Published online Jul 15, 2016. doi: 10.4251/wjgo.v8.i7.563
Peer-review started: December 24, 2015
First decision: January 20, 2016
Revised: March 23, 2016
Accepted: April 20, 2016
Article in press: April 22, 2016
Published online: July 15, 2016
Processing time: 198 Days and 15.4 Hours
AIM: To investigate the prevalence of undernutrition, risk of malnutrition and obesity in the Italian gastroenterological population.
METHODS: The Italian Hospital Gastroenterology Association conducted an observational, cross-sectional multicenter study. Weight, weight loss, and body mass index were evaluated. Undernutrition was defined as unintentional weight loss > 10% in the last three-six months. Values of Malnutrition Universal Screening Tool (MUST) > 2, NRS-2002 > 3, and Mini Nutritional Assessment (MNA) from 17 to 25 identified risk of malnutrition in outpatients, inpatients and elderly patients, respectively. A body mass index ≥ 30 indicated obesity. Gastrointestinal pathologies were categorized into acute, chronic and neoplastic diseases.
RESULTS: A total of 513 patients participated in the study. The prevalence of undernutrition was 4.6% in outpatients and 19.6% in inpatients. Moreover, undernutrition was present in 4.3% of the gastrointestinal patients with chronic disease, 11.0% of those with acute disease, and 17.6% of those with cancer. The risk of malnutrition increased progressively and significantly in chronic, acute and neoplastic gastrointestinal diseases in inpatients and the elderly population. Logistical regression analysis confirmed that cancer was a risk factor for undernutrition (OR = 2.7; 95%CI: 1.2-6.44, P = 0.02). Obesity and overweight were more frequent in outpatients.
CONCLUSION: More than 63% of outpatients and 80% of inpatients in gastroenterological centers suffered from significant changes in body composition and required specific nutritional competence and treatment.
Core tip: The relevance of this study concerns the finding that in patients with gastroenterological disease, both prevalence of undernutrition and risk of malnutrition were higher in patients admitted to the hospital and in patients with cancer disease, while obesity and overweight were more frequently detected in outpatients. In conclusion, we can attest that two-thirds of gastroenterological patients suffered from abnormalities in body composition and required targeted nutritional treatments.
- Citation: Rizzi M, Mazzuoli S, Regano N, Inguaggiato R, Bianco M, Leandro G, Bugianesi E, Noè D, Orzes N, Pallini P, Petroni ML, Testino G, Guglielmi FW. Undernutrition, risk of malnutrition and obesity in gastroenterological patients: A multicenter study. World J Gastrointest Oncol 2016; 8(7): 563-572
- URL: https://www.wjgnet.com/1948-5204/full/v8/i7/563.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i7.563
Malnutrition is defined as a structural and functional alteration of the body composition. Although the term malnutrition is commonly used in the sense of undernutrition, it encompasses both weight loss (undernutrition) and weight gain (overweight and obesity). The physiological basis of undernutrition and obesity is the deficit (undernutrition) or the excess (obesity) of calories that results in measurable adverse effects on clinical outcomes[1-5]. The equilibrium between the total energy requirements, nutrient intake and utilization is mediated by hormonal and cytokine stimuli that induce the activation of intracellular metabolic pathways.
Previous papers have found that the prevalence of hospital undernutrition varies between 27% to more than 50% depending on the identification criteria, the medical or surgical setting and the age of the patients[6-10].
It is important to identify and treat undernutrition because it has been associated with a higher likelihood of hospitalization, a prolonged (by 29%-65%) hospital stay[11-15] and an increase in costs of up to 68%[16,17]. Moreover, it must be emphasized that hospital undernutrition worsens if untreated[18]. Today, many health care workers do not recognize malnutrition and do not consider nutrition as one of the more relevant aspects of the clinical management of patients[19].
The risk of undernutrition can be identified using different validated nutritional screening tools, such as the NRS-2002, MUST, and the MNA[20-22].
Of note, the prevalence of overweight and obesity has been steadily growing in the general population and among inpatients and represent important risk factors of morbidity and mortality[23-27].
Currently few data are available in the literature about the prevalence of undernutrition and the risk of malnutrition among Italian inpatients and outpatients, particularly in the gastroenterological setting. The Italian multicenter HOMIS study identified undernutrition in 19.1% and obesity in 24.8% of its patients[28]. Another Italian multicenter study, PIMAI, reported a prevalence rate of undernutrition of 30.7%, a risk of malnutrition, evaluated by the NRS-2002, of 28.6% and a rate of obesity of 21%[29,30].
The nutritional state of Italian gastroenterological patients has been described in an Italian multicenter study[31] that involved twenty-seven gastroenterology units. The study highlighted a mean of 27% of undernourished patients with a wide range (4% to 55%), probably due to the lack of appropriate nutritional assessment.
Consistent data on the prevalence of malnutrition in gastroenterology units have been limited to a few subsets of patients such as those with cirrhosis[32-36] and Crohn’s disease[37-39].
Similarly, the prevalence of obesity in Italian gastroenterology centers has not been adequately studied, although it is an important risk factor for many gastroenterological diseases[40-42], and it seems to affect approximately 12% of discharged patients[43].
The aim of this study is to measure the prevalence rate of undernutrition, risk of malnutrition and obesity in the Italian gastroenterological population suffering from acute, chronic and neoplastic disease.
This was an observational, cross-sectional and prospective multicenter study that was urged and supported by the Italian Hospital Gastroenterology Association (AIGO).
Nineteen Italian Gastroenterological Units and Services participated in the study, as disclosed by the AIGO website.
All inpatients and outpatients (day hospital and ambulatory) admitted between January 14 and 20, 2013 were consecutively enrolled in the study and submitted to nutritional assessment. As this was an observational study, the sample size was determined by the number of inpatients and outpatients who visited the various centers during the study week.
In this study, we enrolled three different categories of patients with gastrointestinal disease: (1) subjects with a diagnosis of acute gastrointestinal disease: Dysphagia, dyspepsia, heartburn, other abdominal pains, acute diarrhea, exacerbation of chronic diarrhea, acute constipation, digestive hemorrhage, acute hepatitis, cholangitis, rectum hemorrhage, inflammatory bowel disease (IBD) exacerbation, or acute pancreatitis; (2) patients with chronic gastroenterological disease: Reflux disease, achalasia, chronic gastritis, chronic viral hepatitis, chronic alcoholic hepatitis, non-alcoholic fatty liver disease, other chronic hepatitis, cirrhosis, chronic pancreatitis, Crohn’s disease and ulcerative proctocolitis, other chronic enteropathy, or chronic constipation; and (3) patients with gastrointestinal cancer: Esophagus, gastric, small bowel, colon, pancreatic, or biliary cancer and hepatocarcinoma. Patients were categorized as having (1) chronic disease, if the symptoms, signs and diagnosis had persisted for ≥ 6 mo; (2) acute disease, if the symptoms, signs and diagnosis had recently appeared, regardless of the presence of chronic disease; or (3) cancer, if affected by gastroenterological neoplastic disease, regardless of the presence of acute and/or chronic disease, as it was assumed that the presence of neoplastic disease itself was sufficient to determine further deterioration of a patient’s nutritional status.
Weight (kg) and height (centimeters) were measured in the morning in fasting patients dressed in their underwear and without shoes.
The body mass index (BMI) was calculated by dividing the weight in kg by the square of height in meters. Weight loss was calculated as the difference between the current weight and the weight in the last three-six months as reported by the patient. Patients were considered undernourished when they presented an unintentional (i.e., without voluntary dietary restriction) weight loss > 10% in the last 3-6 mo. Obesity was defined as a BMI ≥ 30, and overweight a BMI between 25 and 29.9. Obese patients were considered undernourished when they lost more than 10% of their weight while receiving their usual diet. The risk of malnutrition was calculated using three different specific tools, as shown in Table 1.
| Test | Subscore | Nutritional risk score | |||
| MUST | BMI (score: 0-2) | Unintentional weight loss (score: 0-2) | Acute disease able to significantly reduce nutrient intake in the following 5 d (score: 2) | ≥ 2 | |
| NRS-2002 | Pre-test: BMI < 2 0.5; weight loss, reduced caloric intake, acute disease | Nutritional risk: Weight loss, BMI, caloric intake (score: 0-3) | Severity of disease (score: 0-3) | Age ≥ 70-yr-old (score: 1) | > 3 |
| MNA | First part (six items: Caloric food intake, weight loss, motility, psychological stress, neuropsychological disease, BMI (score: 0-14) | If subscore ≤ 11: Complete second part | Second part (12 items: Home and nutritional autonomy, drugs, bedsores, daily meals, brachial and calf circumference; (maximal score of 16) | 17-25 | |
Specifically, the calculation of the three methods are described in detail in the following text: (1) the Malnutrition Universal Screening Tool (MUST) was used for outpatients. This method was based on the BMI, the unintentional weight loss and the presence of acute disease that was able to significantly reduce nutrient intake in the following five days. The total score ranged from 0-6; a score of 0 indicated null or low risk of malnutrition, a score of 1 suggested a moderate risk of malnutrition, and a score ≥ 2 was indicative of a severe risk of malnutrition[44]. We considered a score ≥ 2 to identify patients at nutritional risk.
The Nutritional Risk Screening Score 2002 (NRS-2002) has been recommended by ESPEN to screen inpatients. This test evaluates the nutritional risk (score: 0-3) and the severity of disease (score: 0-3) with an additional point for patients ≥ 70 years old. The final score ranges from 1 to 7. The patient is considered at nutritional risk for scores higher than 3[45].
The Mini Nutritional Assessment (MNA) is a tool for elderly patients (≥ 65 years). This tool has two parts. The first part consists of six items and results in a score between 0 and 14; a score lower than 12 is considered indicative of risk of undernutrition and leads to the patient answering the second part of the tool, which is composed of 12 items with a possible maximal score of 16. A total score < 17 is indicative of malnutrition, a score between 17 and 25 indicates risk of undernutrition, while scores > 25 are indicative of well-nourished patients[46-48].
We used the MUST and NRS-2002 to evaluate the risk of malnutrition in < 65-year-old patients and the MNA for those ≥ 65 years old.
This study was designed with the aim of obtaining epidemiological data and anthropometric measurements that did not compromise patient’s safety. All data were anonymous. Patients were referred using the first two letters of their name and surname and with a consecutive number.
A written informed consent statement was obtained from each patient prior to study inclusion. The study was conducted with the consensus of the local ethical committees of each center and of all the patients.
The results were presented as the mean ± SD. Categorical data were described as frequencies. The nonparametric Mann-Whitney U test was used to compare continuous variables, and χ2 test was used to compare categorical data. Two-tailed P values less than 0.05 were considered statistically significant.
Univariate and multivariate analyses were used to identify potential predictors of malnutrition. A linear trend test was used to assess associations with severity of disease. A stepwise backward logistical regression analysis, adjusted for age and gender and considering acute, chronic and neoplastic disease independent of each other, was performed to identify significant independent predictors of malnutrition. Statistical analyses were performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, United States).
Five hundred and ninety-seven patients were enrolled. We excluded from the statistical analysis five patients for incomplete data and 79 cirrhotic patients because of the negative effects of ascites and peripheral edema on nutritional parameters.
In Table 2 we report the anthropometric and nutritional data of the 513 patients enrolled in the 19 gastroenterology units participating in the study: 51.4% were males and 48.6% females, and the average age was 59.8 ± 17.8 years without a significant difference between the centers.
| Centers | n | M | F | Age (average ± DS) | Ambulatory | DH | Inpatients | MUST | NRS | MNA | Weight (kg) | Weight loss (%) | BMI |
| Bolzano | 21 | 61.90% | 38.10% | 67.5 ± 17.2 | 0.00% | 0.00% | 100.00% | - | 2.2 ± 1.5 | 16.8 ± 4.3 | 71.6 ± 16.4 | -4.7 ± 6.2 | 24.8 ± 4.4 |
| Fiemme del Cavalese | 17 | 57.1% | 42.9% | 56.2 ± 25.1 | 57.1% | 0.0% | 42.9% | 0.8 ± 1.3 | 1.7 ± 1.4 | 19.0 ± 9.9 | 66.9 ± 5.1 | -2.5 ± 6.9 | 23.1 ± 2.4 |
| Pordenone | 14 | 25.0% | 75.0% | 48.7 ± 24.0 | 100.0% | 0.0% | 0.0% | 1.0 ± 1.2 | - | 22.0 ± 0.0 | 58.3 ± 12.7 | 0.0 ± 0.0 | 21.3 ± 3.6 |
| Udine | 10 | 50.0% | 50.0% | 51.6 ± 8.7 | 70.0% | 0.0% | 30.0% | 0.4 ± 0.7 | - | 13.0 ± 0.0 | 75.5 ± 16.0 | -2.0 ± 5.7 | 25.9 ± 5.2 |
| Como | 12 | 72.2% | 27.8% | 65.3 ± 17.9 | 0.0 | 22.2% | 77.8% | 1.7 ± 2.1 | 2.3 ± 1.5 | 18.6 ± 5.4 | 73.5 ± 22.7 | -7.6 ± 9.4 | 25.8 ± 6.7 |
| Milano | 17 | 64.0% | 36.0% | 64.9 ± 19.2 | 0.0 | 0.0% | 100.0% | - | 1.7 ± 1.4 | 9.5 ± 3.1 | 69.4 ± 15.7 | -2.8 ± 7.4 | 25.2 ± 4.7 |
| Torino | 38 | 50.0% | 50.0% | 58.9 ± 16.1 | 0.0 | 55.3% | 44.7% | 1.6 ± 1.7 | 2.2 ± 2.2 | 15.8 ± 4.9 | 65.3 ± 16.6 | -0.9 ± 9.1 | 23.9 ± 4.5 |
| Alto Vicentino di Santorso | 32 | 40.6% | 59.4% | 68.2 ± 12.7 | 97.3 | 0.0% | 2.7% | 0.2 ± 0.6 | 0.5 ± 0.7 | 15.4± 4.6 | 71.3 ± 12.8 | -2.3 ± 4.9 | 25.0 ± 3.9 |
| Bassano del Grappa | 25 | 48.0% | 52.0% | 48.3 ± 17.9 | 60.0 | 0.0% | 40.0% | 0.5 ± 0.9 | 0.8 ± 1.4 | 17.6 ± 4.8 | 68.0 ± 14.5 | 0.1 ± 9.0 | 23.9 ± 4.3 |
| Legnano | 16 | 87.5% | 12.5% | 53.0 ± 16.1 | 0.0 | 0.0% | 100.0% | - | 1.1 ± 1.6 | 15.2 ± 4.6 | 66.1 ± 13.5 | -4.2 ± 12.3 | 23.0 ± 2.7 |
| Ravenna | 66 | 41.5% | 58.5% | 57.4 ± 19.4 | 52.5 | 21.9 | 25.6% | 0.8 ± 1.3 | 1.3 ± 1.4 | 19.6 ± 2.8 | 67.7 ± 17.4 | -1.5 ± 7.2 | 24.3 ± 5.4 |
| Ancona | 33 | 39.4 | 60.6 | 58.9 ± 19 | 100.0 | 0.0 | 0.0 | 0.1 ± 0.3 | - | 13.5 ± 4.8 | 70.8 ± 13.5 | 0.5 ± 2.5 | 25.7 ± 4.8 |
| Napoli | 26 | 53.8 | 46.1 | 59.1 ± 15.8 | 7.7 | 30.8 | 61.5 | 0.5 ± 0.9 | 1.3 ± 1.2 | 12.2 ± 2.7 | 67.9 ± 14.9 | -1.2 ± 3.8 | 24.7 ± 5.6 |
| Foggia | 33 | 39.4 | 60.6 | 57.7 ± 18.2 | 66.6 | 6,1 | 27.3 | 0.3 ± 0.9 | 0.4 ± 0.8 | 15.0 ± 3.3 | 76.5 ± 21.9 | -1.5 ± 3.0 | 28.6 ± 7.6 |
| Trani | 53 | 58.5 | 41.5 | 55.5 ± 18.1 | 62.1 | 10.3 | 27.6 | 0.5 ± 0.9 | 0.7 ± 1.1 | 13.4 ± 3.2 | 71.6 ± 14.5 | 1.1 ± 5.5 | 27.1 ± 5.5 |
| Cosenza | 58 | 53.9 | 46.1 | 58.8 ± 18.4 | 38.1 | 15.9 | 46.0 | 1.3 ± 1.7 | 2.1 ± 1.1 | 15.3 ± 3.7 | 67.0 ± 15.9 | -3.8 ± 7.9 | 24.5 ± 4.8 |
| Palermo | 15 | 64.7 | 35.3 | 64.2 ± 10.1 | 0.0 | 0.0 | 100.0 | - | 1.3 ± 0.6 | 13.0 ± 4.5 | 77.5 ± 21.3 | -1.3 ± 1.6 | 27.6 ± 6.7 |
| Marsala | 17 | 41.2 | 58.8 | 70.4 ± 9.5 | 88.2 | 0.0 | 11.8 | 0.6 ± 0.8 | 2.6 ± 0.9 | 18.0 ± 5.4 | 72.2 ± 12.4 | -2.2 ± 3.5 | 27.7 ± 3.7 |
| Siracusa | 10 | 60.0 | 40.0 | 67.9 ± 1.3 | 20.0 | 0.0 | 80.0 | 0.8 ± 1.2 | 2.6 ± 1.5 | 15.4 ± 4.6 | 61.8 ± 7.8 | -1.6 ± 4.9 | 23.9 ± 2.6 |
| Total | 513 | 51.4 | 48.6 | 59.8 ± 17.8 | 48.1 | 12.1 | 39.8 | 0.7 ± 0.5 | 1.5 ± 0.8 | 15.8 ± 2.9 | 69.7 ± 16.0 | -1.7 ± 6.9 | 25.3 ± 5.3 |
In total, 39.8% of the patients required hospitalization, 12.1% were DH patients and 48.1% were ambulatory patients. Approximately 30.8% of the patients were urgently hospitalized, 21.2% of the cases had a planned admission, 22.2% needed a follow-up evaluation and 25.8% were at their first medical visit.
In Table 3 we report the age, gender, medical evaluation setting and disease category of patients according to their age category.
| Total cohort (n = 513) | < 65 yr (n = 289) | ≥ 65 yr (n = 218) | P value | |
| Age (yr) (Mean ± SD) | 59.8 ± 17.8 | 47.4 ± 12.8 | 76.1 ± 6.9 | < 0.0011 |
| Male gender, | 260 (51.4) | 138 (47.8) | 122 (56.2) | 0.062 |
| Setting | < 0.0012 | |||
| Inpatient | 204 (39.8) | 93 (32.2) | 110 (50.7) | |
| Outpatient | 308 (60.2) | 196 (67.8) | 107 (49.3) | |
| Disease | < 0.0012 | |||
| Acute | 414 (72.1) | 232 (71.8) | 177 (72.2) | |
| Chronic | 96 (16.7) | 73 (22.6) | 22 (9.0) | |
| Neoplastic | 64 (11.1) | 18 (5.6) | 46 (18.8) |
Gastrointestinal diseases were acute in 72.1% of the cases, chronic in 16.7% and neoplastic in 11.1%. In particular, chronic diseases were more frequent in younger participants (22.6% vs 9.0% in older patients, P < 0.001), while neoplastic diseases were more represented in the elderly patients (5.6% in younger patients vs 18.8%, P < 0.001).
Table 4 shows the prevalence of each pathology included in the three categories: Acute disease, chronic diseases and cancers according to age.
| Chronic disease | % | < 65 yr | ≥ 65 yr | P value | Acute disease | % | < 65 yr | ≥ 65 yr | P value | Cancer | % | < 65 yr | ≥ 65 yr | P value |
| GERD | 8.90 | 35 | 18 | 0.123 | Dysphagia | 2.7 | 7 | 9 | 0.199 | Esophagus | 0.7 | 0 | 4 | 0.033 |
| Achalasia | 0.20 | 1 | 0 | 0.577 | Dyspepsia | 9.3 | 25 | 30 | 0.046 | Stomach | 1.3 | 3 | 5 | 0.219 |
| Chronic gastropathy | 5.90 | 17 | 18 | 0.174 | Epigastralgia | 12.6 | 44 | 31 | 0.44 | Small-large intestine | 3 | 5 | 13 | 0.01 |
| Chronic viral hepatitis | 4.20 | 17 | 8 | 0.194 | Other abdominalgia | 17.7 | 53 | 52 | 0.78 | Pancreas | 0.5 | 1 | 2 | 0.394 |
| Chronic alcoholic hepatitis | 3.20 | 14 | 5 | 0.113 | Acute diarrhea | 2.5 | 8 | 6 | 0.602 | Liver | 3.4 | 7 | 13 | 0.036 |
| NAFLD | 4.50 | 13 | 14 | 0.206 | Acute constipation | 1.5 | 7 | 2 | 0.18 | Biliary tree | 2 | 3 | 9 | 0.024 |
| Other chronic hepatitis | 1.30 | 3 | 5 | 0.211 | Digestive bleeding | 5.6 | 13 | 20 | 0.027 | |||||
| Compensated cirrhosis | 14.70 | 49 | 38 | 0.441 | Acute hepatitis | 3.4 | 13 | 7 | 0.315 | |||||
| Chronic pancreatitis | 1.20 | 4 | 3 | 0.632 | Cirrhosis complicated | 11.5 | 39 | 29 | 0.478 | |||||
| Non-IBD chronic diarrhea | 3.50 | 11 | 10 | 0.391 | Hematochezia | 3.5 | 7 | 14 | 0.022 | |||||
| Crohn's disease | 6.60 | 33 | 6 | 0.0002 | IBD exacerbation | 6.1 | 33 | 3 | 0.0000033 | |||||
| Ulcerative colitis | 4.5 | 24 | 3 | 0.00036 | Acute pancreatitis | 4.9 | 17 | 12 | 0.515 | |||||
| Chronic constipation | 5.2 | 13 | 18 | 0.053 | ||||||||||
| Celiac disease | 1.7 | 10 | 0 | 0.004 |
The mean prevalence of undernutrition in our population was 12.1%:4.6% in the ambulatory and DH settings and 19.6% in patients requiring hospitalization.
The undernourished patients were affected by chronic, acute and neoplastic disease at rates of 8%, 74% and 18%, respectively.
In Table 5 we report the distribution of undernutrition, risk of malnutrition, obesity and overweight among inpatients and outpatients in both categories of age. In both categories of age, the rate of undernourished inpatients was significantly higher compared to that of outpatients.
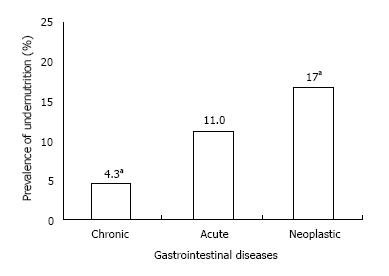
As shown in Figure 1, the prevalence of undernutrition in chronic, acute and neoplastic gastrointestinal diseases was 4.3%, 11.0% and 17.6%, respectively, with a prevalence significantly higher in neoplastic patients than in those with chronic disease (P = 0.03). The linear trend test showed that the prevalence of undernutrition was significantly higher in hospital admitted patients (P = 0.01).
The logistical regression analysis corrected for age, gender and acute, chronic and neoplastic disease showed that gastrointestinal cancer was a risk factor for undernutrition with an odds ratio of 2.7 (95%CI: 1.2-6.4, P = 0.02).
The mean prevalence of risk of malnutrition in our population was 23.8%. In Table 5 we show the prevalence of risk of malnutrition among inpatients and outpatients, and in Table 6 we report the prevalence of risk of malnutrition according to the three screening tests and age group. Of the < 65-year-old patients, the 24.7% of inpatients were at risk of malnutrition; in the elderly participants, the MNA screening tool revealed a risk of malnutrition in inpatients that was higher than that of elderly outpatients (69.2% vs 40.6%, respectively, P < 0.001).
| Nutritional risk test | Total cohort (n = 513) | < 65 yr (n = 289) | ≥65 yr (n = 218) | P value |
| MUST | 0.45 | |||
| < 2 (%) | 274 (89.0) | 172 (87.8) | 97 (90.7) | |
| ≥ 2 (%) | 34 (11.0) | 24 (12.2) | 10 (9.3) | |
| NRS 2002 | 0.002 | |||
| < 3 (%) | 112 (63.3) | 61 (75.3) | 51 (53.1) | |
| ≥ 3 (%) | 65 (36.7) | 20 (24.7) | 45 (46.9) | |
| MNA 17%-25% | 115 (54.2) | - | 115 (54.5) |
Of the outpatients at risk of malnutrition, acute and chronic gastrointestinal diseases had the same prevalence (50%); ten outpatients were affected by cancer, but none were at risk of malnutrition. In this population, only the 17.7% of patients with chronic gastrointestinal disease and 8.8% of those with acute gastrointestinal disease were at risk of malnutrition.
The inpatients at risk of malnutrition were affected by acute disease in 61.5% and by gastrointestinal cancer in 38.5% of the cases.
The elderly patients at risk of malnutrition presented chronic, acute and neoplastic disease in 5.2%, 67.0%, and 27.8% of the cases, respectively.
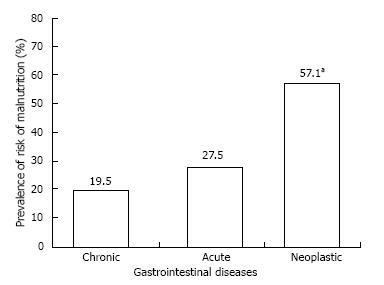
Figure 2 shows that the frequency of risk of malnutrition increased progressively with hospitalization, reaching statistical significance (P < 0.007) in the presence of neoplasm.
The prevalence of overweight and obesity in our population was, respectively, 28.6% and 15.6%. In Table 5, we report the rate of overweight and obesity in outpatients (31.7% and 18.3%, respectively) and in inpatients (25.5% and 13.0%, respectively) according to age group. Moreover, the rate of overweight and obesity did not show a difference in age. The prevalence of overweight and obesity was, respectively, 28.7% and 19.1% in chronic, 29.1% and 15.7% in acute, and 26.4% and 11.3% in neoplastic disease.
In our population, we found that no obese outpatients presented a weight loss > 10% in the last 3-6 mo, while the prevalence of risk of malnutrition was 15% higher in elderly subjects (36.8%). The rate of undernutrition was 12% in the hospitalized obese patients, and the prevalence of risk of malnutrition was 13.3% in young obese inpatients and increased up to 80% in those > 65 years old.
Of the overweight outpatients, the rate of undernutrition was 2%, while it was 8.9% in overweight inpatients. The rate of overweight outpatients at risk of malnutrition was 28.3%, but this rate reached 33.3% in those hospitalized.
This study was designed to define the nutritional state of patients with gastroenterological disease. Regarding the diseases included in this study, we want to specify that cirrhotic patients were excluded because of the obscuring effect of compartmentalized ascites on body weight[31,36,49]. However, diseases causing reduced albumin levels, which decreases oncotic pressure and allows for diffuse fluid leakage into the interstitial space, were not excluded, as fluid retention has to be considered a direct consequence of malnutrition.
Our data found that undernutrition affected approximately 12% of all patients; in particular, we documented that the rate of prevalence of undernutrition was significantly higher in inpatients (20%) than in outpatients. This is a consequence of the fact that acute diseases requiring hospitalization reduce caloric intake because of the presence of abdominal pain, diarrhea and vomiting. The use of tools to evaluate the risk of malnutrition should allow us to focus our attention on patients who have not yet begun to lose weight.
It was also interesting to note that younger and neoplastic inpatients were more frequently malnourished than the other groups, and this was due to the high rate of chronic inflammatory gastrointestinal diseases in our younger population (IBD: 11.4% vs 1.4% and celiac disease: 3.5% vs 0% in < 65-year-old and > 65-year-old patients, respectively) and the metabolic abnormalities characterizing cancer cachexia. Indeed, logistic regression confirmed that (1) the rate of undernutrition increased with the severity of disease; and (2) gastrointestinal cancer was a risk factor for undernutrition.
Very little data can be found in the literature concerning the prevalence rates of undernutrition in gastroenterology units. In our population, the prevalence of undernutrition was similar to those reported in other European[8] and Italian studies[28] but differed from other studies[9,29,30,50] because of both (1) the different tools used to evaluate malnutrition; and (2) the diverse stages of disease. In this study, we reported for the first time (1) the rate of undernutrition in gastroenterology outpatients; and (2) the correlation of undernutrition with hospital admission.
Regarding the risk of malnutrition, we want to emphasize that all of the screening tools we used were well-validated in identifying the patients who would develop undernutrition in the absence of an adequate nutritional care plan and that the NRS-2002 has shown a good predictive value for mortality, length of stay and complications. Few data have been published about the risk of malnutrition in the gastroenterology population[9,10,50]. In this study, we demonstrated that, in the gastroenterology department, inpatient and elderly patients had a greater frequency of risk of malnutrition than outpatients and younger patients, especially if affected by cancer; additionally, this risk was lower for those with chronic gastrointestinal disease. We must also highlight the fact that the NRS-2002 in inpatients was more frequently influenced by a reduction in caloric intake than by weight loss.
In our population, the NRS-2002 disclosed a risk of malnutrition in 36.7% of the inpatients. This value was lower than that reported in a Danish study evaluating gastro-surgery patients[9] but higher than data from Romanian gastroenterology departments[50].
Moreover, while young inpatients were undernourished or at risk of malnutrition at a similar prevalence, elderly inpatients were at a greater risk of malnutrition. These data indicate that the management of malnutrition in gastrointestinal departments should have different targets according to patients’ age: We should probably treat undernutrition with artificial nutrition in younger patients, while oral supplementation should be recommended to prevent malnutrition in elderly patients. Very limited data are available on the evaluation of the risk of malnutrition in outpatients. In this setting, our data indicated that patients with nutritional risk were more often affected by chronic disease, in which the catabolic effects of inflammatory cytokines influence nutritional status[4,51,52].
Overweight and obesity represent another aspect of malnutrition that entails excessive fat mass in body composition. In recent years, a number of works have reported an increasing incidence of obesity in the general population, but data on the prevalence of obesity and overweight in the gastroenterological population have been limited. The average prevalence of overweight-obesity in our study was 22.7%; this rate of obesity was lower than those reported in a hospital setting in previous Italian[28-30] reports but was higher than the Italian general population rate, especially in outpatients.
We also found that approximately 10% of the obese and overweight hospitalized patients were undernourished and that the risk of malnutrition was present in more than one-third of the obese and overweight gastroenterological patients, with rates that reached 80% in > 65-year-old obese inpatients.
The prevalence of obesity and overweight in outpatients was higher than in inpatients but was not statistically significant.
It is not surprising that in obese patients, undernutrition and risk of malnutrition could be present at the same time. An involuntary weight loss > 10% and/or reduction in caloric intake allowed us to identify patients who, despite having excess weight, met the criteria for malnutrition or risk of malnutrition. These data indicate that we must keep in mind that even obese patients can be malnourished and that we must investigate the risk of malnutrition especially among elderly gastroenterological patients, regardless of their weight at admission.
A possible bias of this study was the voluntary participation of the gastrointestinal units including centers that paid major attention to nutritional aspects. Another limitation of this work was the difference in the population size and in the frequency of disease, which was often determined by each center’s particular experience; it was thus difficult to study undernutrition and the risk of malnutrition for each single disease.
Overall, our data noted that 55% of inpatients and 22% of outpatients were undernourished and at risk of malnutrition and that half of the outpatients and nearly one-third of inpatients were obese or overweight. In our population, only 19.7% and 36.6% of inpatients and outpatients, respectively, did not present nutritional abnormalities (weight loss, risk of malnutrition, overweight or obesity). These data indicate that 80.3% of inpatients and 63.4% of outpatients would require nutritional competence in gastrointestinal units to assess the degree of malnutrition, to correctly design appropriate therapeutic programs to improve protein-caloric alterations and to prevent complications.
We thank the Collaborators: Simona Fregnan (Trani), Giusy Leogrande (Trani), Michael Kob (Bolzano), Marion Schrei (Bolzano), Monika Staffler (Bolzano), Ernestina Callegari (Bolzano), Lando Leila (Bolzano), Delia Tornifoglia (Bolzano), Fausto Chilovi (Bolzano), Giada Natali (Como), Anna Toldi (Como), Giancarlo Spinzi (Como), Lorella Bordandini (Ravenna), Tino Casetti (Ravenna), Marco Rosanna (Cosenza), Rosa Maria Lucibello (Cosenza), Leo Pietro (Cosenza), Rodolfo De Rocca (Torino), Cristina Rossino (Torino), Monica Roma (Torino), Francesco Manguso (Napoli), Elisabetta Riccio (Napoli), Matteo Antonino (Foggia), Maria Cocco (Foggia), Nicola Muscatiello (Foggia), Egiziano Peruzzi (Ancona), Sofia Bencivenni (AltoVicentino di Santorso-Vicenza), Gianluca Baldassarre (AltoVicentino di Santorso-Vicenza), Fabio Monica (Bassano del Grappa-Vicenza), Sonia Seligmann (Fiemme di Cavalese-Trento), Maria Cappello (Palermo), Maria Giovanna Cilluffo (Palermo), Antonio Craxì (Palermo), Giuseppe Milazzo (Marsala-Trapani), Antonietta Maria Scarpitta (Marsala-Trapani), Roberto Risicato (Augusta-Siracusa), Marco Marino (Udine), Valerio Zorzetto (Legnago-Verona), Stefania Maiero (Aviano-Pordenone), Renato Cannizzaro (Aviano-Pordenone). All the collaborators contributed to data acquisition.
Malnutrition has adverse effects on clinical outcomes, but many health care workers do not adequately consider it as a relevant aspect of the clinical management of patients. In the literature, few data are available regarding the prevalence of malnutrition (undernutrition, obesity,) and risk of malnutrition in European and Italian gastroenterology departments, with rates that depend on the criteria adopted for their identification, the medical or surgical setting and the age of the patients.
A better understanding of the prevalence of malnutrition and nutritional risk according to the severity of disease (chronic, acute and cancer) in outpatients and inpatients would facilitate the identification of patients with impairment of nutritional status and with adequate nutritional management.
For the first time, they studied undernutrition, risk of malnutrition (using three different nutritional screening tools) and obesity according to the severity of gastroenterological disease (chronic, acute and cancer) in both admitted patients and outpatients.
The authors’ data showed that there was a different distribution of undernutrition, risk of malnutrition and obesity according to the severity of disease and age group among inpatients and outpatients, which indicates that an appropriate nutritional care plan in gastrointestinal departments to achieve different nutritional targets may be needed.
Undernutrition: When patients presented an unintentional (i.e., without voluntary dietary restriction) weight loss > 10% in the last 3-6 mo; The MUST (Malnutrition Universal Screening Tool), NRS-2002 (Nutritional Risk Screening Score 2002) and MNA (Mini Nutritional Assessment) are three screening nutritional tests that identify patients at risk of malnutrition; BMI: Body mass index (calculated by dividing the weight in kg by the square of height in meters). Obesity: BMI ≥ 30; overweight: BMI between 25 and 29.9.
The authors have excluded cirrhotics because of the obscuring effect of ascitis and or edema. Inflammatory diseases may cause a drop in serum albumin levels that decrease oncotic pressure and favors fluid leakage to the interstitial space, that may reach up to 5 l before edema is clinically evident.
P- Reviewer: Garcia-Rodriguez MT, Gonzalez-Reimers E, Maiti S S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr. 2015;34:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1171] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 2. | Lancha A, Frühbeck G, Gómez-Ambrosi J. Peripheral signalling involved in energy homeostasis control. Nutr Res Rev. 2012;25:223-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul. 2009;43:157-168. [PubMed] |
| 4. | Roubenoff R. Molecular basis of inflammation: relationships between catabolic cytokines, hormones, energy balance, and muscle. JPEN J Parenter Enteral Nutr. 2008;32:630-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Amitani M, Asakawa A, Amitani H, Inui A. Control of food intake and muscle wasting in cachexia. Int J Biochem Cell Biol. 2013;45:2179-2185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ. 1994;308:945-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 850] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 7. | Imoberdorf R, Meier R, Krebs P, Hangartner PJ, Hess B, Stäubli M, Wegmann D, Rühlin M, Ballmer PE. Prevalence of undernutrition on admission to Swiss hospitals. Clin Nutr. 2010;29:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Edington J, Boorman J, Durrant ER, Perkins A, Giffin CV, James R, Thomson JM, Oldroyd JC, Smith JC, Torrance AD. Prevalence of malnutrition on admission to four hospitals in England. The Malnutrition Prevalence Group. Clin Nutr. 2000;19:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 253] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Rasmussen HH, Kondrup J, Staun M, Ladefoged K, Kristensen H, Wengler A. Prevalence of patients at nutritional risk in Danish hospitals. Clin Nutr. 2004;23:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Pirlich M, Schütz T, Norman K, Gastell S, Lübke HJ, Bischoff SC, Bolder U, Frieling T, Güldenzoph H, Hahn K. The German hospital malnutrition study. Clin Nutr. 2006;25:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 361] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 11. | Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 937] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 12. | Kyle UG, Pirlich M, Schuetz T, Lochs H, Pichard C. Is nutritional depletion by Nutritional Risk Index associated with increased length of hospital stay? A population-based study. JPEN J Parenter Enteral Nutr. 2004;28:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Caccialanza R, Klersy C, Cereda E, Cameletti B, Bonoldi A, Bonardi C, Marinelli M, Dionigi P. Nutritional parameters associated with prolonged hospital stay among ambulatory adult patients. CMAJ. 2010;182:1843-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1119] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 15. | Takahashi PY, Sauver JL, Olson TC, Huber JM, Cha SS, Ebbert JO. Association between underweight and hospitalization, emergency room visits, and mortality among patients in community medical homes. Risk Manag Healthc Policy. 2013;6:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Pérez de la Cruz A, Lobo Támer G, Orduña Espinosa R, Mellado Pastor C, Aguayo de Hoyos E, Ruiz López MD. [Malnutrition in hospitalized patients: prevalence and economic impact]. Med Clin (Barc). 2004;123:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Beck AM, Balknäs UN, Fürst P, Hasunen K, Jones L, Keller U, Melchior JC, Mikkelsen BE, Schauder P, Sivonen L. Food and nutritional care in hospitals: how to prevent undernutrition--report and guidelines from the Council of Europe. Clin Nutr. 2001;20:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Kyle UG, Kossovsky MP, Karsegard VL, Pichard C. Comparison of tools for nutritional assessment and screening at hospital admission: a population study. Clin Nutr. 2006;25:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Mowe M, Bosaeus I, Rasmussen HH, Kondrup J, Unosson M, Rothenberg E, Irtun Ø. Insufficient nutritional knowledge among health care workers? Clin Nutr. 2008;27:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1900] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 21. | Sorensen J, Kondrup J, Prokopowicz J, Schiesser M, Krähenbühl L, Meier R, Liberda M. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr. 2008;27:340-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 337] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 22. | Velasco C, García E, Rodríguez V, Frias L, Garriga R, Alvarez J, García-Peris P, León M. Comparison of four nutritional screening tools to detect nutritional risk in hospitalized patients: a multicentre study. Eur J Clin Nutr. 2011;65:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Ambrose T, Cullen S, Baker G, Smith M, Elia M, Leach R, De Silva A. Obesity: a window of opportunity to intervene? Characteristics and management of morbidly obese adult inpatients in three trusts in Southern England. Clin Med (Lond). 2013;13:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Migliore E, Pagano E, Mirabelli D, Baldi I, Gregori D, Zocchetti C, Tuzzi C, Balzola F, Petroni ML, Merletti F. Hospitalization rates and cost in severe or complicated obesity: an Italian cohort study. BMC Public Health. 2013;13:544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Luchsinger JA, Lee WN, Carrasquillo O, Rabinowitz D, Shea S. Body mass index and hospitalization in the elderly. J Am Geriatr Soc. 2003;51:1615-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Han E, Truesdale KP, Taber DR, Cai J, Juhaeri J, Stevens J. Impact of overweight and obesity on hospitalization: race and gender differences. Int J Obes (Lond). 2009;33:249-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Ringbäck Weitoft G, Eliasson M, Rosén M. Underweight, overweight and obesity as risk factors for mortality and hospitalization. Scand J Public Health. 2008;36:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Comi D, Palmo A, Brugnani M, D’Amicis A, Costa A, D’Andrea F, Del Toma E, Domeniconi D, Fusco MA, Gatti E. The hospital malnutrition Italian study. Clin Nutr. 1998;17:52. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Lucchin L, D’Amicis A, Gentile MG, Battistini NC, Fusco MA, Palmo A, Muscaritoli M, Contaldo F, Cereda E, the PIMAI group. An Italian investigation on nutritional risk at hospital admission: The PIMAI (Project: Iatrogenic MAlnutrition in Italy) study. Clin Nutrit Metabol. 2009;4:199-202. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Cereda E, Pedrolli C, Lucchin L, D’Amicis A, Gentile MG, Battistini NC, Fusco MA, Palmo A, Muscaritoli M. Fluid intake and nutritional risk in non-critically ill patients at hospital referral. Br J Nutr. 2010;104:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Guglielmi FW, Mastronuzzi T, Laddaga L, De Marco M, Scatigna F, Panella C, Francavilla A. Clinical utility of the bioelectrical impedance for evaluation body composition in patients with gastroenterological disease. Ital J Gastroenterol. 1990;22:250. |
| 32. | Guglielmi FW, Mastronuzzi T, De Marco M, Laddaga L, Scatigna F, Panella C, Francavilla A. Calorimetric data of cirrhotic patients with and without hepatocellular carcinoma: role of malnutrition. Ital J Gastroenterol. 1991;23:308-309. |
| 33. | Guglielmi FW, Panella C, Buda A, Budillon G, Caregaro L, Clerici C, Conte D, Federico A, Gasbarrini G, Guglielmi A. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism. Multicentre prospective study by the ‘Nutritional Problems in Gastroenterology’ Section of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis. 2005;37:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Loguercio C, Sava E, Marmo R, del Vecchio Blanco C, Coltorti M. Malnutrition in cirrhotic patients: anthropometric measurements as a method of assessing nutritional status. Br J Clin Pract. 1990;44:98-101. [PubMed] |
| 35. | Madden AM, Morgan MY. A comparison of skinfold anthropometry and bioelectrical impedance analysis for measuring percentage body fat in patients with cirrhosis. J Hepatol. 1994;21:878-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Morgan MY, Madden AM, Soulsby CT, Morris RW. Derivation and Validation of a New Global Method for Assessing Nutritional Status in Patients with Cirrhosis. Hepatology. 2006;44:823-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 37. | Capristo E, Addolorato G, Mingrone G, Greco AV, Gasbarrini G. Effect of disease localization on the anthropometric and metabolic features of Crohn’s disease. Am J Gastroenterol. 1998;93:2411-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Guglielmi FW, Rizzi M, Mazzuoli S, Regano N, Leogrande G, Addante A, Guglielmi A, Panella C, Francavilla A, Di Leo A. Negative energy balance in Crohn’s disease causes malnutrition and impaired muscle function. Dig Liver Dis. 2009;41S. [DOI] [Full Text] |
| 39. | Mazzuoli S, Addante I, Regano N, Rizzi M, Fregnan S, Leogrande G, Guglielmi A, Guglielmi FW. Prevalence of malnutrition in patients with moderate-severe Crohn’s disease: the role of NRS2002. Nutritional Therapy and Metabolism SINPE News. 2011;4:9. |
| 40. | Hussain Z, Quigley EM. Gastrointestinal issues in the assessment and management of the obese patient. Gastroenterol Hepatol (N Y). 2007;3:559-569. [PubMed] |
| 41. | Hurt RT, Kulisek C, Buchanan LA, McClave SA. The obesity epidemic: challenges, health initiatives, and implications for gastroenterologists. Gastroenterol Hepatol (NY). 2010;6:780-792. [PubMed] |
| 42. | Kirovski G, Schacherer D, Wobser H, Huber H, Niessen C, Beer C, Schölmerich J, Hellerbrand C. Prevalence of ultrasound-diagnosed non-alcoholic fatty liver disease in a hospital cohort and its association with anthropometric, biochemical and sonographic characteristics. Int J Clin Exp Med. 2010;3:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 43. | Vellinga A, O’Donovan D, De La Harpe D. Length of stay and associated costs of obesity related hospital admissions in Ireland. BMC Health Serv Res. 2008;8:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M, King C, Elia M. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br J Nutr. 2004;92:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 682] [Cited by in RCA: 794] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 45. | Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1754] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 46. | Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. 1996;54:S59-S65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 834] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 47. | Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, Albarede JL. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1317] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 48. | Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin Geriatr Med. 2002;18:737-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 622] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 49. | Fernandes SA, Bassani L, Nunes FF, Aydos ME, Alves AV, Marroni CA. Nutritional assessment in patients with cirrhosis. Arq Gastroenterol. 2012;49:19-27. [PubMed] |
| 50. | Gheorghe C, Pascu O, Iacob R, Goldis A, Tantau M, Dumitru E, Dobru D, Miutescu E, Saftoiu A, Fraticiu A. Nutritional Risk Screening and Prevalence of Malnutrition on Admission to Gastroenterology Departments: A Multicentric Study. Chirurgia (Bucur). 2013;108:535-41. |
| 51. | Catapano G, Pedone C, Nunziata E, Zizzo A, Passantino A, Incalzi RA. Nutrient intake and serum cytokine pattern in elderly people with heart failure. Eur J Heart Fail. 2008;10:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Yang YM, Sun TY, Liu XM. The role of serum leptin and tumor necrosis factor-alpha in malnutrition of male chronic obstructive pulmonary disease patients. Chin Med J (Engl). 2006;119:628-633. [PubMed] |