INTRODUCTION
Obstruction of the extrahepatic bile ducts from a malignant process presents both a diagnostic and therapeutic challenge. It is a common problem, with as many of 70% of pancreatic cancer patients presenting with obstruction upon diagnosis[1]. Obstruction may serve as the initial sign of disease - such as in the classic presentation of painless jaundice in pancreatic ductal adenocarcinoma - or may occur during progression of malignancy once the diagnosis is established. The two most common malignant neoplasms known to occlude the bile ducts are pancreatic ductal adenocarcinoma and primary bile duct cancer (cholangiocarcinoma). Other causes of malignant biliary obstruction can include ampullary carcinoma, primary duodenal adenocarcinoma, pancreatic neuroendocrine tumors, or occlusion of the hepatic hilum due to lymphadenopathy at the porta hepatis (as seen in metastatic colon cancer or lymphoma). Of note, some premalignant lesions such as biliary papillomatosis may cause an obstructive picture similar to malignancy. Benign conditions such as autoimmune cholangiopathy must also be ruled out, so obtaining tissue via endoscopic retrograde cholangiography (ERCP) with brush biopsy or core biopsy, or endoscopic ultrasound with fine needle aspiration (FNA) is paramount[2]. Only once a firm diagnosis of malignancy is secured can the final choice of treatment be made.
Occlusion of the bile ducts may cause debilitating symptoms such as pruritus and malaise, and thus treatment is often recommended on that basis alone. This may come in the form of surgical resection if the patient presents with resectable disease. However, both pancreatic cancer and cholangiocarcinoma are notorious for presenting at an advanced stage in which immediate surgery is contraindicated. Treatment goals for these patients include downstaging of the tumor with chemoradiotherapy, or strictly palliative measures. Relief of biliary obstruction is recommended in either setting. Treatment of distal malignant biliary obstruction from pancreatic cancer is typically managed by an endoscopically placed single biliary prosthesis, whereas hilar strictures can be more challenging to manage due to the need to access the left and right systems of the biliary tree.
Within the past decade endoscopic techniques have been developed to treat tumor ingrowth into the bile duct with photodynamic therapy or radiofrequency ablation, and recent studies show promise in expanding the role of endoscopic treatment. While the primary role at this time is to provide biliary decompression and relieve jaundice, the ability to provide therapy for these tumors represents a major shift in the role of the endoscopist. This review will consider the options for management based on the location of obstruction, as well as the stage of the underlying malignancy.
THE EFFECT OF JAUNDICE
The decision whether to decompress obstructed bile ducts in a patient with resectable disease has traditionally been quite controversial. Jaundice has long been recognized as an important preoperative risk factor in the setting of malignancy[3,4]. Several mechanisms have been described through which jaundice exerts its negative effects. Jaundice is thought to impair cellular immunity, allowing tumor growth and metastasic progression if left untreated[5]. In addition, obstruction to flow of bile decreases its availability in the enteric system for the absorption of lipid-soluble vitamins, including vitamin K, leading to coagulopathy and increased surgical bleeding risk. Even additional administration of oral vitamin K may be inadequate to reach appropriate levels of coagulation in obstructive cases[6], which may further complicate any planned surgery. Bacterial and endotoxin translocation through the intestinal mucosa has also been demonstrated in jaundiced patients, making SIRS and sepsis a serious complication that can develop even prior to surgery[6]. Jaundice has also been shown to increase the risk of infection if not treated before surgery[7], as well as complications after surgery[8]. Thus, there is a theoretical benefit to biliary drainage for relief of jaundice prior to surgical resection of these tumors.
It is thought the benefit of preoperative biliary drainage (PBD) may vary depending on the level of obstruction and planned surgery: Distal biliary obstruction from pancreatic cancer or distal cholangiocarcinoma may be treated surgically without the need for preoperative decompression, while hilar obstruction may require decompression to improve surgical outcomes. The differences in strategy likely stem from the need for partial hepatic resection in the treatment of hilar tumors, which may benefit from preoperative decompression.
MALIGNANT DISTAL BILIARY OBSTRUCTION
As many as 70% of patients with newly diagnosed pancreatic cancer have some degree of biliary tract obstruction at the time of diagnosis. Decompression via endoscopic stent placement can palliate jaundice and pruritus for symptomatic relief[9]. Stent placement may also speed allow the patient to begin chemotherapy regimens by reducing the risk of chemotoxicity in a cholestatic liver[10]. Endoscopic stent placement into the common bile duct is a fairly routine procedure (technically successful in over 90% of cases) and has thus become the most common method of achieving biliary decompression[11]. The choice of plastic or metallic stents depends on factors such as cost-effectiveness, expected length of survival, and diagnostic certainty. Over the past decade the use of self-expanding metal stents (SEMS) has become more common for treatment of both benign and malignant biliary strictures. While surgeons initially discouraged use of SEMS for pancreatic cancer due to concerns of increasing the difficulty of resection, SEMS do not interfere with planned pancreaticoduodenectomy as long as the stent does not involve the hilum[12]. Thus the role for SEMS in treatment of obstruction from pancreatic cancer has grown in recent years.
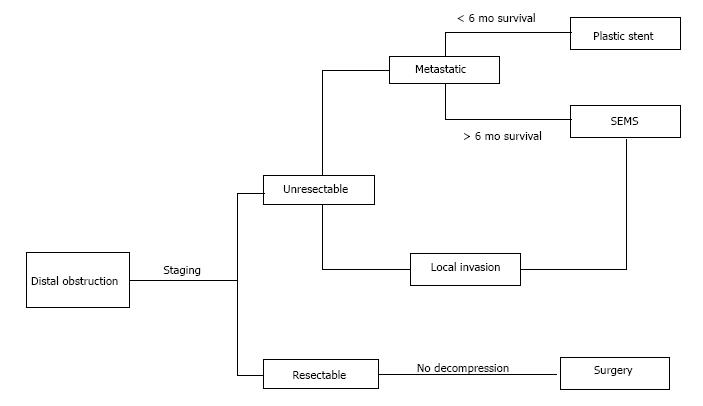
Distal cholangiocarcinoma has a similar presentation, pattern of spread, and poor prognosis when compared to pancreatic ductal adenocarcinoma. At times the two diseases may be indistinguishable from each other upon initial presentation. Distal cholangiocarcinoma tends to infiltrate the adjacent pancreas, duodenum, and vasculature as well as nearby lymphatics, leading to locally advanced disease and eventually metastases. Thus, the same staging evaluation can be performed to assess for local invasion, with the goal of curative surgical resection when possible[13]. Once the stage is known, similar principles of biliary decompression are applied as in pancreatic ductal adenocarcinoma. The management is based on disease stage as depicted in Figure 1.
Figure 1 Algorithm for treatment of distal malignant biliary obstruction based on disease stage.
Patients who are not candidates for ERCP with stent placement may undergo EUS-BD or percutaneous drainage. Adapted from Boulay BR, Parepally M. World J Gastroenterol 2014; 20: 9345-9353. SEMS: Self-expanding metallic stent; ERCP: Endoscopic retrograde cholangiography; EUS-BD: Endoscopic ultrasound-guided biliary drainage.
RESECTABLE DISEASE
Surgical resection is the definitive treatment for patients who present with early-stage pancreatic cancer[14]. PBD in resectable pancreatic cancer is not automatically recommended. Despite the beneficial effects of relieving jaundice, PBD has been associated with increased complications including various types of infections as well as pancreatic fistulas[15-17]. In 2010, van der Gaag et al[18] reported a randomized trial of 202 patients demonstrating that preoperative biliary drainage with stents was linked to increased complications compared to surgery alone in resectable pancreatic cancer. In this seminal study, rates of serious complications were 39% in the early-surgery group and 74% in the preoperative drainage group. Of note, the preoperative biliary drainage group waited 4 to 6 wk for surgery and were treated with plastic stents, both of which may have contributed to the poor performance of the biliary decompression group. However, based largely upon the experience noted by van der Gaag et al[18], preoperative biliary decompression for distal biliary obstruction is not recommended except to treat cholangitis or intractable pruritus.
LOCALLY ADVANCED DISEASE AND NEOADJUVANT THERAPY
Even those patients who undergo surgical resection of pancreatic cancer have poor long-term survival rates, so there is growing interest in the use of neoadjuvant therapy to boost outcomes, even for resectable pancreatic tumors[19]. Neoadjuvant chemoradiotherapy has also been used for locally advanced tumors to downstage them and permit eventual surgical resection. Biliary stents are placed prior to neoadjuvant chemoradiotherapy, with the expectation of remaining patent until the time of surgery. Unfortunately, this expectation has not always been met when plastic stents are used during the preoperative period.
Nuerous studies have now shown that SEMSs are preferable to plastic stents in patients undergoing neoadjuvant therapy[20-24]. Retrospective reviews of plastic stent performance during neoadjuvant therapy have demonstrated poor performance with the frequent need for unplanned stent exchange due to stent occlusion or cholangitis[20,25]. Adams et al[22] described a complication rate nearly 7 times higher with plastic stents, with a 3 times higher rate of hospitalization among a 52 patient cohort. SEMSs are clearly more expensive than plastic stents, but their lower occlusion rates (and thus fewer unplanned stent exchanges) make them a more cost effective choice for patients undergoing neoadjuvant chemoradiotherapy with planned surgical resection[26].
There are several choices of SEMS type for use in patients with malignant distal biliary obstruction undergoing neoadjuvant therapy or in palliative cases. When considering uncovered (USEMSs) or covered stents (CSEMSs), the difference in design leads to a trade-off in adverse events between tissue ingrowth in USEMS and stent migration in CSEMS. One recent meta-analysis concluded that CSEMSs afforded an average of 61 d longer patency than USEMSs in palliative cases, with the cost of an increased incidence in migration (RR 8.11)[27]. In contrast, a retrospective cohort study showed no difference in overall obstruction (CSEMSs 35% vs USEMSs 38%) among 749 patients, merely that the mechanisms of obstruction varied by stent design (tumor ingrowth vs debris )[28]. Partially covered SEMSs appear to have similar performance characteristics to fully covered SEMSs and USEMS[27,29].
Novel stent designs may further improve the performance of SEMS. A modified CSEMS with low axial force and uncovered flare ends has been developed with the goal of reducing stent migration, and when compared to USEMS had significantly longer patency (mean 219.3 d vs 166.9 d) and fewer unplanned procedures (23% vs 37%) compared to USEMSs[30]. Drug eluting stents have been designed in an attempt to improve SEMS prevent tumor ingrowth and stent occlusion[31]. An early multicenter prospective study using a paclitaxel-eluting stent did not show improved performance compared to conventional USEMS, though other stents are currently in development[32]. Anti-reflux stents have been developed to limit duodenal contents into the bile ducts and limit stent occlusion[23]. Initial experience with anti-reflux SEMS has yielded conflicting results, with one study showing long-term patency possibly exceeding conventional SEMS[33] while a smaller study showed a disappointing rate of early occlusion[34]. Further studies are needed to demonstrate the efficacy of anti-reflux and drug-eluting SEMS to determine their role in maintaining long-term stent patency.
Despite the wealth of data demonstrating the superiority of SEMS over plastic stents for malignant biliary obstruction, endoscopists may prefer to place a removable plastic stent if the diagnosis of malignancy is uncertain at that time of ERCP (such as in facilities where endoscopic ultrasound with FNA and rapid on-site evaluation by cytopathologists is not available). In this situation, benign conditions such as chronic pancreatitis or autoimmune cholangiopathy may be suspected as the etiology of the biliary stricture, and a removable stent is preferable. The use of a fully covered SEMS is ideal when suspicion of malignancy is high and life expectancy exceeds 4 mo.
PALLIATIVE DECOMPRESSION IN DISTAL OBSTRUCTION WITH METASTATIC DISEASE
In the setting of incurable pancreatic cancer, patients may often present with advanced disease and limited life expectancy. SEMS may not be cost-effective for these patients, since their main advantage of durable patency is not applicable. SEMSs cost 15-40 times more than most plastic stents, and are only cost effective if the patient survives > 4 mo[26]. Some authors have suggested that SEMSs should be used only in patients without distant metastases[35]. The presence of liver metastases has been shown to predict mortality in pancreatic cancer, though the data is sparse and generally used only to determine operative risk[36-38]. Although determination of life expectancy can be difficult and more research is needed to identify prognostic factors, endoscopists should be aware of the cost savings with plastic stents in patients with poor functional status and limited expected survival.
NON-ENDOSCOPIC BILIARY DRAINAGE IN MALIGNANT DISTAL OBSTRUCTION
In cases where ERCP fails or cannot be performed, percutaneous transhepatic biliary drainage (PTBD) has traditionally been used to create a tract for internal and external drainage. External drains have the potential downside of requiring emptying and flushing of the drain as well as routine drain exchange[39]. Recent trials of percutaneous SEMS placement have also demonstrated good safety and effectiveness[40-42]. An alternate second-line approach is endoscopic ultrasound-guided biliary drainage (EUS-BD), which has been increasingly shown to be both safe and effective when standard ERCP approaches fail. This technique can be used to achieve decompression via EUS-guided rendezvous procedure, EUS-guided choledochoduodenostomy, and EUS- guided hepatic gastrostomy[43]. Complications can include bile leak, bleeding, or pneumoperitoneum. EUS-BD remains technically complex and limited to high-volume expert centers[44].
Surgical biliary bypass remains an option, particularly when life expectancy exceeds 6 mo. Trials comparing surgical bypass to endoscopic therapy have shown similar mortality and fewer incidents of recurrent biliary obstruction when surgery was performed, though these trials preceded the widespread use of SEMS and do not reflect current endoscopic practice[45,46]. Surgical bypass can also relieve biliary and gastric outlet obstruction at the same time via creation of a surgical gastrojejunostomy and biliary bypass. However, biliary and gastroduodenal obstruction can also be relieved endoscopically during the same procedure, with placement of a duodenal SEMS followed by an endoscopic approach to the papilla for ERCP guided biliary stent placement or even EUS-BD[47-50]. Thus, while ERCP is the preferred method for management of malignant distal biliary obstruction, a multidisciplinary team including interventional radiologists and surgeons is ideal for management of unusually difficult strictures when ERCP fails.
HILAR CHOLANGIOCARCINOMA: SURGICALLY TREATABLE DISEASE
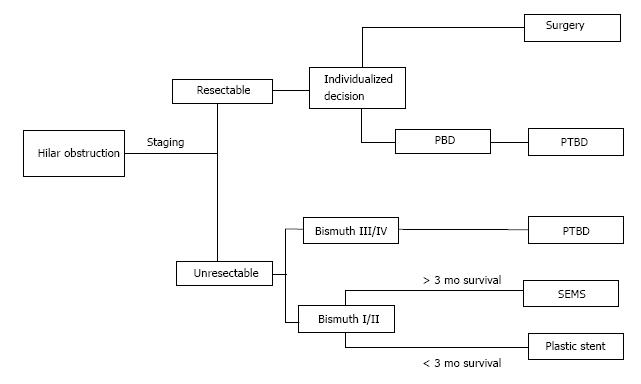
Malignant obstruction of the extrahepatic bile ducts at the liver hilum can be much more difficult to treat, given the possible involvement of the left and right hepatic ducts and the need to decompress the left and right lobes individually. Complete resection is the only curative treatment for cholangiocarcinoma. Unfortunately, most patients will present with obstructive symptoms or frank jaundice later in the disease course[51]. Resection modalities vary depending on the location of the malignancy: Intrahepatic tumors are treated with hepatic resection while extrahepatic tumors can be classified as hilar cholangiocarcinoma (Klatskin tumor) or distal cholangiocarcinoma. The level of the cystic duct demarcates hilar vs distal tumors. Hilar cholangiocarcinoma may still require partial hepatic resection as part of definitive management[52]. In patients with Bismuth class 4 strictures involving the left and right hepatic duct, or vascular involvement of the hepatic artery or portal vein, surgical resection is contraindicated and neoadjuvant or palliative techniques are indicated[53]. Figure 2 depicts the overall management of hilar obstruction based on disease stage.
Figure 2 Algorithm for treatment of hilar malignant biliary obstruction.
PBD is an individualized decision based on local expertise. PTBD: Percutaneous transhepatic biliary drainage; SEMS: Self-expanding metallic stent; PBD: Preoperative biliary drainage.
Several different imaging modalities can be used to determine the degree of proximal tumor extension. This is of critical importance in deciding on resectability and the optimal means of achieving biliary decompression. Computed tomography (CT) is the most commonly used type of imaging at the time of diagnosis, and multidetector CT (MDCT) has an accuracy of 86% in evaluating the ductal extent of hilar cholangiocarcinoma[54]. Magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP) provides detailed images of the biliary tree, though the presence of biliary stents can reduce the accuracy of staging[55]. The accuracy of MRI in determining hilar involvement is estimated at 89%, similar to that of MDCT. Both types of imaging also provide information regarding lymph nodes and direct invasion of nearby structures. In contrast, direct cholangiography by either ERCP or percutaneous means may not allow imaging of the entire biliary tree due to complete obstruction of some segments by tumor. Direct cholangiography is also invasive and presents risks of bleeding or infection which are avoidable with cross-sectional imaging. Thus, MDCT or MRCP are both acceptable initial imaging strategies for treatment planning, though the presence of stents may favor the use of MDCT.
When a patient with hilar cholangiocarcinoma presents with resectable disease, the treatment team must consider whether to pursue PBD. As with pancreatic cancer, preoperative biliary drainage has long been a debated topic, though most authorities advocate against it in the absence of cholangitis[56,57]. Drainage is recommended in special situations such as cholangitis as well as patients with symptomatic jaundice (e.g., pruritus) or renal failure[10]. In the absence of these factors, preoperative drainage has been previously discouraged on the basis that it confers no mortality benefit but may cause adverse events. Meta-analyses and systematic reviews of randomized controlled trials of preoperative biliary drainage have found increased complications and morbidity when PBD was performed, though overall mortality does not differ between the two groups[58,59].
Nonetheless, it is standard practice to perform preoperative biliary drainage in countries such as Japan and South Korea, and there is reason to believe it can be helpful in optimizing surgical outcomes with proper patient selection. One reason for this enthusiasm has been increased recognition of the flaws in earlier studies which argued against preoperative drainage. A large number of studies from 1980s to early 2000s did not have standardized timing to clear obstruction and jaundice, with some prolonged periods that contributed to stent occlusions and associated complications[60]. Even now, the optimal bilirubin level for surgery and optimal duration of PBD have not been established. The risk of drain occlusion or inflammatory change within the bile duct with prolonged drainage must be weighed against the risk of progressive obstruction on the outcome of liver resection during curative surgery. Thus preoperative biliary drainage is indicated when there will be a delay for surgery, such as in patients who undergo selective portal vein embolization in the setting of inadequate future liver remnant. In addition, the high-quality cholangiograms obtained in PBD can assist with treatment planning by delineating the extent of tumor involvement within the segmental bile ducts (though staging with MDCT or MRCP can provide adequate staging information without the risks of decompression).
It is possible that the benefit of PBD depends on the location of biliary obstruction within the hilum. Farges et al[61] performed a multicenter retrospective analysis of 366 patients undergoing extended right or left hepatectomy for hilar cholangiocarcinoma. PBD was not found to improve mortality overall, though a subgroup analysis revealed improved mortality in patients undergoing right hepatectomy (through reduced incidence of postoperative liver failure) while mortality was worse with left hepatectomy (through increased risk of sepsis)[61]. This intriguing data will require confirmation with additional studies but may help guide the controversial decision whether to perform PBD in the future.
HILAR CHOLANGIOCARCINOMA: TECHNIQUES FOR PREOPERATIVE BILIARY DRAINAGE
Currently three techniques are available for preoperative biliary drainage of hilar malignancy: PTBD, endoscopic nasobiliary drainage (ENBD), and endoscopic retrograde biliary drainage (ERBD) with stent placement. No randomized controlled trial has been performed to compare these techniques. For hilar tumors, ERBD can be technically challenging with the need to place multiple stents to drain obstructed biliary segments. The rate of complications is high, with reports showing morbidity of 25%-50% and mortality rates of 3%-5% with hilar tumors[62]. The complications are mainly due to cholangitis with stent failure or inadequate drainage[3,8].
ENBD has the advantage of providing drainage while allowing repeat cholangiography as needed prior to surgery with easy access to the drained intrahepatic segments[7]. Nasobiliary drainage is also a safe technique with fewer complications than PTBD[8]. However, nasobiliary tubes can be easily dislodged and may be poorly tolerated due to patient discomfort[63]. The use of multiple nasobiliary tubes to provide bilateral drainage for Bismuth IV hilar tumors has been performed but is technically demanding.
Percutaneous transhepatic biliary drainage has become the preferred method for preoperative biliary decompression in some centers. This technique is attractive due to its low complication rate when compared to endoscopic stent placement. Kloek et al[3] reviewed 101 patients who had undergone PBD and found 48% infection rate with ERBD, while PTBD was associated with only 9% infection rate. The chief problem with PTBD is the risk of tumor seeding along the drain tract, which is estimated at 5%-20%[7,8]. External transhepatic drains may cause patient discomfort and sometimes require additional oral intake of bile acid supplements, representing an uncomfortable nuisance to the patient[8]. Despite these drawbacks, the safety and comparative ease of drain placement (compared to ENBD) make PTBD a reasonable option depending on the local expertise of the treatment team.
HILAR CHOLANGIOCARCINOMA: PALLIATIVE THERAPY FOR MALIGNANT BILIARY OBSTRUCTION
While preoperative treatment tends to employ ENBD or PTBD, these therapies are less practical in the setting of unresectable disease. For patients with Bismuth I or II tumors, ERBD has similar performance to PTBD while being less invasive. However, patients with more advanced hilar obstruction (Bismuth III or IV) are more difficult to palliate with biliary stents. A retrospective review of 126 patients with Bismuth III-IV obstruction demonstrated higher success rates with PTBD over SEMS (93% vs 77%), though median survival was similar between the two groups[64]. Thus, PTBD is generally favored over endoscopic therapy even for palliation in advanced hilar obstruction.
The goal for palliative drainage is to relieve jaundice by draining an adequate liver volume (50% or more). This can be achieved with a single stent in Bismuth I tumors, though the strategy with more advanced hilar disease is more complex. Drainage of > 50% of the liver volume may require more than one catheter or stent, though the right lobe of the liver takes up 50%-60% of the liver volume and successful drainage of the right lobe with a single stent may be sufficient to relieve jaundice. The question of whether to place a single or multiple drains can be answered by volume assessment of the liver (volumetry) using cross-sectional imaging by CT or MRI. MRI imaging can be used to guide the placement of a single stent to access the dominant lobe and provide adequate drainage with a single stent[65].
Much like the treatment of distal obstruction with pancreatic malignancies, the past decade has seen increasing use of SEMS over plastic stents. There have been numerous studies that show significant advantages to metal stents vs plastic polyethylene stents[56]. The length of patency for SEMS compared to plastic is much higher (as much as 12 mo vs 3 mo), owing to the 8-10 mm diameter of SEMS compared to the narrower 7, 8.5 or 10 French plastic stents. Complications from occlusion or migration are less common, though SEMS are notably much more expensive than plastic stents[66]. Of course, patients with low performance status or advanced illness may not be expected to benefit from the prolonged duration of patency in SEMS; patients with life expectancy of < 3 mo would achieve the same benefit of palliation with lower costs using plastic stents. Cost analysis has demonstrated superiority of SEMS when patient survival is expected to exceed 3 mo[56,66-68]. As with pancreatic cancer patients, the treating endoscopist must consider expected length of survival when choosing an appropriate and cost-effective stent for palliation.
When bilateral or multisegmental stents are required for adequate biliary drainage, various techniques are available to place SEMS and take advantage of the prolonged duration of patency relative to plastic stents. Not all patients will require multiple SEMS; a meta-analysis by Sawas et al[69] shows no statistical difference in rate of failures or cholangitis between unilateral and bilateral SEMS placement for hilar tumors. Other retrospective data has favored the use of bilateral stents over unilateral stenting, with superior length of patency using multiple stents[70]. The two main techniques for use of multiple stents for hilar tumors are the “side by side” method, in which both stents are placed in parallel, or the “stent-in-stent” or “Y” method, in which the second stent is placed through the mesh interstices of the first stent with their distal ends overlapping[71]. There are no large studies indicating which method is superior in terms of technical or clinical success. Additional studies have looked at a 3-branch stent-in-stent to allow for better patency of stenting, with promising results but high degree of challenge for the endoscopist[72,73]. It is generally recommended that endoscopic therapy of advanced hilar strictures be performed by experienced endoscopists in a tertiary center with available backup by interventional radiologists and surgeons.
ENDOSCOPIC ADJUVANT TREATMENT OF BILIARY OBSTRUCTION: MOVING BEYOND STENTING
Patients with cholangiocarcinoma and pancreatic cancer continue to have notoriously poor prognoses when surgical cure is not an option. Meta-analyses have not shown significant improvement with standard chemoradiation regimens for biliary malignancies[74], likely due to late presentation and aggressive nature on presentation. However, two endoscopic therapies aimed at providing local control of malignant biliary obstruction have shown some promise in early studies.
Over the past decade the use of photodynamic therapy (PDT) has been studied for palliation of unresectable cholangiocarcinoma. This technique employs a photosensitizing molecule such as porfimer sodium which accumulates in tissue with rapid turnover such as malignant cells. After 48 h laser irradiation is then used to treat the tumor, leading to selective apoptosis within the tumor mass via generation of oxidative radicals. The application of oxygen and light can be performed through a cholangioscope for precise phototherapy administration to limit damage on normal tissue[75]. The first randomized controlled trial of PDT when compared to biliary stenting alone showed a dramatic increase in survival time from 98 d to 493 d[76]. Another RCT also showed median survival increased from 210 to 630 d[77]. Retrospective data also contributes to the body of information supporting increased survival and quality of life when PDT is used in additional to biliary stents as well as chemotherapy[78,79]. Side effects from phototherapy are mainly related to photosensitivity, requiring patients to avoid direct sunlight for 4-6 wk. In addition, the high cost of PDT may be a factor preventing its widespread use for local control of unresectable cholangiocarcinoma.
Radiofrequency ablation, previously used for colonic or esophageal malignancies as well as hepatocellular carcinoma, has also been increasingly studied for local treatment of biliary obstructive malignancies. Compared to PDT, it offers low cost and is technically simple to perform. RFA induces ablative necrosis and can be used to palliate known biliary malignancies by using a bipolar probe placed at the site of obstruction[75]. RFA can be performed percutaneously or via a catheter inserted via ERCP. Ablation uses 7-10 W bursts to create coagulative necrosis of the intraductal tumor mass and when performed via ERCP is followed by biliary stent placement[51,75]. Plastic stents are applied when future ablations are planned, while SEMS may be used when a single session is planned. The risk of adverse events is low but includes hemobilia and biliary fistulas. The body of literature supporting RFA for biliary malignancies is not as robust as that for PDT, consisting mostly of retrospective series[80]. A retrospective comparison by Strand et al[81] compared results in 48 patients (16 RFA, 32 PDT) which demonstrated similar median survival (9.6 mo in RFA, 7.5 mo in PDT). Future studies will be required to determine the optimal techniques for RFA, as well as the patient populations who are most likely to benefit. European studies have also investigated the use of RFA therapy to treat occlusion of SEMS without the need for additional stent placement[82].
The role of RFA in distal malignant biliary obstruction has not been defined, though early experience is encouraging. In a retrospective study of 20 patients undergoing RFA of biliary strictures, 8 patients had distal obstruction due to pancreatic adenocarcinoma or intraductal papillary mucinous neoplasm. The study showed a median increase of 3.5 mm in bile duct diameter following RFA treatment, with maintenance of stent patency at 30 d[83]. Similarly, a registry of 69 patients who underwent RFA for malignant biliary obstruction included 19 patients with pancreatic cancer. Again, the median diameter of the bile duct improved following RFA treatment. Interestingly, the pancreatic cancer patients responded better to RFA than cholangiocarcinoma patients, with RR 1.8 for stricture improvement[84]. Other outcomes such as length of survival have not yet been studied in these patients. While further data is needed, including high-quality prospective data, the ability to achieve local control and prolong survival in these diseases using endoscopic therapies is an exciting prospect.
CONCLUSION
The management of malignant biliary obstruction requires consideration of several factors prior to the act of decompression via endoscopic or percutaneous means. The location of the stricture and underlying malignancy will affect the approach, as hilar stricture may be much more difficult to treat compared to simple strictures of the distal common bile duct. The stage of underlying malignancy also plays a role, as resectable disease may not typically require preoperative biliary drainage. For most cases, preoperative biliary drainage is discouraged due to the high incidence of infectious complications. In patients undergoing neoadjuvant therapy for locally advanced disease, SEMS appear to be the optimal approach for distal strictures while most data for hilar strictures appears to favor PTBD or ENBD. For palliation in advanced malignancy, SEMS are generally favored if life expectancy exceeds 3-4 mo, while plastic stents are favored in patients with particularly poor prognosis. The use of photodynamic therapy and radiofrequency ablation to achieve local control of these malignancies is an important step in prolonging survival, and presents an opportunity for endoscopists to have an increased role beyond stent placement in improving the quality and quantity of life for patients with cancer and biliary obstruction.
P- Reviewer: Espinel J, Kassir R, Li W, Mercado MA, Pinho R S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ