Published online May 15, 2016. doi: 10.4251/wjgo.v8.i5.474
Peer-review started: October 21, 2015
First decision: November 27, 2015
Revised: February 1, 2016
Accepted: March 7, 2016
Article in press: March 9, 2016
Published online: May 15, 2016
Processing time: 203 Days and 16.7 Hours
AIM: To evaluate impact of radiation therapy dose escalation through intensity modulated radiation therapy with simultaneous integrated boost (IMRT-SIB).
METHODS: We retrospectively reviewed the patients who underwent four-dimensional-based IMRT-SIB-based neoadjuvant chemoradiation protocol. During the concurrent chemoradiation therapy, radiation therapy was through IMRT-SIB delivered in 28 consecutive daily fractions with total radiation doses of 56 Gy to tumor and 5040 Gy dose-painted to clinical tumor volume, with a regimen at the discretion of the treating medical oncologist. This was followed by surgical tumor resection. We analyzed pathological completion response (pCR) rates its relationship with overall survival and event-free survival.
RESULTS: Seventeen patients underwent dose escalation with the IMRT-SIB protocol between 2007 and 2014 and their records were available for analysis. Among the IMRT-SIB-treated patients, the toxicity appeared mild, the most common side effects were grade 1-3 esophagitis (46%) and pneumonitis (11.7%). There were no cardiac events. The Ro resection rate was 94% (n = 16), the pCR rate was 47% (n = 8), and the postoperative morbidity was zero. There was one mediastinal failure found, one patient had local failure at the anastomosis site, and the majority of failures were distant in the lung or bone. The 3-year disease-free survival and overall survival rates were 41% (n = 7) and 53% (n = 9), respectively.
CONCLUSION: The dose escalation through IMRT-SIB in the chemoradiation regimen seems responsible for down-staging the distal esophageal with well-tolerated complications.
Core tip: There are more data supporting neoadjuvant chemoradiation for locally advanced esophageal cancer. The best regimen of neoadjuvant chemoradiation remains to be defined, current available data using three-dimensional vs intensity modulated radiation therapy deliver modest dose to downstage the tumor. In this report, we reviewed our experience using dose escalation technique to Gross Tumor Volume with compromising dose to organ at risk, the high R0 resection rate results suggest the feasibility of using this approach for future prospective study.
- Citation: Zeng M, Aguila FN, Patel T, Knapp M, Zhu XQ, Chen XL, Price PD. Intensity modulated radiation therapy with simultaneous integrated boost based dose escalation on neoadjuvant chemoradiation therapy for locally advanced distal esophageal adenocarcinoma. World J Gastrointest Oncol 2016; 8(5): 474-480
- URL: https://www.wjgnet.com/1948-5204/full/v8/i5/474.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i5.474
Distal esophageal cancer is a commonly lethal malignancy, with the annual death rate in the United States about 15590[1]. Most single-modality treatment regimens provide poor cure rate. For example, surgical treatment alone has a 5-year survival of approximately 15% to 20%[2,3]. Radiation therapy alone for resectable highly selected squamous cell cancer results in a 5-year survival rate of approximately 34%[4]. In recent decades, more data have emerged for locally regional esophageal cancer management. There are reports that suggest triple-modality treatment regimens provide better local control, better control of distant metastases, and better overall survival[5]. Meta-analysis showed a survival benefit for patients treated with preoperative chemoradiotherapy (CRT) compared with surgery alone[6]. We have been using triple-modality approach for locally advanced distal esophageal cancer. Although the results from CROSS studies demonstrated the triple-modality approach to be well-tolerated with survival benefit, the absolute benefit for adenocarcinoma remains small and the survival benefit for adenocarcinoma is not statistically significant[7], with a 17% pathological complete response (pCR). There is clear evidence correlating pCR and better local control with improved survival[8]. However, there are few reports regarding the role of radiation dose variation in triple-modality therapy. Understanding dose change and its impact on tumor response provides insight into the effectiveness of the combined treatment and may lead to improved survival. Therefore, we analyzed our experience with dose escalation through the intensity modulated radiation therapy with simultaneous integrated boost (IMRT-SIB) technique, with emphasis on the relationship of the site of pCR to the radiation dose escalation delivered to the gross tumor volume.
Our Institutional Review Board approved this study. The patients included in this study were treated at our institution for locally advanced esophageal cancer between January 2007 and December 2014. The inclusion criteria were pathologically confirmed adenocarcinoma and no past or current history of malignancy or radiation treatment in the chest or abdomen. Written informed consent was obtained from all patients before starting treatment. All patients underwent staging and those with no distant disease and medically operable patients received neoadjuvant CRT for 5-6 wk. The resectable disease included stage cT1N1M0 or cT2-3N0-1M0 (Table 1)[9].
| Characteristic | Value |
| Mean age (range) | 65 yr (45-76) |
| Men/women (n) | 14/3 |
| ECOG PS (n) | |
| 0 | 14 |
| 1 | 3 |
| 2 | 0 |
| 3 | 0 |
| T stage (n) | |
| T1 | 0 |
| T2 | 2 |
| T3 | 15 |
| T4 | 0 |
| N stage (n) | |
| N0 | 12 |
| N1 | 5 |
| Concurrent chemotherapy (n) | |
| 5-FU/cisplatin | 11 |
| Carboplatin/Taxel | 6 |
| Tumor location: < 35 cm/> 35 cm (n) | 14/3 |
| Mean gross tumor volume (cm3) (range) | 43.7 (33.3-62.4) |
| Mean clinical tumor volume (cm3) (range) | 503.2 (419.3-577.1) |
Pretreatment staging included elaborate history taking, physical examination, routine blood studies, pulmonary function tests, an upper gastrointestinal endoscopy with biopsies, and computed tomography (CT) of chest, abdomen and neck. Endoscopic ultrasound was used routinely for staging of esophageal tumors if technically possible. All patients in this analysis underwent positron emission tomographic (PET) scanning as part of staging to better define gross tumor volume.
All treatment began with concurrent chemoradiation. Chemotherapy consisted of five cycles of concurrent platin-based weekly chemotherapy or 5-fluorouracil (5-FU)-based daily chemotherapy. The platin-based chemotherapy was given at a dose of 40-100 mg/m2, starting on days 1, 8, 15, 22 and 29. The 5-FU-based chemotherapy was either continuous infusion CIV 5-FU (225 mg/m2) or oral capecitabine (capecitabine, Genentech, San Francisco, CA) 750 mg/m2 twice daily starting on day 1 to day 28.
The radiation therapy could be delivered through four-dimensional (4D) plus IMRT with SIB. All patients who received radiation therapy started with CT-based 4D treatment simulations. The simulation was performed with the patient in the supine position using immobilization with the patient’s arms over the head. The 4D simulations were performed if respiratory gating was feasible, otherwise, free-breathing 3D CT acquisition data would be obtained during simulation, the patients then were excluded from the IMRT-SIB protocol. All treatment planning in this series was performed by the same radiation oncologist. During the treatment planning, two target volumes were drawn gross tumor volume and clinical target volume, PET/CT imaging obtained within 1-3 wk prior to simulation data. Patients who did not have PET/CT data were excluded from the IMRT-SIB protocol. Clinical target volume and the clinical internal target volume reflected the microscopic sites of highest risk. The treatment planning target volume (PTV) for clinical target volume is about 5 mm beyond clinical target volume; the clinical target volume was contoured based on the Radiation Therapy Oncology Group consensus study protocol[10]. The treatment PTV for gross tumor volume had no margins.
There are total 17 patients received SIB technique and included in this study, after resection the postoperative T stages of the analyzed tumors were as follows: ypT0 (n = 8, 47%); ypT1 (n = 4, 23.5%); ypT2 (n = 3, 17.6%); ypT3 (n = 2, 11.7%). Nodal disease was confirmed in three patients (17.6%) by pathological staging and the median number of assessed lymph nodes was 13 (range 3-27) (Table 2). There were five pulmonary complications (29%), one cardiac complication (5.8%), and six surgical complications (35%). There were no treatment-related or operative deaths (Table 3).
| Pathological staging | Patient n (%) |
| ypT | |
| 0 | 8 (47) |
| 1 | 4 (23) |
| 2 | 3 (18) |
| 3 | 2 (12) |
| 4 | 0 |
| ypN | |
| 0 | 13 (76.5) |
| 1 | 4 (23.5) |
| Pulmonary | 5 |
| Pneumonia | 3 |
| Pneumonitis | 2 |
| Cardiac1 | 1 |
| Surgery related | 6 |
| Anastomotic leakage | 4 |
| Anastomotic stricture | 1 |
| Wound infection | 1 |
| Death within admission | 0 |
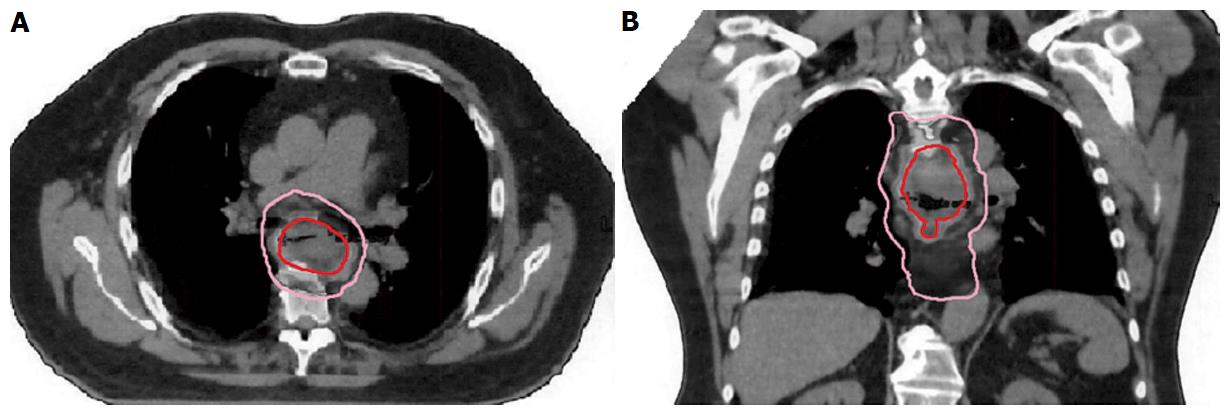
The time required to finish radiation treatment ranged from 28-35 d. Clinical tumor volume (CTV) represents the conventional dose coverage, 5040 in 28 fractions and PET-positive alone target area will receive SIB to 5600 in 28 fractions, which is labeled as gross tumor volume (Figure 1). The PTV of 180 cGy per fraction provided a proximal and distal margin of 5 cm and a radial margin of 7 mm around the CTV volume except to the heart with approximately 3-5 mm margins. The average beam number was 6.3 (range, 5-9). All organs at risk met their dose constraints. The daily prescription dose of 2 Gy was specified at the International Commission on Radiation Units and Measurement reference point, and at least the 95% isodose had to encompass the entire PTV. The maximum dose to the PTV was not to exceed the prescription dose by 7%. Tissue density inhomogeneity correction was used. The 4D plan using respiratory gating technique applied to all patients.
Patients were followed routinely after finishing neoadjuvant chemoradiation. Surgical resection was performed between 6-8 wk after completion of CRT. The operative technique consisted of a transthoracic approach with a two-field lymph node dissection or a transhiatal approach, depending on tumor localization. A wide local excision of the N1 lymph nodes, including standard excision of the celiac nodes, was carried out in both techniques. Continuity of the digestive tract was restored by gastric tube reconstruction or colonic interposition procedure with cervical anastomosis.
For grading of the therapy response, the degree of histomorphologic regression was classified into four modified categories, as described by Mandard et al[11]. Surgical margins were designated in accordance with the criteria of the AJCC staging manual. All resection margins, including circumferential margins, were evaluated for vital tumor with a cutoff point of 1 mm. Margin status was confirmed by frozen and permanent sections and the close distance to the nearest millimeter between cancer cells, and the margin was measured microscopically and recorded prospectively. The operation was defined as an R0 resection if there was no microscopic tumor found at the margin and as an R1 resection if a margin was positive microscopically.
Duration of follow-up was defined as the interval between the day of completed surgery, death, or the last follow-up visit or telephone call. The Kaplan-Meier method was used to calculate survival probabilities. Survival analyses were performed using Prism Graph Pad Version 5.00 (GraphPad Software, Inc., LaJolla, CA).
During the study period, 57 patients underwent neoadjuvant chemoradiation therapy followed by esophagostomy. After exclusion of patients with insufficient data, those who did not meet study criteria, and those without adequate follow-up (n = 22), the records of 35 patients were reviewed. Of the 35, 19 patients were eligible and enrolled in the IMRT-SIB treatment protocol and two patients without histological identification of adenocarcinoma were excluded from this analysis. Thus, 17 patients completed the IMRT-SIB treatment and were included in this study. There were 15 men and 2 women and the mean postoperative follow-up was 2.5 years (range from 0.22-6.5).
All staging was performed before any treatment began. The mean age at time of diagnosis was 65 years (range, 45-76 years) and 15 patients were men. Of all patients, 88% had a uT3 tumor. A microscopically radical (R0) resection was achieved in 94% of patients. One patient had an R1 resection due to persistent disease in the gastric cardia. Total pathologic complete response ypT0No is 47%. The node-negative patient after neoadjuvant chemoRT is 82% (Table 2). Table 2 shows the pathologic staging and effects of SIB-based neoadjuvant CRT. The pathologic stages of cancer were T0, T1, T2, T3, and T4 in 8 (47%), 4 (23%), 3 (18%), 2 (12%), and 0 (0%), respectively. A comparison of clinical and pathologic stages revealed that SIB-based neoadjuvant CRT resulted in down-staging of either the T or the N status of 13 (76.5%) patients.
Hematologic toxicity from neoadjuvant chemoradiation with SIB was mild for all patients. A few patients received granulocyte-colony stimulating factor for neutropenia that did not occur during CRT. Among non-hematologic adverse effects, esophagitis (n = 10, 58.8%) and pneumonia (n = 3, 17%) was more common than pneumonitis (n = 2, 11%). Surgical leak was the most common surgical-related complication (n = 4, 23.5%) (Table 3). No survivors had symptoms due to late toxicities such as accumulated pleural or cardiac effusions during long-term follow-up. There were no treatment-related deaths.
After a minimum follow-up of 22 mo and a mean survival of 29 mo, the local recurrence rate was 11% (n = 2). Most patients had distant failure (35%) or combined local/regional and distant failure (5%). The majority of local recurrences were within 2 years of follow-up. In addition, the anastomosis was the only recurrence site in 5.8% of patients. There was one mediastinal relapse associated with positive nodes that was treated with a full dose of IMRT-SIB. There was no peritoneal carcinomatosis found.
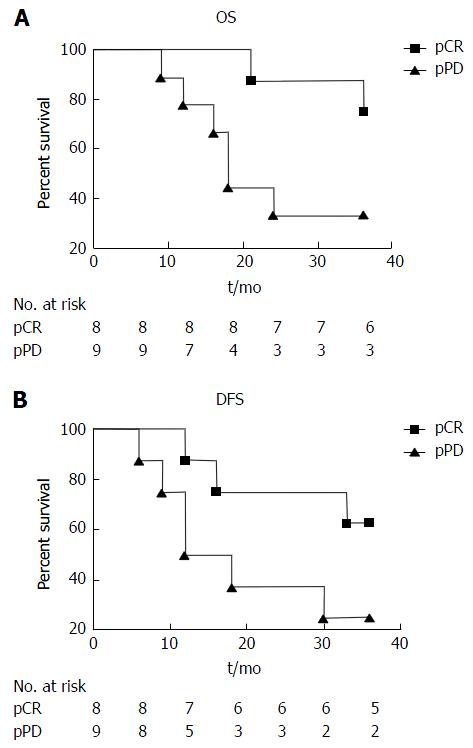
For the 17 patients analyzed, nine were alive and eight had died at the end of follow-up, for a 3-year overall survival rate of 52% (Figure 2). Among the pCR, the 3-year overall survival was 75%, and compared with pathological persistent disease (pPD) the survival was 33% (Figure 2A). The 3-year disease-free survival rate for the 17 patients analyzed was 41.2%. The disease-free survival for the pCR and pPD subgroups were 63.55% and 22.2%, respectively (Figure 2B). There is no statistical significance among the overall survival analysis (P = 0.0523) and disease-free survival analysis (P = 0.0897). However, there is trend toward improved prognosis when comparing pCR vs not complete response, although this did not reach statistical significance.
In univariate survival analysis, among the various factors, post-triple modality node stages were a strong independent favorable predicting factor for survival (P < 0.01). Sex, the tumor size, the type of chemotherapy, the tumor location, and tumor configuration were not predicting factors for survival (Table 4).
| Overall survival (%) | ||||
| 1 yr | 3 yr | P value | ||
| Sex | ||||
| Female | 2 | 100 (2) | 100 (2) | 0.017584 |
| Male | 15 | 73 (11) | 47 (7) | |
| Tumor size | ||||
| > 3 cm | 9 | 78 (7) | 44 (4) | 0.34649 |
| < 3 cm | 6 | 83 (5) | 67 (4) | |
| Unknown | 2 | 50 (1) | 50 (1) | |
| Concurrent chemo | ||||
| 5-FU based | 11 | 82 (9) | 55 (6) | 0.078225 |
| Cis based | 6 | 83 (5) | 50 (3) | |
| Tumor configuration | ||||
| > 180 cm | 8 | 63 (5) | 50 (4) | 0.382319 |
| < 180 cm | 9 | 89 (8) | 55 (5) | |
| Node status | ||||
| Negative | 13 | 85 (11) | 62 (8) | 0.009262 |
| Positive | 4 | 75 (3) | 25 (1) | |
| Location of primary tumor | ||||
| 20-35 cm | 12 | 83 (10) | 58 (7) | 0.024229 |
| > 35 ~ | 5 | 80 (4) | 40 (2) | |
| Total | 17 | 82 (14) | 53 (9) | |
Patients who had pCR to neoadjuvant treatment had a trend of benefit for the probability of survival compared to patients with pPD after neoadjuvant treatment. The 3-year overall survival rates were 75% vs 33% and the 3-year disease-free survival rates were 62.5% vs 22.2%, respectively. Interestingly, the analysis of histological regression after neoadjuvant showed improved survival rates if no or only rare residual tumor cells were found in the node specimen (Table 4).
Many reports show a consistent finding that response to preoperative therapy, particularly the absence of residual disease in the surgical specimen, is a good outcome indicator of better disease-free and overall survival[8,12-14]. In a comprehensive literature review of 22 studies in which patients with esophageal or esophagogastric junction cancer underwent esophagectomy after neoadjuvant CRT, patients with a pCR were two to three times more likely to survive than were those with residual disease in the esophagectomy specimen[15]. These benefits translate into a 33% to 36% mean absolute survival benefit when a pCR is achieved than when it is not. These assumptions provide the rationale for intensification of preoperative treatment via a higher biological dose delivered to the tumor mass, without increasing the surrounding organs’ risk of toxicity. The IMRT-SIB approach was based upon better radiographic findings of the biological target through PET scanning prior to CRT. This approach provides a better outline of the biological tumor within a mass, and allows RT dose intensity increase by 10% to the biological activity of tumor, without increasing the overall treatment time and the dose to the surrounding organs at risk[16].
In the Cross study, although the improvement in overall survival is 14%, the improvement for adenocarcinoma is limited[7]. The trend of improvement is not statistically significant. The greatest benefit was for squamous cell cancer. Few data were available on how to improve the outcome for esophageal adenocarcinoma and gastroesophageal cancers. Most reports had histology of adenocarcinoma and squamous cell cancer.
We reviewed our single-institute experience on adenocarcinoma using IMRT-SIB dose escalation technique, and found that the toxicity is low and local and that the incidence of distal recurrence is consistent with previous reports[11,17,18]. Moreover, we found a possible association between that dose escalation with IMRT-SIB and the high pCR rate. The idea behind preoperative CRT in the treatment of esophageal and gastroesophageal junction cancer was to improve survival by reducing locoregional failure[13,18]. The high pCR rate through IMRT-SIB dose escalation to the tumor mass itself is a strong favorable prognostic factor for both locoregional and systemic recurrence and maybe even for overall survival[19]. However, further studies are needed to explore the potential association between SIB-induced pCR and overall survival rate for adenocarcinoma.
The Cross study[7] and the report by Hoeppner et al[20] used less than 4500 cGy dose protocol. In the Cross study, the low dose could explain the less favorable results for adenocarcinoma compared with squamous cell cancer histology[7]. In the Hoeppner et al[20] report, the low-dose radiation could explain the less favorable results for neoadjuvant chemoradiation compared with perioperative chemotherapy for adenocarcinoma. At least, the suggestion of the low dose of radiation is one of factor contributed to low pCR was reported[21]. In addition, whether predicting value of the SIB induced pCR and conventional dose resulted pCR are the same? Is there anything else be young dose response relationship between the gross tumor volume and delivered dose? Does dose escalation impact on risk of distant metastasis? More future studies need to explore above questions.
Since all cancers in the present study were in the lower distal esophagus, the radiation field was limited to the lower mediastinum and did not include in supraclavicular regions, and there were no recurrences in the supraclavicular areas. Strict dose constrains to the heart were used and no significant cardiac events were noted. Although some reports suggested that higher treatment morbidity and mortality were associated with neoadjuvant CRT[20,22], the current cohort’s patients had acceptable tolerance.
To our knowledge, ours is the first report of using IMRT-SIB with dose escalation resulting in high pCR in adenocarcinoma of esophagus. However, our study has limitations. First, this was a retrospective review that mostly consisted of the patients who were treated by a dedicated multidisciplinary team at a single institution. Selection biases from the study existed. Second, the sample size was small and only ypN was a positive predictive factor in univarate analysis; a larger size study will provide more reliable conclusions. Moreover, although we have reviewed all esophageal adenocarcinoma cases in our institute from the past 10 years, the IMRT-SIB has been implemented only recently and, as a result, the follow-up for this subgroup of patients was short. In a prospective setting, the patients could be stratified by different prognostic clinical variables in an effort to better elucidate the role of SIB dose escalation in certain patient groups.
In conclusion, the dose escalation through IMRT-SIB in the chemoradiation regimen seems responsible for the down staging of the distal esophageal or gastroesophageal junction tumors. The protocol is well-tolerated, postoperative complications were acceptable, and the complete resection rate is high. This radiation therapy dose escala–tion strategy warrants further investigation.
This report is in memory of Phillip D Price, MD, one of surgeons involved with the multidisciplinary pancreatic cancer committee at Zangmeister Cancer Center and Mount Carmel Health System, who passed away from malignancy. We thank Chris Tobolski for providing support for the treatment plan.
Improvement in resection rate is very important for advanced esophageal cancer, balance between toxicity and benefit from neoadjuvant treatment is always the focus of multidisciplinary oncology team. Neadjuvent concurrent chemoradiation is becoming a standard of care for locally advanced esophageal cancer.
The authors reported here a protocol that improved resection rate through simultaneous integrated boost (SIB) based dose escalation technique without comprising the toxicities. Few reports using this protocol.
Intensity modulated radiation therapy with SIB (IMRT-SIB) has much safe toxicity profile and less equipment restrain. This finding needs further large phase III clinical studies to confirm.
IMRT-SIB is a novo radiation technique to deliver much higher dose to the biological gross tumor volume defined by positron emission tomographic positive area without increase radiation dose to all other area surrounding to it.
The authors present a retrospective analysis of an intensified regimen in neoadjuvant chemoirradiation of advanced distal adenocarcinoma of the esophagus. This work is of interest for the oncology community.
P- Reviewer: Sterzing F S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9956] [Article Influence: 995.6] [Reference Citation Analysis (0)] |
| 2. | Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236:177-183. [PubMed] |
| 3. | Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1144] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 4. | Sun XD, Yu JM, Fan XL, Ren RM, Li MH, Zhang GL. [Randomized clinical study of surgery versus radiotherapy alone in the treatment of resectable esophageal cancer in the chest]. Zhonghua Zhongliu Zazhi. 2006;28:784-787. [PubMed] |
| 5. | Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 1053] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 6. | Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1265] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 7. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4071] [Article Influence: 313.2] [Reference Citation Analysis (0)] |
| 8. | Meguid RA, Hooker CM, Taylor JT, Kleinberg LR, Cattaneo SM, Sussman MS, Yang SC, Heitmiller RF, Forastiere AA, Brock MV. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg. 2009;138:1309-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6456] [Article Influence: 430.4] [Reference Citation Analysis (0)] |
| 10. | Jabbour SK, Hashem SA, Bosch W, Kim TK, Finkelstein SE, Anderson BM, Ben-Josef E, Crane CH, Goodman KA, Haddock MG. Upper abdominal normal organ contouring guidelines and atlas: a Radiation Therapy Oncology Group consensus. Pract Radiat Oncol. 2014;4:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Cheedella NK, Suzuki A, Xiao L, Hofstetter WL, Maru DM, Taketa T, Sudo K, Blum MA, Lin SH, Welch J. Association between clinical complete response and pathological complete response after preoperative chemoradiation in patients with gastroesophageal cancer: analysis in a large cohort. Ann Oncol. 2013;24:1262-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Davies AR, Gossage JA, Zylstra J, Mattsson F, Lagergren J, Maisey N, Smyth EC, Cunningham D, Allum WH, Mason RC. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol. 2014;32:2983-2990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle JS, Vaitkevicius VK, Cooper J, Byhardt R, Davis L, Emami B. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15:277-284. [PubMed] |
| 15. | Scheer RV, Fakiris AJ, Johnstone PA. Quantifying the benefit of a pathologic complete response after neoadjuvant chemoradiotherapy in the treatment of esophageal cancer. Int J Radiat Oncol Biol Phys. 2011;80:996-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Warren S, Partridge M, Carrington R, Hurt C, Crosby T, Hawkins MA. Radiobiological determination of dose escalation and normal tissue toxicity in definitive chemoradiation therapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2014;90:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, Ackland S, Gotley DC, Joseph D, Millar J. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 704] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 18. | Pennathur A, Luketich JD, Landreneau RJ, Ward J, Christie NA, Gibson MK, Schuchert M, Cooper K, Land SR, Belani CP. Long-term results of a phase II trial of neoadjuvant chemotherapy followed by esophagectomy for locally advanced esophageal neoplasm. Ann Thorac Surg. 2008;85:1930-1936; discussion 1936-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Hamai Y, Hihara J, Emi M, Murakami Y, Kenjo M, Nagata Y, Okada M. Results of Neoadjuvant Chemoradiotherapy With Docetaxel and 5-Fluorouracil Followed by Esophagectomy to Treat Locally Advanced Esophageal Cancer. Ann Thorac Surg. 2015;99:1887-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Hoeppner J, Zirlik K, Brunner T, Bronsert P, Kulemann B, Sick O, Marjanovic G, Hopt UT, Makowiec F. Multimodal treatment of locally advanced esophageal adenocarcinoma: which regimen should we choose? Outcome analysis of perioperative chemotherapy versus neoadjuvant chemoradiation in 105 patients. J Surg Oncol. 2014;109:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Königsrainer A. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 695] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 22. | Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 448] [Article Influence: 20.4] [Reference Citation Analysis (0)] |