Published online Apr 15, 2016. doi: 10.4251/wjgo.v8.i4.351
Peer-review started: May 5, 2015
First decision: July 1, 2015
Revised: January 24, 2016
Accepted: February 16, 2016
Article in press: February 17, 2016
Published online: April 15, 2016
Processing time: 336 Days and 20.4 Hours
Surgical therapy for ulcerative colitis (UC) depends on the medical therapy administered for the patient’s condition. UC is a benign disease. However, it has been reported that the rare cases of cancer in UC patients are increasing, and such cases have a worse prognosis. Recently, surgical therapy has greatly changed, there has been quite an increase in the number of UC patients with high-grade dysplasia and/or cancer. These lesions are known as colitis-associated cancer (CAC). The relationship between inflammation and tumorigenesis is well-established, and in the last decade, a great deal of supporting evidence has been obtained from genetic, pharmacological, and epidemiological studies. Inflammatory bowel disease, especially UC, is an important risk factor for the development of colon cancer. We should determine the risk factors for UC patients with cancer based on a large body of data, and we should attempt to prevent the increase in the number of such patients using these newly identified risk factors in the near future. Actively introducing the surgical treatment in addition to medical treatment should be considered. Several physicians should analyze UC from their unique perspectives in order to establish new clinically relevant diagnostic and treatment methods in the future. This article discusses CAC, including its etiology, mechanism, diagnosis, and treatment in UC patients.
Core tip: Inflammatory bowel disease, especially ulcerative colitis, is an important risk factor for development of colon cancer. There has been quite increased in the number of patients who had high-grade dysplasia and/or cancer. The relationship between inflammation and tumorigenesis is well-established and in the last decade has received a great deal of supporting evidence from genetic, pharmacological, and epidemiological data. To avoid such a problem, there is a need for appropriate diagnosis and treatment. It should be considered that actively introduce the surgical treatment in addition to medical treatment.
- Citation: Kinugasa T, Akagi Y. Status of colitis-associated cancer in ulcerative colitis. World J Gastrointest Oncol 2016; 8(4): 351-357
- URL: https://www.wjgnet.com/1948-5204/full/v8/i4/351.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i4.351
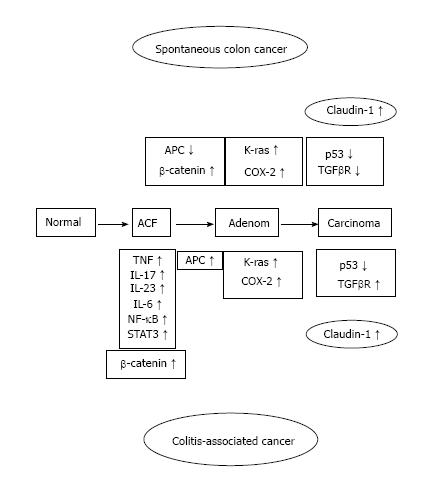
Ulcerative colitis (UC) is of unknown cause inflammatory bowel disease (IBD) associated with inflammation of the large intestine mucosa. UC number of the patients that have been recorded in Japan in 2014 at 166110 people, this number is increasing every year. As a recent problem, in patients with UC, it has been reported that it is easy to develop colorectal cancer (CRC). The longer the period of UC, the higher is the risk of developing UC-associated CRC. The mechanism of IBD-related colon cancer is different from sporadic CRC. The former is an inflammation-dysplasia-carcinoma sequence[1] and the latter is from the adenoma-carcinoma sequence in sporadic CRC (Figure 1).
Therefore the UC patient becomes the indication of the operation when the pathological findings are demonstrated high-grade or multifocal low-grade dysplasia in colonic mucosal which means that the entire mucosal lining of the colon exposed to chronic inflammation is at increased risk of cancer[1,2].
Crohn’s disease (CD) and UC, which include IBD, is a chronic, relapsing inflammatory condition of the gastrointestinal tract. IBD has a clear pathological and clinical characteristic. Though many studies were performed about this cause of IBD for the past several decades, the cause is not yet clear. The only consensus was obtained about IBD which was deregulated the mucosal immune response in the host. Some various components, which including environmental intestinal epithelial cells, and microbial factors, genetic susceptibility, and components of the innate and adaptive immune system, are implicated in the pathogenesis of IBD.
The connection between inflammation and tumorigenesis is well established, and in the last decade, a great deal of supporting evidence has been obtained through genetic, pharmacological, and epidemiological studies. IBD is an important risk factor for the development of colon cancer.
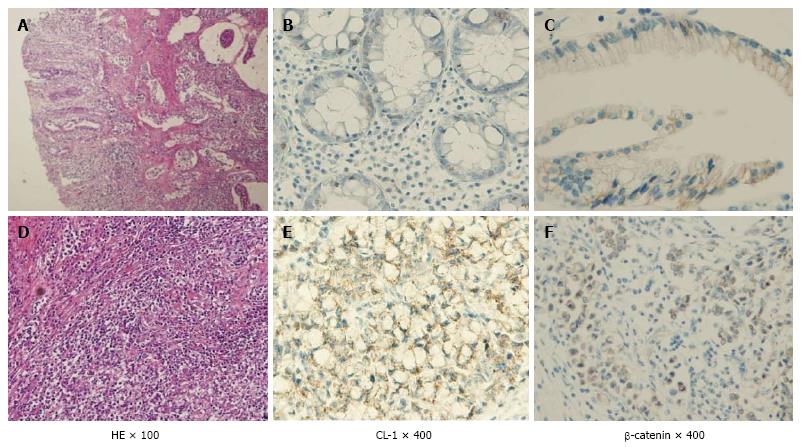
As a result has high mortality, it is difficult to treat colitis-associated cancer (CAC), which is the CRC subtype that is associated with IBD[3]. When the patients with IBD are the higher the probability within 30 years disease duration, and > 50% of these patients will die from CAC[4]. Some of the essential stages of cancer development, including the formation of aberrant crypt foci, polyps, adenomas, and carcinomas, are similar in noninflammatory CRC and CAC. However, several different pathogenic sequences CAC, has been reported including a well-defined inflammation and damage dysplasia cancer that occurs without the formation of the gland. Nevertheless, both of in the CRC and CAC had changed same genetic and signaling pathways which involving Wnt, β-catenin, K-ras, p53, and transforming growth factor (TGF)-β. However, the difference activation timing between CRC and CAC were reported about p53, adenomatous polyposis coli (APC) and K-ras[4,5]. A > 2-fold higher risk for colon cancer development with a family history of CRC have than without in IBD patients, suggesting an overlap in the mechanisms driving CRC and CAC[6]. Kinugasa et al[7] also showed that high-grade dysplasia (HGD) and CAC in patients with UC results in an increase in β-catenin transcriptional activity that may contribute to increased claudin (CL)-1 expression. According to above things, the expression of CL-1 was increased in CAC and dysplasia than normal mucosa is likely to be involved in neoplastic progression in UC patients. Thus, I propose that there is a possibility that increased CL-1 expression may contribute to carcinogenesis in UC. CL-1 is a tight junction specific protein that was first described by Furuse et al[8] in 1998. The signaling pathways, including TGF-β/SMAD and β-catenin[9,10] were interacted with the CL protein that is separate from their effects on the barrier function. We reported that CL-1 is regulated by β-catenin/TCF/lymphocyte-enhancer factor signaling[11,12] suggests that the expression of CL-1 is concerned in β-catenin activation (Figure 2).
These mechanisms are the basis for the current discussion about CAC as well as for new approaches to prevention and therapy.
Patients with IBD are at an increased risk of developing colorectal advanced neoplasia, including colorectal HGD and CRC[13-16]. Chronic intestinal inflammation is considered to be a promoter of carcinogenesis[17]. The two major drivers of the excess risk of CRC in IBD are disease extension[18] and duration[13]. Concomitant primary sclerosing cholangitis[19], family history of CRC[6], and active intestinal inflammation[20-23] are the other established risk factors.
The risk of CRC in patients with IBD was recognized as far back as 1925 for UC and 1948 for CD[24]. There were many reports of risk for colon cancer development about UC patients mainly, and which ratio was indicated from 16% to 43%[25-28]. In 2001 Eaden et al[13] estimated CRC risk as 2% at 10 years, 8% at 20 years and 18% at 30 years using a widely cited meta-analysis of 116 studies with age stratified data. Interestingly, recent studies we could get two different conclusions about IBD with CRC. Jess et al[29] suggested that the risk of CAC is almost same between UC patients and the general population in a population-based study from Denmark. In contrast, the risk of CRC is 60% higher with IBD than without IBD matched cohorts of people from California, and the risk remained the same throughout the study period of 14.5 years by Herrinton et al[15]. There are few reports from Asia on CRC in UC which reports that the pressure ranges from 0.87% to 1.8% in the general population, and it can be as high as 13.5% in patients with extensive type of UC. Thus, the risk of CRC is increased in IBD, though there is variation due to various factors, such as hospital- or population-based data, referral center bias, and small numbers of patients.
IBD is a lifelong diseases that primarily affects young patients. Sustained mucosal healing is becoming the standard objective of the long-term treatment of IBD[30]. This objective can be achieved currently by maintenance treatment with immunosuppressants, including thiopurines, methotrexate, and anti-tumor necrosis factor (TNF)[31,32]. Unfortunately, there is no major trend towards the spontaneous extinction of IBD activity with time[33,34], and disease activity often recurs after the withdrawal of immunosuppressants[35,36]. Similarly, another report showed that there is an unclear risk of colonic HGD and CRC among patients with IBD treated with immunosuppressants. Beaugerie et al[37] analyzed data on CRC development among patients with IBD and found that patients with IBD and long-standing extensive colitis are at increased of CRC, although the risk is lower among patients receiving thiopurine therapy. Patients without long-standing extensive colitis have a risk for CRC similar to that of the general population, but they can develop IBD-related lesions within 10 years after their diagnosis of IBD. Table 1 compares CAC with CRC.
| CAC: Up to 20% of UC patients develop CAC within 30 yr of disease onset |
| CAC: High overall mortality rate |
| NSAID use reduces the risk of CRC, suggesting a potential role for anticytokine therapy |
| CRC: Classic adenoma to carcinoma sequence |
| CAC: Chronic inflammation, injury, dysplasia, and CRC |
| The common genetic and signaling pathways are different between |
| CRC and CAC including β-catenin, p53, k-ras, B-raf |
| Both CRC and CAC are associated with transcription factors, such as |
| NF-κB and/or STAT3 which mediate the immune response and oncogenesis |
| Both CRC and CAC depend on the quality and quantity of intestinal microflora |
We have to debate about the surveillance techniques for CAC and high grade dysplasia whether adequate or not under currently way[38]. Presently, surveillance of using colonoscopy for UC patients is recommended every 2 years over 8 years after the onset of UC diagnosis. However, sometimes it is very hard to determinate the CRC findings under the UC mucosa, and do not match between the biopsy results and excisional tissue results in pathological diagnosis, this is that colonoscopy is often burdensome for the patient with UC patients[39-41]. Surprisingly, 20% to 50% of UC patients with CRC were diagnosed with only dysplasia in preoperative pathological diagnosis. This indicates that it is difficult surveillance for UC patients using colonoscopy.
Furthermore, endoscopic findings of IBD-related cancers have been found to easily overlook by colonoscopy because it is not a mass-like lesions to compared with sporadic CRC. As a result, it is very difficult to appropriate for diagnosis of adenocarcinoma for UC patients using biopsied tissue taken from the lesion. Meanwhile, we could find unexpectedly in a specimen resected for medically refractive IBD without previous diagnosis of dysplasia or adenocarcinoma[42,43].
It is clear that colonoscopic surveillance in the present form is neither ideal nor practical. We should reconsider the guidelines about the colonoscopy surveillance based on the other new reliable date and method. The ideal frequency of surveillance is not clear. Further studies are necessary to optimize the frequency of surveillance, including cost-effectiveness, and to make guidelines considering emerging methods and technologies.
Despite the proven usefulness of colonoscopy surveillance protocols and increased risk of CRC with UC, we could not determinant a useful in clinical diagnosing such as genetic or serological marker. After analyzing the association of the whole genome, the single nucleotide polymorphism (SNP) 300 or more and the genetic loci 160 or more were found to be associated with IBD[44]. There was another genetic instability report[45] that sought to identify IBD-associated SNPs that are potential markers for CAC by comparing groups of UC patients with and without neoplasia matched for sex, disease duration and age at diagnosis. Their conclusion was that none of the 314 studied IBD-associated SNPs were strongly associated with UC-neoplasia which may be the result of genetic mutations in molecular pathways other than those that predispose to inflammation.
On the other hand, there was a unique report that applied the tree models by considering the response variable as the CAC or UC group and the explanatory variable as the criteria studied by univariate analysis[46].
We proposed the useful method which analysis is a tree model permits the automatic execution of the process of determining the factors, setting the threshold value and by differentiating the patients successively into two groups during the process automatically. Therefore, it might be another possibility to help identify the indication and timing for surgery in UC patients, because the ideal surveillance methods have not yet been established.
The overall prevalence of CRC in patients with UC has been estimated as 3.7% in a meta-analysis[13]. Chronic UC for > 10 years and pancolitis are known risk factors for CAC[47]. On the other hand, sporadic adenoma and adenocarcinoma can arise coincidentally in patients with UC. From the perspective of clinical considerations, accurate pathological diagnosis is very important for distinguishing between different pathological entities, given their different therapeutic consequences such as sporadic adenocarcinoma and CAC.
Anal function and quality of life differ substantially between total proctocolectomy with ileal pouch anal anastomosis (IPAA) and low anterior resection (LAR). A key point is that further proctocolectomy and IPAA may be suitable for sporadic cancers in the lower rectum. In patients with UC, irrespective of the degree of colitis, LAR should not be selected for sporadic cancer in the lower rectum except in older patients, based on considerations of quality of life and risk of further colitis. In elderly patients with poor anal function, surgical procedures should obviously be considered based on overall considerations including prognosis of the cancer, degree of inflammation with colitis, and potential requirements for further treatment. In view of the risk of recurrent colitis and cancer, partial resection might be less advantageous than proctocolectomy. Proctocolectomy with IPAA may be safe for advanced CRC regardless of the origin as colitic or sporadic cancer, because of the difficulty of differentiation, ready invasive behavior against an inflamed background and worsened potential for further progressive colitis in younger patients. Decisions on surgical procedures should be made based on full consideration of background factors including age, degree of colitis and cancer prognosis.
There was an interesting report on the risk of ileoanal pouch neoplasia in patients with IBD[48]. Although restorative proctocolectomy with IPAA substantially reduces the risk of CRC in patients with IBD, subsequent pouch neoplasia can develop. The purpose of their study was to determine the cumulative incidence of pouch neoplasia in patients with IBD and to identify risk factors for developing pouch neoplasia. The incidence and prevalence of pouch neoplasia in patients with IBD are probably low. According to the latest review, only 42 pouch adenocarcinomas have been described in the literature[49]. A previous study reported a cumulative incidence of pouch neoplasia of 1.9% after 15 years and 5.1% after 25 years[50]. However, these data were collected in a single tertiary pouch referral center and may not be representative of the general IBD population with IPAA. Furthermore, the relatively low incidence makes it difficult to assess risk factors for the development of pouch neoplasia. The result of the study indicated that the incidence of pouch neoplasia in patients with IBD without a history of colorectal neoplasia is relatively low. Prior dysplasia or colon cancer is associated with an approximately 4- and 25-fold increase in risk, respectively, of developing pouch neoplasia.
UC is a benign disease. However, according to the recent reports that the rate of CAC is increasing in UC patients, and such cases have a worse prognosis. As such, there is a need for appropriate diagnosis and treatment. Actively introducing the surgical treatment in addition to medical treatment should be considered. Several physicians should analyze UC from their unique perspectives in order to establish new clinically relevant diagnostic and treatment methods in the future. We should determine the risk factors for UC patients with cancer based on a large body of data, and we should attempt to prevent the increase in the number of such patients using these newly identified risk factors in the near future.
P- Reviewer: Nakajima N S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 907] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 2. | Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 246] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14:3937-3947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 339] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 5. | Sheng H, Shao J, Williams CS, Pereira MA, Taketo MM, Oshima M, Reynolds AB, Washington MK, DuBois RN, Beauchamp RD. Nuclear translocation of beta-catenin in hereditary and carcinogen-induced intestinal adenomas. Carcinogenesis. 1998;19:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Askling J, Dickman PW, Karlén P, Broström O, Lapidus A, Löfberg R, Ekbom A. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Kinugasa T, Akagi Y, Yoshida T, Ryu Y, Shiratuchi I, Ishibashi N, Shirouzu K. Increased claudin-1 protein expression contributes to tumorigenesis in ulcerative colitis-associated colorectal cancer. Anticancer Res. 2010;30:3181-3186. [PubMed] |
| 8. | Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1524] [Cited by in RCA: 1569] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 9. | Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, Neff J, Washington MK, Beauchamp RD. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 435] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 10. | Shiou SR, Singh AB, Moorthy K, Datta PK, Washington MK, Beauchamp RD, Dhawan P. Smad4 regulates claudin-1 expression in a transforming growth factor-beta-independent manner in colon cancer cells. Cancer Res. 2007;67:1571-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Miwa N, Furuse M, Tsukita S, Niikawa N, Nakamura Y, Furukawa Y. Involvement of claudin-1 in the beta-catenin/Tcf signaling pathway and its frequent upregulation in human colorectal cancers. Oncol Res. 2001;12:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 219] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Mankertz J, Hillenbrand B, Tavalali S, Huber O, Fromm M, Schulzke JD. Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Commun. 2004;314:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2073] [Article Influence: 86.4] [Reference Citation Analysis (1)] |
| 14. | Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 403] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 15. | Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 244] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 16. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 658] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 17. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 852] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 18. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1197] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 19. | Torres J, Pineton de Chambrun G, Itzkowitz S, Sachar DB, Colombel JF. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:497-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Bansal P, Sonnenberg A. Risk factors of colorectal cancer in inflammatory bowel disease. Am J Gastroenterol. 1996;91:44-48. [PubMed] |
| 21. | Mathy C, Schneider K, Chen YY, Varma M, Terdiman JP, Mahadevan U. Gross versus microscopic pancolitis and the occurrence of neoplasia in ulcerative colitis. Inflamm Bowel Dis. 2003;9:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 878] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 23. | Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, Williams CB, Price AB, Talbot IC, Forbes A. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Warren S, Sommers SC. Cicatrizing enteritis as a pathologic entity; analysis of 120 cases. Am J Pathol. 1948;24:475-501. [PubMed] |
| 25. | Slaney G, Brooke BN. Cancer in ulcerative colitis. Lancet. 1959;2:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 83] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | de Dombal FT, Watts JM, Watkinson G, Goligher JC. Local complications of ulcerative colitis. Stricture, pseudopolyps and cancer of the colon and rectum. Am J Proctol. 1967;18:198-201. [PubMed] |
| 27. | Devroede GJ, Taylor WF, Sauer WG, Jackman RJ, Stickler GB. Cancer risk and life expectancy of children with ulcerative colitis. N Engl J Med. 1971;285:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 287] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Broström O, Löfberg R, Nordenvall B, Ost A, Hellers G. The risk of colorectal cancer in ulcerative colitis. An epidemiologic study. Scand J Gastroenterol. 1987;22:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 98] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375-381.e1; quiz e13-14. [PubMed] |
| 30. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 657] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 31. | Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1030] [Article Influence: 68.7] [Reference Citation Analysis (1)] |
| 32. | Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 701] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 33. | Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3-11. [PubMed] |
| 34. | Munkholm P, Langholz E, Davidsen M, Binder V. Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol. 1995;30:699-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 344] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 35. | Lémann M, Mary JY, Colombel JF, Duclos B, Soule JC, Lerebours E, Modigliani R, Bouhnik Y. A randomized, double-blind, controlled withdrawal trial in Crohn’s disease patients in long-term remission on azathioprine. Gastroenterology. 2005;128:1812-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 36. | Louis E, Mary JY, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63-70.e5; quiz e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Beaugerie L, Svrcek M, Seksik P, Bouvier AM, Simon T, Allez M, Brixi H, Gornet JM, Altwegg R, Beau P. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166-175.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 38. | Connelly TM, Koltun WA. The cancer “fear” in IBD patients: is it still REAL? J Gastrointest Surg. 2014;18:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Eaden JA, Ward BA, Mayberry JF. How gastroenterologists screen for colonic cancer in ulcerative colitis: an analysis of performance. Gastrointest Endosc. 2000;51:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Rutter MD. Surveillance programmes for neoplasia in colitis. J Gastroenterol. 2011;46 Suppl 1:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 807] [Article Influence: 53.8] [Reference Citation Analysis (2)] |
| 42. | Averboukh F, Ziv Y, Kariv Y, Zmora O, Dotan I, Klausner JM, Rabau M, Tulchinsky H. Colorectal carcinoma in inflammatory bowel disease: a comparison between Crohn’s and ulcerative colitis. Colorectal Dis. 2011;13:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Blackstone MO, Riddell RH, Rogers BH, Levin B. Dysplasia-associated lesion or mass (DALM) detected by colonoscopy in long-standing ulcerative colitis: an indication for colectomy. Gastroenterology. 1981;80:366-374. [PubMed] |
| 44. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3979] [Cited by in RCA: 3587] [Article Influence: 275.9] [Reference Citation Analysis (0)] |
| 45. | Connelly TM, Berg AS, Harris LR, Brinton DL, Hegarty JP, Deiling SM, Stewart DB, Koltun WA. Ulcerative colitis neoplasia is not associated with common inflammatory bowel disease single-nucleotide polymorphisms. Surgery. 2014;156:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Kinugasa T, Akagi Y, Murotani K, Romeo K, Yoshida T, Ryu Y, Shiratuchi I, Shirouzu K. Relationship between ulcerative colitis patients treated with leukocytapheresis and ulcerative colitis-associated colorectal cancer. Anticancer Res. 2011;31:2547-2552. [PubMed] |
| 47. | Ullman T, Odze R, Farraye FA. Diagnosis and management of dysplasia in patients with ulcerative colitis and Crohn’s disease of the colon. Inflamm Bowel Dis. 2009;15:630-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Derikx LA, Kievit W, Drenth JP, de Jong DJ, Ponsioen CY, Oldenburg B, van der Meulen-de Jong AE, Dijkstra G, Grubben MJ, van Laarhoven CJ. Prior colorectal neoplasia is associated with increased risk of ileoanal pouch neoplasia in patients with inflammatory bowel disease. Gastroenterology. 2014;146:119-128.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 49. | Liu ZX, Kiran RP, Bennett AE, Ni RZ, Shen B. Diagnosis and management of dysplasia and cancer of the ileal pouch in patients with underlying inflammatory bowel disease. Cancer. 2011;117:3081-3092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Kariv R, Remzi FH, Lian L, Bennett AE, Kiran RP, Kariv Y, Fazio VW, Lavery IC, Shen B. Preoperative colorectal neoplasia increases risk for pouch neoplasia in patients with restorative proctocolectomy. Gastroenterology. 2010;139:806-812, 812.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |