significantly associated with lymph node metastasis[44]. The potency of the functional significance of LAT1 in metastasis has been shown. Knockdown of LAT1 by RNAi inhibited the migration and invasion of gastric cancer[45] and a cholangiocarcinoma cell line[46]. BCH inhibited the proliferation and migration of a human epithelial ovarian cancer cell line[47]. On the basis of these findings, inhibition of LAT1 will be good strategy to prevent metastasis of cancer. However, it remains to be determined whether the metastasis defect is derived from amino acid starvation or from other factors such as an aberrance of adhesion molecules. It would thus be valuable to investigate the relevance of LAT1 and integrin in metastasis, since they form a complex[48].
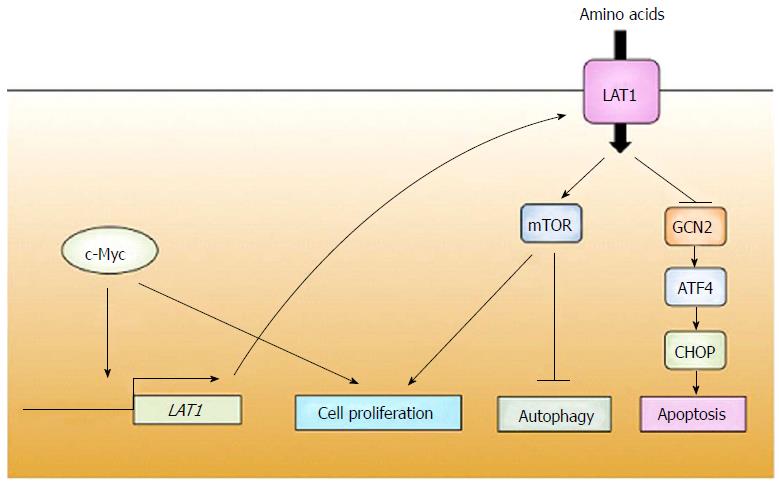
Although it remains unknown how LAT1 expression is facilitated in cancers, some possible molecular mechanisms have been proposed. c-Myc, a proto-oncogenic transcription factor, has been demonstrated to be an upstream of LAT1. The expression of c-Myc in normal adults is generally low[49], but overexpression of c-Myc triggered by some cues such as gene amplification, gene translocation or other gene mutations[50] is responsible for malignant transformation. Numerous human cancer tissues strongly express c-Myc. Target genes of c-Myc include many factors involved in progression of the cell cycle[51]. On the other hand, the consensus binding sequence of c-Myc is also located at the LAT1 promoter[27]. Moreover, knockdown of c-Myc leads to reduction of LAT1 expression in cancer cell lines[27]. These results suggest that up-regulation of LAT1 is mediated, at least in part, by c-Myc (Figure 2). Of note is that c-Myc also enhances the metabolic reprogram in cancers by promoting the expression of enzymes of glycolysis and glucose transporter[52,53]. This is an ingenious strategy of cancers since they can coordinate multiple events required for cell growth by just one factor.
Some other factors appear to regulate LAT1 expression. Hypoxia-inducible factor (Hif) is a critical regulator in response to hypoxia. Hif2α, an isoform of the Hif family, binds to the LAT1 promoter and enhances LAT1 expression in renal carcinoma cell lines[54]. Artificial manipulation to elevate Hif2α activity induces LAT1 expression in lung and liver tissues, in which LAT1 expression is usually low[54]. Aryl hydrocarbon receptor (AHR) is a transcription factor that is activated by interaction with its ligands such as dioxin, and it promotes tumorigenesis[55]. AHR binds to its consensus binding sequence in LAT1 and drives LAT1 expression in breast cancer cell lines[56], suggesting that LAT1 contributes to tumorigenesis induced by an environmental carcinogen. As described previously, T cell activation induces LAT1 expression[5,6]. Nuclear factor kappa B, AP-1 and nuclear factor of activated T-cells are critical transcription factors that are activated by T cell stimulation and enhance immunological reactions. The expression of LAT1 is prevented by inhibitors of these transcription factors[5,6]. This means that LAT1 expression is induced by the common regulators that also boost immunological reaction in T cells.
Ensuring a sufficient supply of nutrients is an issue of vital importance for cancers. The majority of cancers are thought to constantly monitor the availability of amino acids. Starvation of amino acids rapidly induces a stress response that puts a brake on cellular biochemical reactions to avoid wasting energy and materials. The most extensively studied system for monitoring the amino acid availability is mechanistic target of rapamycin (mTOR)[57], a serine-threonine kinase. Plenty of amino acids maintains mTOR kinase activity, resulting in progression of the cell cycle, protein synthesis, or inhibition of autophagy induction (Figure 2). Some mTOR regulators such as SLC38A9[58-60], Cellular arginine sensor for mTORC1 (CASTOR1)[61] and Sestrin2[62] have been demonstrated to associate with amino acids to dictate mTOR activity. Dissociation of those interactions caused by amino acid deficiency inactivates mTOR and inverses the reaction of its downstream, resulting in a halt of cancer growth. Growing evidence suggests that LAT1 disruption leads to the inhibition of mTOR. LAT1 inhibition decreases mTOR activity in many cancer cell lines[28,33,63-65]. These findings suggest that the arrest of cell growth of cancers by a defect of LAT1 is derived from inactivation of mTOR (Figure 2). mTOR inhibitors are being used in practical trials for therapeutic management of several cancers[66]. Application of JPH203 together with an mTOR inhibitor probably creates a synergistic effect and might be useful for maximizing the benefit of treatment with a low-dose drug, which would help to minimize adverse effects.
General control non-derepressible 2 (GCN2) is another factor for detection of amino acid starvation[67]. GCN2 is a serine-threonine kinase that is activated by amino acid deficiency. Uncharged tRNAs caused by a decline of amino acid concentration activates GCN2, which eventually induces activity of activating transcription factor 4 (ATF4). ATF4 regulates the expression of genes responsible for coping with amino acid deficiency[68]. Several studies have shown that dysfunction of LAT1 initiates the GCN2 signal. JPH203 promotes the expression of C/EBP homologous protein [CHOP, also known as DNA damage inducible transcript 3 (DDIT3)], which is up-regulated by ATF4[68] and probably takes part in apoptosis in leukemia[33]. Gene disruption of LAT1 in cancer cell lines activates the GCN2-ATF4 cascade[12]. Activation of ATF4 by LAT1 defect was also shown in cells other than cancer. JPH203 triggers the expression of CHOP[5,69] and homeobox B9[70], a novel target of ATF4, in human T cells to repress cytokine production. These findings suggest that GCN2-ATF4 is another critical system for detecting amino acid deficiency evoked by LAT1 inhibition (Figure 2).
After the importance of LAT1 in cancer cells had been established, basic studies on LAT1 have progressed with remarkable speed. Better still, research achievements are potentially capable of technical developments for the use of LAT1 as a molecular target in clinical practice. However, although JPH203 is more effective and specific than BCH, it still requires a high concentration for sufficient suppression of the growth of cancers, and wariness of adverse effect persists. Nevertheless, such concerns might be overcome, at least for the time being, by virtue of the proper combinational use of multiple drugs with different action points in cellular metabolism (e.g., mTOR inhibitor). However, further improvements in selectivity of the inhibitor, boron donor of BNCT and PET probe to LAT1 will raise the quality of cancer treatment. Besides, although not to the extent to LAT1, there are several cancers that rely on LAT3 for their growth and development of a LAT3-specific inhibitors is also encouraged. Advances in technologies are expected to resolve such issues.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bugaj AM, Camacho J, Song JJ S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Hediger MA, Clémençon B, Burrier RE, Bruford EA. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med. 2013;34:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 429] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 2. | Kanai Y, Segawa H, Miyamoto Ki, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem. 1998;273:23629-23632. [PubMed] |
| 3. | Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 444] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Nakada N, Mikami T, Hana K, Ichinoe M, Yanagisawa N, Yoshida T, Endou H, Okayasu I. Unique and selective expression of L-amino acid transporter 1 in human tissue as well as being an aspect of oncofetal protein. Histol Histopathol. 2014;29:217-227. [PubMed] |
| 5. | Hayashi K, Jutabha P, Endou H, Sagara H, Anzai N. LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J Immunol. 2013;191:4080-4085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14:500-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 693] [Cited by in RCA: 711] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 7. | Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, Kühn LC. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem. 1999;274:34948-34954. [PubMed] |
| 8. | Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745-19751. [PubMed] |
| 9. | Pineda M, Fernández E, Torrents D, Estévez R, López C, Camps M, Lloberas J, Zorzano A, Palacín M. Identification of a membrane protein, LAT-2, that Co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274:19738-19744. [PubMed] |
| 10. | Braun D, Wirth EK, Wohlgemuth F, Reix N, Klein MO, Grüters A, Köhrle J, Schweizer U. Aminoaciduria, but normal thyroid hormone levels and signalling, in mice lacking the amino acid and thyroid hormone transporter Slc7a8. Biochem J. 2011;439:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 537] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 12. | Cormerais Y, Giuliano S, LeFloch R, Front B, Durivault J, Tambutté E, Massard PA, de la Ballina LR, Endou H, Wempe MF. Genetic Disruption of the Multifunctional CD98/LAT1 Complex Demonstrates the Key Role of Essential Amino Acid Transport in the Control of mTORC1 and Tumor Growth. Cancer Res. 2016;76:4481-4492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Babu E, Kanai Y, Chairoungdua A, Kim DK, Iribe Y, Tangtrongsup S, Jutabha P, Li Y, Ahmed N, Sakamoto S. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem. 2003;278:43838-43845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Chuaqui RF, Englert CR, Strup SE, Vocke CD, Zhuang Z, Duray PH, Bostwick DG, Linehan WM, Liotta LA, Emmert-Buck MR. Identification of a novel transcript up-regulated in a clinically aggressive prostate carcinoma. Urology. 1997;50:302-307. [PubMed] |
| 15. | Cole KA, Chuaqui RF, Katz K, Pack S, Zhuang Z, Cole CE, Lyne JC, Linehan WM, Liotta LA, Emmert-Buck MR. cDNA sequencing and analysis of POV1 (PB39): a novel gene up-regulated in prostate cancer. Genomics. 1998;51:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Fukuhara D, Kanai Y, Chairoungdua A, Babu E, Bessho F, Kawano T, Akimoto Y, Endou H, Yan K. Protein characterization of NA+-independent system L amino acid transporter 3 in mice: a potential role in supply of branched-chain amino acids under nutrient starvation. Am J Pathol. 2007;170:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Sekine Y, Nishibori Y, Akimoto Y, Kudo A, Ito N, Fukuhara D, Kurayama R, Higashihara E, Babu E, Kanai Y. Amino acid transporter LAT3 is required for podocyte development and function. J Am Soc Nephrol. 2009;20:1586-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Wang Q, Bailey CG, Ng C, Tiffen J, Thoeng A, Minhas V, Lehman ML, Hendy SC, Buchanan G, Nelson CC. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res. 2011;71:7525-7536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Wang Q, Tiffen J, Bailey CG, Lehman ML, Ritchie W, Fazli L, Metierre C, Feng YJ, Li E, Gleave M. Targeting amino acid transport in metastatic castration-resistant prostate cancer: effects on cell cycle, cell growth, and tumor development. J Natl Cancer Inst. 2013;105:1463-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Bodoy S, Martín L, Zorzano A, Palacín M, Estévez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002-12011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Guetg A, Mariotta L, Bock L, Herzog B, Fingerhut R, Camargo SM, Verrey F. Essential amino acid transporter Lat4 (Slc43a2) is required for mouse development. J Physiol. 2015;593:1273-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, Ueno A, Kanai Y, Endou H, Okayasu I. L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;59:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 23. | Ichinoe M, Mikami T, Yoshida T, Igawa I, Tsuruta T, Nakada N, Anzai N, Suzuki Y, Endou H, Okayasu I. High expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesions. Pathol Int. 2011;61:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Yanagisawa N, Ichinoe M, Mikami T, Nakada N, Hana K, Koizumi W, Endou H, Okayasu I. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J Clin Pathol. 2012;65:1019-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Kim CH, Park KJ, Park JR, Kanai Y, Endou H, Park JC, Kim DK. The RNA interference of amino acid transporter LAT1 inhibits the growth of KB human oral cancer cells. Anticancer Res. 2006;26:2943-2948. [PubMed] |
| 26. | Marshall AD, van Geldermalsen M, Otte NJ, Anderson LA, Lum T, Vellozzi MA, Zhang BK, Thoeng A, Wang Q, Rasko JE. LAT1 is a putative therapeutic target in endometrioid endometrial carcinoma. Int J Cancer. 2016;139:2529-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Hayashi K, Jutabha P, Endou H, Anzai N. c-Myc is crucial for the expression of LAT1 in MIA Paca-2 human pancreatic cancer cells. Oncol Rep. 2012;28:862-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Xu M, Sakamoto S, Matsushima J, Kimura T, Ueda T, Mizokami A, Kanai Y, Ichikawa T. Up-Regulation of LAT1 during Antiandrogen Therapy Contributes to Progression in Prostate Cancer Cells. J Urol. 2016;195:1588-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Liang Z, Cho HT, Williams L, Zhu A, Liang K, Huang K, Wu H, Jiang C, Hong S, Crowe R. Potential Biomarker of L-type Amino Acid Transporter 1 in Breast Cancer Progression. Nucl Med Mol Imaging. 2011;45:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Morimoto E, Kanai Y, Kim DK, Chairoungdua A, Choi HW, Wempe MF, Anzai N, Endou H. Establishment and characterization of mammalian cell lines stably expressing human L-type amino acid transporters. J Pharmacol Sci. 2008;108:505-516. [PubMed] |
| 31. | Oda K, Hosoda N, Endo H, Saito K, Tsujihara K, Yamamura M, Sakata T, Anzai N, Wempe MF, Kanai Y. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010;101:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 32. | Yun DW, Lee SA, Park MG, Kim JS, Yu SK, Park MR, Kim SG, Oh JS, Kim CS, Kim HJ. JPH203, an L-type amino acid transporter 1-selective compound, induces apoptosis of YD-38 human oral cancer cells. J Pharmacol Sci. 2014;124:208-217. [PubMed] |
| 33. | Rosilio C, Nebout M, Imbert V, Griessinger E, Neffati Z, Benadiba J, Hagenbeek T, Spits H, Reverso J, Ambrosetti D. L-type amino-acid transporter 1 (LAT1): a therapeutic target supporting growth and survival of T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia. Leukemia. 2015;29:1253-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Sharma P, Mukherjee A. Newer positron emission tomography radiopharmaceuticals for radiotherapy planning: an overview. Ann Transl Med. 2016;4:53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 35. | Wei L, Tominaga H, Ohgaki R, Wiriyasermkul P, Hagiwara K, Okuda S, Kaira K, Oriuchi N, Nagamori S, Kanai Y. Specific transport of 3-fluoro-l-α-methyl-tyrosine by LAT1 explains its specificity to malignant tumors in imaging. Cancer Sci. 2016;107:347-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Barth RF, Coderre JA, Vicente MG, Blue TE. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res. 2005;11:3987-4002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 685] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 37. | Das BC, Thapa P, Karki R, Schinke C, Das S, Kambhampati S, Banerjee SK, Van Veldhuizen P, Verma A, Weiss LM. Boron chemicals in diagnosis and therapeutics. Future Med Chem. 2013;5:653-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 38. | Detta A, Cruickshank GS. L-amino acid transporter-1 and boronophenylalanine-based boron neutron capture therapy of human brain tumors. Cancer Res. 2009;69:2126-2132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Yoshimoto M, Kurihara H, Honda N, Kawai K, Ohe K, Fujii H, Itami J, Arai Y. Predominant contribution of L-type amino acid transporter to 4-borono-2-(18)F-fluoro-phenylalanine uptake in human glioblastoma cells. Nucl Med Biol. 2013;40:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Wongthai P, Hagiwara K, Miyoshi Y, Wiriyasermkul P, Wei L, Ohgaki R, Kato I, Hamase K, Nagamori S, Kanai Y. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015;106:279-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 41. | Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I-III nonsmall cell lung cancer. Br J Cancer. 2008;98:742-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 42. | Betsunoh H, Fukuda T, Anzai N, Nishihara D, Mizuno T, Yuki H, Masuda A, Yamaguchi Y, Abe H, Yashi M. Increased expression of system large amino acid transporter (LAT)-1 mRNA is associated with invasive potential and unfavorable prognosis of human clear cell renal cell carcinoma. BMC Cancer. 2013;13:509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Ichinoe M, Yanagisawa N, Mikami T, Hana K, Nakada N, Endou H, Okayasu I, Murakumo Y. L-Type amino acid transporter 1 (LAT1) expression in lymph node metastasis of gastric carcinoma: Its correlation with size of metastatic lesion and Ki-67 labeling. Pathol Res Pract. 2015;211:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Kawashima O, Iijima H, Ishizuka T. Expression of L-type amino acid transporter 1 (LAT1) in neuroendocrine tumors of the lung. Pathol Res Pract. 2008;204:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Shi L, Luo W, Huang W, Huang S, Huang G. Downregulation of L-type amino acid transporter 1 expression inhibits the growth, migration and invasion of gastric cancer cells. Oncol Lett. 2013;6:106-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Janpipatkul K, Suksen K, Borwornpinyo S, Jearawiriyapaisarn N, Hongeng S, Piyachaturawat P, Chairoungdua A. Downregulation of LAT1 expression suppresses cholangiocarcinoma cell invasion and migration. Cell Signal. 2014;26:1668-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Kaji M, Kabir-Salmani M, Anzai N, Jin CJ, Akimoto Y, Horita A, Sakamoto A, Kanai Y, Sakurai H, Iwashita M. Properties of L-type amino acid transporter 1 in epidermal ovarian cancer. Int J Gynecol Cancer. 2010;20:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Zent R, Fenczik CA, Calderwood DA, Liu S, Dellos M, Ginsberg MH. Class- and splice variant-specific association of CD98 with integrin beta cytoplasmic domains. J Biol Chem. 2000;275:5059-5064. [PubMed] |
| 49. | Wierstra I, Alves J. The c-myc promoter: still MysterY and challenge. Adv Cancer Res. 2008;99:113-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 50. | Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol. 2006;16:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 413] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 51. | Kress TR, Sabò A, Amati B. MYC: connecting selective transcriptional control to global RNA production. Nat Rev Cancer. 2015;15:593-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 372] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 52. | Kim JW, Zeller KI, Wang Y, Jegga AG, Aronow BJ, O’Donnell KA, Dang CV. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol. 2004;24:5923-5936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 296] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 53. | Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275:21797-21800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 670] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 54. | Elorza A, Soro-Arnáiz I, Meléndez-Rodríguez F, Rodríguez-Vaello V, Marsboom G, de Cárcer G, Acosta-Iborra B, Albacete-Albacete L, Ordóñez A, Serrano-Oviedo L. HIF2α acts as an mTORC1 activator through the amino acid carrier SLC7A5. Mol Cell. 2012;48:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 55. | Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat Rev Cancer. 2014;14:801-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 56. | Tomblin JK, Arthur S, Primerano DA, Chaudhry AR, Fan J, Denvir J, Salisbury TB. Aryl hydrocarbon receptor (AHR) regulation of L-Type Amino Acid Transporter 1 (LAT-1) expression in MCF-7 and MDA-MB-231 breast cancer cells. Biochem Pharmacol. 2016;106:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Goberdhan DC, Wilson C, Harris AL. Amino Acid Sensing by mTORC1: Intracellular Transporters Mark the Spot. Cell Metab. 2016;23:580-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 58. | Rebsamen M, Pochini L, Stasyk T, de Araújo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519:477-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 539] [Cited by in RCA: 530] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 59. | Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2015;347:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 649] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 60. | Jung J, Genau HM, Behrends C. Amino Acid-Dependent mTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9. Mol Cell Biol. 2015;35:2479-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 61. | Chantranupong L, Scaria SM, Saxton RA, Gygi MP, Shen K, Wyant GA, Wang T, Harper JW, Gygi SP, Sabatini DM. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell. 2016;165:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 615] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 62. | Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 931] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 63. | He B, Zhang N, Zhao R. Dexamethasone Downregulates SLC7A5 Expression and Promotes Cell Cycle Arrest, Autophagy and Apoptosis in BeWo Cells. J Cell Physiol. 2016;231:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Imai H, Kaira K, Oriuchi N, Shimizu K, Tominaga H, Yanagitani N, Sunaga N, Ishizuka T, Nagamori S, Promchan K. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30:4819-4828. [PubMed] |
| 65. | Fan X, Ross DD, Arakawa H, Ganapathy V, Tamai I, Nakanishi T. Impact of system L amino acid transporter 1 (LAT1) on proliferation of human ovarian cancer cells: a possible target for combination therapy with anti-proliferative aminopeptidase inhibitors. Biochem Pharmacol. 2010;80:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Rodrik-Outmezguine VS, Okaniwa M, Yao Z, Novotny CJ, McWhirter C, Banaji A, Won H, Wong W, Berger M, de Stanchina E. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 2016;534:272-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 67. | Castilho BA, Shanmugam R, Silva RC, Ramesh R, Himme BM, Sattlegger E. Keeping the eIF2 alpha kinase Gcn2 in check. Biochim Biophys Acta. 2014;1843:1948-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 68. | Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 475] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 69. | Hayashi K, Anzai N. Role of LAT1 in the promotion of amino acid incorporation in activated T cells. Crit Rev Immunol. 2014;34:467-479. [PubMed] |
| 70. | Hayashi K, Ouchi M, Endou H, Anzai N. HOXB9 acts as a negative regulator of activated human T cells in response to amino acid deficiency. Immunol Cell Biol. 2016;94:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Kaira K, Sunose Y, Ohshima Y, Ishioka NS, Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N. Clinical significance of L-type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC Cancer. 2013;13:482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 72. | Kim DK, Kanai Y, Choi HW, Tangtrongsup S, Chairoungdua A, Babu E, Tachampa K, Anzai N, Iribe Y, Endou H. Characterization of the system L amino acid transporter in T24 human bladder carcinoma cells. Biochim Biophys Acta. 2002;1565:112-121. [PubMed] |
| 73. | Koshi H, Sano T, Handa T, Yanagawa T, Saitou K, Nagamori S, Kanai Y, Takagishi K, Oyama T. L-type amino acid transporter-1 and CD98 expression in bone and soft tissue tumors. Pathol Int. 2015;65:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Kobayashi K, Ohnishi A, Promsuk J, Shimizu S, Kanai Y, Shiokawa Y, Nagane M. Enhanced tumor growth elicited by L-type amino acid transporter 1 in human malignant glioma cells. Neurosurgery. 2008;62:493-503; discussion 503-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Haining Z, Kawai N, Miyake K, Okada M, Okubo S, Zhang X, Fei Z, Tamiya T. Relation of LAT1/4F2hc expression with pathological grade, proliferation and angiogenesis in human gliomas. BMC Clin Pathol. 2012;12:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 76. | Shennan DB, Thomson J, Gow IF, Travers MT, Barber MC. L-leucine transport in human breast cancer cells (MCF-7 and MDA-MB-231): kinetics, regulation by estrogen and molecular identity of the transporter. Biochim Biophys Acta. 2004;1664:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Shennan DB, Thomson J, Barber MC, Travers MT. Functional and molecular characteristics of system L in human breast cancer cells. Biochim Biophys Acta. 2003;1611:81-90. [PubMed] |
| 78. | Furuya M, Horiguchi J, Nakajima H, Kanai Y, Oyama T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012;103:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 79. | Kobayashi H, Ishii Y, Takayama T. Expression of L-type amino acid transporter 1 (LAT1) in esophageal carcinoma. J Surg Oncol. 2005;90:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 80. | Honjo H, Kaira K, Miyazaki T, Yokobori T, Kanai Y, Nagamori S, Oyama T, Asao T, Kuwano H. Clinicopathological significance of LAT1 and ASCT2 in patients with surgically resected esophageal squamous cell carcinoma. J Surg Oncol. 2016;113:381-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 81. | Namikawa M, Kakizaki S, Kaira K, Tojima H, Yamazaki Y, Horiguchi N, Sato K, Oriuchi N, Tominaga H, Sunose Y. Expression of amino acid transporters (LAT1, ASCT2 and xCT) as clinical significance in hepatocellular carcinoma. Hepatol Res. 2014; Oct 9; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 82. | Nikkuni O, Kaira K, Toyoda M, Shino M, Sakakura K, Takahashi K, Tominaga H, Oriuchi N, Suzuki M, Iijima M. Expression of Amino Acid Transporters (LAT1 and ASCT2) in Patients with Stage III/IV Laryngeal Squamous Cell Carcinoma. Pathol Oncol Res. 2015;21:1175-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 83. | Imai H, Kaira K, Oriuchi N, Yanagitani N, Sunaga N, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M. L-type amino acid transporter 1 expression is a prognostic marker in patients with surgically resected stage I non-small cell lung cancer. Histopathology. 2009;54:804-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 84. | Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Kanai Y, Endou H. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in early stage squamous cell carcinoma of the lung. Cancer Sci. 2009;100:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Kawashima O, Kamide Y, Ishizuka T. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in surgically resectable stage III non-small cell lung cancer. Exp Ther Med. 2010;1:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 86. | Shimizu A, Kaira K, Kato M, Yasuda M, Takahashi A, Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Oyama T. Prognostic significance of L-type amino acid transporter 1 (LAT1) expression in cutaneous melanoma. Melanoma Res. 2015;25:399-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 87. | Wang Q, Beaumont KA, Otte NJ, Font J, Bailey CG, van Geldermalsen M, Sharp DM, Tiffen JC, Ryan RM, Jormakka M. Targeting glutamine transport to suppress melanoma cell growth. Int J Cancer. 2014;135:1060-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 88. | Kühne A, Tzvetkov MV, Hagos Y, Lage H, Burckhardt G, Brockmöller J. Influx and efflux transport as determinants of melphalan cytotoxicity: Resistance to melphalan in MDR1 overexpressing tumor cell lines. Biochem Pharmacol. 2009;78:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Barollo S, Bertazza L, Watutantrige-Fernando S, Censi S, Cavedon E, Galuppini F, Pennelli G, Fassina A, Citton M, Rubin B. Overexpression of L-Type Amino Acid Transporter 1 (LAT1) and 2 (LAT2): Novel Markers of Neuroendocrine Tumors. PLoS One. 2016;11:e0156044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 90. | Kaira K, Nakamura K, Hirakawa T, Imai H, Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Tsukamoto N, Oyama T. Prognostic significance of L-type amino acid transporter 1 (LAT1) expression in patients with ovarian tumors. Am J Transl Res. 2015;7:1161-1171. [PubMed] |
| 91. | Kaira K, Sunose Y, Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br J Cancer. 2012;107:632-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 92. | Kaira K, Oriuchi N, Takahashi T, Nakagawa K, Ohde Y, Okumura T, Murakami H, Shukuya T, Kenmotsu H, Naito T. L-type amino acid transporter 1 (LAT1) expression in malignant pleural mesothelioma. Anticancer Res. 2011;31:4075-4082. [PubMed] |
| 93. | Toyoda M, Kaira K, Ohshima Y, Ishioka NS, Shino M, Sakakura K, Takayasu Y, Takahashi K, Tominaga H, Oriuchi N. Prognostic significance of amino-acid transporter expression (LAT1, ASCT2, and xCT) in surgically resected tongue cancer. Br J Cancer. 2014;110:2506-2513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 94. | Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Kanai Y, Endou H. L-type amino acid transporter 1 (LAT1) is frequently expressed in thymic carcinomas but is absent in thymomas. J Surg Oncol. 2009;99:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Hayashi K, Jutabha P, Maeda S, Supak Y, Ouchi M, Endou H, Fujita T, Chida M, Anzai N. LAT1 acts as a crucial transporter of amino acids in human thymic carcinoma cells. J Pharmacol Sci. 2016;132:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Nakanishi K, Ogata S, Matsuo H, Kanai Y, Endou H, Hiroi S, Tominaga S, Aida S, Kasamatsu H, Kawai T. Expression of LAT1 predicts risk of progression of transitional cell carcinoma of the upper urinary tract. Virchows Arch. 2007;451:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |