Published online Apr 15, 2016. doi: 10.4251/wjgo.v8.i4.410
Peer-review started: January 5, 2016
First decision: January 30, 2016
Revised: February 22, 2016
Accepted: March 14, 2016
Article in press: March 16, 2016
Published online: April 15, 2016
Processing time: 85 Days and 7.7 Hours
AIM: To compared the prognosis of middle third gastric carcinoma (MGC) patients with those of patients with proximal/distal gastric carcinoma (PGC/DGC).
METHODS: Of 3299 patients diagnosed with gastric carcinoma who underwent surgery at our hospital over a 15-year period, 919 (27.9%) were diagnosed with MGC. For each patient, the following information was obtained from hospital records: Age, sex, tumor size, depth of invasion, histologic type, nodal involvement, extent of lymph node dissection, hepatic metastasis, peritoneal dissemination, stage at initial diagnosis, operative type, curability, and survival rate.
RESULTS: T1 category tumors were more common in patients with MGC than in patients with PGC (P < 0.001). Tumor stage (stage I), N category (N0), and T category (T1) significantly influenced the 5-year survival rates for patients with curatively resected tumors. A multivariate analysis showed that age, tumor size, serosal invasion, lymph node metastasis, and curability were significant predictors of survival in patients with MGC. The survival rate for MGC patients was similar to that for PGC/DGC patients (52.8% vs 44.4%/51.4%, P = 0.1138). The 5-year survival rate for MGC patients with curative resection was higher than that for MGC patients with non-curative resection (62.9% vs 8.7%, P < 0.001).
CONCLUSION: These results indicate that tumor location did not affect the prognosis. Curative resection is important for improving the prognosis of patients with MGC.
Core tip: The clinicopathological features of the patients with middle third gastric carcinoma (MGC) were reviewed retrospectively. Tumor location did not affect the prognosis. When the MGC group was divided into patients with or without curative resection, the survival rates were higher for patients with curative resection. Therefore, curative resection is important for improving the prognosis of patients with MGC.
- Citation: Kim JH, Joo JK, Ryu SY, Kim HG, Lee JH, Kim DY. Clinicopathological features of patients with middle third gastric carcinoma. World J Gastrointest Oncol 2016; 8(4): 410-415
- URL: https://www.wjgnet.com/1948-5204/full/v8/i4/410.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i4.410
Although the incidence of gastric carcinoma is declining, it remains one of the leading causes of death from malignant tumors worldwide and advanced gastric carcinoma patients still have unfavorable prognoses[1]. Generally, the prognosis of patients with middle third gastric carcinoma (MGC) is better than that of patients with proximal or distal third gastric carcinoma (PGC/DGC)[2]; however, few studies have described the follow-up of patients with MGC. Therefore, it is important to analyze the prognostic factors in patients with MGC. We compared the clinicopathological features and outcomes of MGC with those of more proximally or distally located gastric carcinomas.
Between 1987 and 2004, 3299 patients with gastric carcinoma were admitted to the Division of Gastroenterologic Surgery, Department of Surgery, Chonnam National University Medical School, Gwangju, South Korea. Of these, 919 (27.9%) had MGC. The clinicopathological features of the patients with MGC were reviewed retrospectively. Patients with carcinomas involving the entire stomach were excluded. Following the Japanese classification of gastric carcinoma outlined by the Japanese Research Society for Gastric Cancer[3], the location of each tumor was described as the proximal, middle, or distal third of the stomach. The following information about each patient was obtained from hospital records: Age, sex, tumor size, depth of invasion, histologic type, nodal involvement, extent of lymph node dissection, hepatic metastasis, peritoneal dissemination, stage at initial diagnosis, operative type, curability, and survival rate.
The survival rates of the patients were calculated using the Kaplan-Meier method, and the relative prognostic importance of the parameters was investigated using the Cox proportional hazards model. The χ2 test was used to evaluate the statistical significance of differences, and P values less than 0.05 were considered significant.
Of the 3299 patients diagnosed with gastric carcinoma who underwent surgery at our hospital over a 17-year period, 919 (27.9%) were diagnosed with MGC. Table 1 describes the clinicopathological features of these 919 patients and the 2312 patients with PGC/DGC. There was a significant difference in the mean age of the patients with MGC (55.8 years) compared to the patients with DGC (57.6 years) (P < 0.001). Of the 919 patients with MGC, 602 (65.5%) were male and 317 (34.5%) were female. There were more males than females in each group, but there was no significant difference in the sex ratio of each group. Carcinomas in the middle third of the stomach were smaller than were carcinomas in the proximal third of the stomach (4.2 cm vs 4.7 cm), and the difference in mean tumor size was significant (P < 0.001).
| Variables | MGC (n = 919) (%) | PGC (n = 312) (%) | DGC (n = 2000) (%) | P value |
| Age (mean, yr) | 55.8 ± 11.7 | 55.8 ± 12.5 | 57.6 ± 10.7 | < 0.001 |
| Gender | 0.277 | |||
| Male | 602 (65.5) | 219 (70.2) | 1327 (66.4) | |
| Female | 317 (34.5) | 93 (29.8) | 673 (33.6) | |
| Tumor size (mean, cm) | 4.2 ± 2.8 | 4.7 ± 2.6 | 3.7 ± 2.3 | < 0.001 |
| Depth of invasion | < 0.001 | |||
| T1 | 296 (32.2) | 41 (13.1) | 648 (32.4) | |
| T2 | 112 (12.2) | 45 (14.4) | 307 (15.4) | |
| T3 | 410 (44.6) | 177 (56.8) | 844 (42.2) | |
| T4 | 101 (11.0) | 49 (15.7) | 201 (10.0) | |
| Histologic type | < 0.001 | |||
| Differentiated | 324 (35.3) | 108 (34.6) | 941 (47.1) | |
| Undifferentiated | 595 (64.7) | 204 (65.4) | 1059 (52.9) | |
| Lymph node dissection | 0.018 | |||
| < D2 | 215 (23.4) | 51 (16.3) | 475 (23.7) | |
| ≥ D2 | 704 (76.6) | 261 (83.7) | 1525 (76.3) | |
| Lymph node metastasis | 0.045 | |||
| N (-) | 561 (61.1) | 125 (40.1) | 1217 (60.9) | |
| N (+) | 358 (38.9) | 187 (59.9) | 783 (39.1) | |
| Operative type | < 0.001 | |||
| Total gastrectomy | 297 (32.3) | 267 (85.6) | 75 (3.8) | |
| Others | 622 (67.7) | 45 (14.4) | 1925 (96.2) | |
| Hepatic metastasis | 0.068 | |||
| H (-) | 899 (97.8) | 300 (96.2) | 1914 (95.7) | |
| H (+) | 20 (2.2) | 12 (3.8) | 86 (4.3) | |
| Peritoneal dissemination | 0.556 | |||
| P (-) | 838 (91.2) | 282 (90.4) | 1829 (91.4) | |
| P (+) | 81 (8.8) | 30 (9.6) | 171 (8.6) | |
| Stage | < 0.001 | |||
| I | 356 (38.7) | 71 (22.7) | 840 (42.0) | |
| II | 167 (18.2) | 66 (21.2) | 322 (16.1) | |
| III | 223 (24.3) | 109 (34.9) | 432 (21.6) | |
| IV | 173 (18.8) | 66 (21.2) | 406 (20.3) | |
| Curability | 0.796 | |||
| Curative | 764 (83.1) | 254 (81.4) | 1658 (82.9) | |
| Non-curative | 155 (16.9) | 58 (18.6) | 342 (17.1) |
Using the pTNM system, 296 patients with MGC were classified as pT1, 112 as pT2, 410 as pT3, and 101 as pT4. T1 tumors were more common in patients with MGC than in patients with PGC (32.2% vs 13.1%, P < 0.001). Using the grade of anaplasia, 324 (35.3%) of the MGC tumors were differentiated and 595 (64.7%) were undifferentiated adenocarcinomas. Of the patients with MGC, 561 (61.1%) had no lymph node metastases (pN0) and 358 (38.9%) had lymph node metastases. Lymph node metastasis was less common in patients with MGC than in patients with PGC (P < 0.05).
Hepatic metastases from MGC were found in 20 patients (2.2%), and peritoneal dissemination was present in 81 patients (8.8%). No significant differences were found in the frequency of hepatic metastasis or peritoneal dissemination among the groups. Of the patients with MGC, 396 (43.1%) were classified as either stage III or IV at the initial diagnosis. In MGC patients, 55.6% of the tumors extended to the serosa or adjacent organs (pT3 and pT4), while 72.5% of the PGCs extended beyond the serosa.
Compared with its use in MGC/DGC, total gastrectomy was performed significantly more frequently for the treatment of PGC (85.6% of cases, P < 0.001). The curative resection rate for patients with MGC was 83.1%, similar to that for patients with PGC/DGC (81.4%/82.9%, P > 0.05).
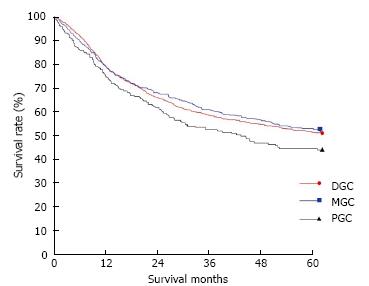
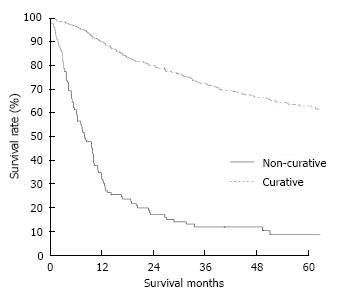
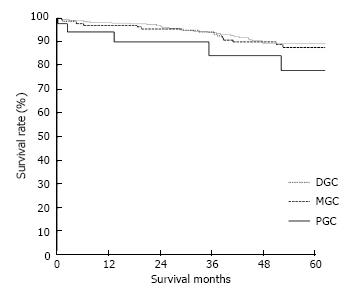
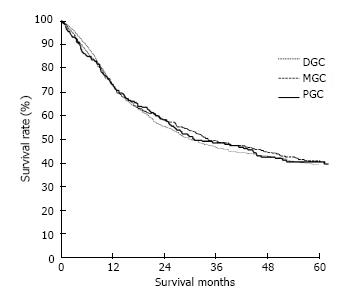
The clinicopathological variables tested in our univariate analysis are shown in Table 2. Factors influencing the 5-year survival rate were patient age, sex, tumor size, depth of invasion, histologic type, presence of hepatic metastasis, lymph node invasion, extent of lymph node dissection, and stage at initial diagnosis. When corrected for depth of invasion, tumor stage, and lymph node invasion in the two groups, tumor stage (stage I), N category (N0), and T category (T1) significantly influenced the 5-year survival rates for patients with curatively resected tumors (Table 3). A multivariate analysis showed that age, tumor size, serosal invasion, lymph node metastasis, and operative curability were significant predictors of survival for patients with MGC (Table 4). Figure 1 shows the patient survival rate according to tumor location. The 5-year survival rate for patients with MGC (52.8%) was higher than that for patients with PGC/DGC (44.4%/51.4%), but not significantly (P > 0.05). When the MGC group was divided into patients with or without curative resection, the respective 5-year survival rates were 62.9% and 8.7% (P < 0.001) (Figure 2). There were no significant differences in the survival rates among MGC, PGC, and DGC when the patients were divided into early and advanced gastric carcinoma (Figures 3 and 4).
| Variables | No. of patients | 5-yr survival rate | P value |
| Age | 0.0057 | ||
| < 65 | 688 | 56.0 | |
| ≥ 65 | 231 | 40.0 | |
| Gender | 0.0161 | ||
| Male | 602 | 48.9 | |
| Female | 317 | 59.9 | |
| Tumor size (cm) | < 0.001 | ||
| < 5 | 616 | 68.5 | |
| ≥ 5 | 303 | 28.0 | |
| Depth of invasion | < 0.001 | ||
| T1 | 296 | 88.3 | |
| T2 | 112 | 75.9 | |
| T3 | 410 | 37.8 | |
| T4 | 101 | 15.4 | |
| Histologic type | 0.0294 | ||
| Differentiated | 324 | 62.8 | |
| Undifferentiated | 595 | 48.2 | |
| Hepatic metastasis | < 0.001 | ||
| (-) | 899 | 53.7 | |
| (+) | 20 | 10.8 | |
| Operative type | 0.4327 | ||
| Total | 297 | 52.4 | |
| Others | 622 | 63.6 | |
| Lymph node invasion | < 0.001 | ||
| N (-) | 561 | 77.5 | |
| N (+) | 358 | 32.7 | |
| Lymph node dissection | < 0.001 | ||
| < D2 | 215 | 18.3 | |
| ≥ D2 | 704 | 60.0 | |
| Stage | < 0.001 | ||
| I | 356 | 87.5 | |
| II | 167 | 62.5 | |
| III | 223 | 35.2 | |
| IV | 173 | 14.7 |
| Variables | PGC (n = 254) (%) | MGC (n = 764) (%) | P value |
| Depth of invasion | |||
| T1 | 77.6 | 88.8 | 0.0477 |
| T2 | 76.0 | 76.1 | 0.7534 |
| T3 | 44.6 | 45.1 | 0.9900 |
| T4 | 17.3 | 16.8 | 0.1698 |
| Lymph node metastasis | |||
| N0 | 72.3 | 79.9 | 0.0270 |
| N1 | 42.7 | 49.6 | 0.6285 |
| N2 | 33.4 | 31.0 | 0.6933 |
| Stage | |||
| I | 77.2 | 87.9 | 0.0420 |
| II | 68.4 | 63.2 | 0.5566 |
| III | 33.7 | 36.3 | 0.5823 |
| IV | 17.6 | 21.8 | 0.3635 |
| Variables | Risk ratio | 95%CI | P value |
| Age (< 65 vs≥ 65) | 1.78 | 1.24-2.55 | 0.002 |
| Tumor size (mm) (< 50 vs≥ 50) | 1.51 | 1.03-2.21 | 0.036 |
| Serosal invasion (negative vs positive) | 2.46 | 1.45-4.15 | 0.001 |
| Lymph node metastasis (negative vs positive) | 2.48 | 1.59-3.87 | 0.000 |
| Curability (curative vs non-curative) | 3.46 | 2.29-5.23 | < 0.001 |
The prognosis of gastric carcinoma varies with tumor location[4-6]. Although MGCs are reported to have relatively better outcomes than carcinomas in other parts of the stomach[2], there is limited information on the prognostic factors for MGC. Therefore, we compared the clinicopathological features and prognosis of MGC patients with those of patients with PGC/DGC.
Investigators have discussed various prognostic factors for MGC. Serosal invasion, lymph node metastasis, and lymphatic involvement were found to have significant correlations with prognosis in univariate analyses, and serosal invasion and lymphatic involvement were independent prognostic factors in a multivariate analysis[7]. Other authors have reported similar findings[8,9]. An anterior location was clearly an independent prognostic factor for patients with MGC based on a multivariate analysis. It has been postulated that tumors in the anterior wall metastasize more easily to the peritoneum compared with tumors elsewhere because there are no organs on the abdominal side of the anterior wall[5]. This explanation seems reasonable, although others do not agree[7]. In this study, we found that age, tumor size, serosal invasion, lymph node metastasis, and curability were independent predictors of survival in patients with MGC in a multivariate analysis.
The operation type for patients with MGC is controversial. One prospective randomized trial conducted in Italy stated that distal gastrectomy was sufficient for treating tumors located in the middle third of the stomach if a cancer-free microscopic margin could be achieved[10]. However, that study included a relatively small number of MGCs. Therefore, many surgeons still recommend total gastrectomy for MGC because they are concerned about the possibility of local recurrence due to the short proximal resection margin and less extensive lymph node dissection in distal gastrectomy[11,12]. In a separate report, distal gastrectomy was performed in only 39.3% of patients with a middle third advanced gastric carcinoma for the same reasons, although the authors stated that the type of gastric resection and length of the proximal resection margin did not affect the long-term prognosis. They also reported that distal gastrectomy was sufficient to achieve a tumor-free resection margin in many cases[13]. Other authors have reported that if curative surgery can be performed, the long-term prognosis of patients with MGC is not affected by the extent of gastric resection, and a distal gastrectomy is feasible[14-16]. When determining the type of operation for MGC, we also stress tumor-free resection. The statistical analysis in this study showed that operation type was not a prognostic factor.
Generally, the prognosis of patients with MGC is better than that of patients with PGC/DGC[2]; the present study showed that tumor location did not affect the prognosis. We thought that the possible reason was due to similar curative resection rates. A significant difference in survival between patients with early and advanced gastric carcinomas has been reported[7]. We also found a significant difference in survival rates between patients with early (87.8%) and advanced (40.8%) gastric carcinomas. The survival rate for patients with MGC was 52.8%, and the cumulative survival rate for patients with MGC was slightly better than that for patients with PGC/DGC. When the MGC group was divided into patients with or without curative resection, the respective 5-year survival rates were 62.9% and 8.7%. Furthermore, we evaluated the relationship between the survival of patients with gastric carcinoma after curative resection and the depth of invasion. There was no significant difference in cumulative survival between the groups when the depth of invasion was that of T2-T4 tumors.
In patients with MGC, as the tumors progress the lymph nodes around the splenic artery and hilum are also frequently involved[5]. Several studies have reported that the incidence of lymph node metastasis is 9.7%-20% along the splenic artery and 9.2%-17% at the splenic hilum in advanced PGC and MGC[17-19]. In our department, splenectomy is not routine for patients with advanced MGC. However, we perform a splenectomy when the tumor invades the spleen directly or when metastasis to the splenic hilar lymph nodes or lymph nodes around the splenic artery is suspected.
Regarding adjuvant chemotherapy, we administered postoperative chemotherapy to select patients according to the pathologic findings instead of tumor location. Since the chemotherapeutic regimen varied during the study period, we did not analyze the effect of postoperative adjuvant chemotherapy.
In conclusion, our results show that tumor location did not affect the prognosis of MGC. When the MGC group was divided into patients with or without curative resection, the survival rates were higher for patients with curative resection. Therefore, curative resection is important for improving the prognosis of patients with MGC.
The prognosis of patients with middle third gastric carcinoma (MGC) is better than that of patients with proximal or distal third gastric carcinoma; however, few studies have described the follow-up of patients with MGC.
The prognosis of gastric carcinoma varies with tumor location. Although MGCs are reported to have relatively better outcomes than carcinomas in other parts of the stomach, there is limited information on the prognostic factors for MGC.
The authors did not find any difference in survival rates according to the tumor location. When the MGC group was divided into patients with or without curative resection, the survival rates were higher for patients with curative resection.
The study shows the importance of curative resection in patients with MGC.
The stomach is anatomically divided into three portions: The upper (U), middle (M), and lower (L) parts. If more than one portion is involved, all involved portions should be described in order of degree of involvement, the first indicating the portion in which the bulk of the tumor is situated.
These authors provided an overall review of the middle third gastric cancer. These authors described several clinico pathological parameters of MGC compared to PGC/DGC. In this article, authors also demonstrated the significant difference between curative resection is one of the prognostic factors for MGC. It is interesting and acceptable for publication.
P- Reviewer: Chen JN, Hsu LS S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Nakamura K, Ueyama T, Yao T, Xuan ZX, Ambe K, Adachi Y, Yakeishi Y, Matsukuma A, Enjoji M. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Li C, Kim S, Lai JF, Oh SJ, Hyung WJ, Choi WH, Choi SH, Zhu ZG, Noh SH. Lymph node dissection around the splenic artery and hilum in advanced middle third gastric carcinoma. Eur J Surg Oncol. 2009;35:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Coyle JT. Reviewing potential malpractice cases. JAMA. 1992;267:2604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2869] [Article Influence: 204.9] [Reference Citation Analysis (0)] |
| 4. | Okamura T, Tsujitani S, Marin P, Haraguchi M, Korenaga D, Baba H, Sugimachi K. Adenocarcinoma in the upper third part of the stomach. Surg Gynecol Obstet. 1987;165:247-250. [PubMed] |
| 5. | Wu CW, Hsieh MC, Tsay SH, Lui WY, P’eng FK. Adenocarcinoma of midstomach. Clinical and pathoanatomic relation to lymph node metastases. J Clin Gastroenterol. 1994;19:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Lee WJ, Lee WC, Houng SJ, Shun CT, Houng RL, Lee PH, Chang KJ, Wei TC, Chen KM. Survival after resection of gastric cancer and prognostic relevance of systematic lymph node dissection: twenty years experience in Taiwan. World J Surg. 1995;19:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Sheen-Chen SM, Chou CW, Chen MC, Chen FC, Chen YS, Chen JJ. Adenocarcinoma in the middle third of the stomach--an evaluation for the prognostic significance of clinicopathological features. Hepatogastroenterology. 1997;44:1488-1494. [PubMed] |
| 8. | Michelassi F, Takanishi DM, Pantalone D, Hart J, Chappell R, Block GE. Analysis of clinicopathologic prognostic features in patients with gastric adenocarcinoma. Surgery. 1994;116:804-809; discussion 809-810. [PubMed] |
| 9. | Baba H, Korenaga D, Okamura T, Saito A, Sugimachi K. Prognostic factors in gastric cancer with serosal invasion. Univariate and multivariate analyses. Arch Surg. 1989;124:1061-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg. 1999;230:170-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 269] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Stein HJ, Sendler A, Siewert JR. Site-dependent resection techniques for gastric cancer. Surg Oncol Clin N Am. 2002;11:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Clark CJ, Thirlby RC, Picozzi V, Schembre DB, Cummings FP, Lin E. Current problems in surgery: gastric cancer. Curr Probl Surg. 2006;43:566-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Jang YJ, Park MS, Kim JH, Park SS, Park SH, Kim SJ, Kim CS, Mok YJ. Advanced gastric cancer in the middle one-third of the stomach: Should surgeons perform total gastrectomy? J Surg Oncol. 2010;101:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Tao KL, Huang CM, Lin JX, Zheng CH, Li P, Xie JW, Wang JB. [Impact of the extent of gastric resection on the prognosis of patients with middle one-third gastric cancer]. Zhonghua Weichang Waike Zazhi. 2013;16:155-159. [PubMed] |
| 15. | Kim HG, Ghu HD, Yun SK, Ryu SY, Kim DY. Clinicopathological features of female gastric carcinoma patients with curative resection: comparison with male patients. Chonnam Med J. 2012;48:86-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Kim DY, Joo JK, Ryu SY, Park YK, Kim YJ, Kim SK. Clinicopathologic characteristics of gastric carcinoma in elderly patients: a comparison with young patients. World J Gastroenterol. 2005;11:22-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Mönig SP, Collet PH, Baldus SE, Schmackpfeffer K, Schröder W, Thiele J, Dienes HP, Hölscher AH. Splenectomy in proximal gastric cancer: frequency of lymph node metastasis to the splenic hilus. J Surg Oncol. 2001;76:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Qin H, Lin C. Radical resection of gastric carcinoma with pancreas and spleen preservation and functional cleaning of lymph nodes. Chin Med J. 2002;115:736-739. [PubMed] |