Published online Mar 15, 2016. doi: 10.4251/wjgo.v8.i3.271
Peer-review started: June 8, 2015
First decision: August 7, 2015
Revised: September 11, 2015
Accepted: December 13, 2015
Article in press: December 15, 2015
Published online: March 15, 2016
Processing time: 275 Days and 21.2 Hours
Gastric cancer (GC) develops as a result of inflammation-associated carcinogenesis due to Helicobacter pylori (H. pylori) infection and subsequent defects in genetic/epigenetic events. Although the indication for eradication therapy has become widespread, clinical studies have revealed its limited effects in decreasing the incidence of GC. Moreover, research on biopsy specimens obtained by conventional endoscopy has demonstrated the feasibility of the restoration of some genetic/epigenetic alterations in the gastric mucosa. Practically, the number of sporadic cases of primary/metachronous GC that emerge after successful eradication has increased, while on-going guidelines recommend eradication therapy for patients with chronic gastritis and those with background mucosa after endoscopic resection for GC. Accordingly, regular surveillance of numerous individuals who have received eradication therapy is recommended despite the lack of biomarkers. Recently, the focus has been on functional reversibility after successful eradication as another cue to elucidate the mechanisms of restoration as well as those of carcinogenesis in the gastric mucosa after H. pylori eradication. We demonstrated that Congo-red chromoendoscopy enabled the identification of the multi-focal distribution of functionally irreversible mucosa compared with that of restored mucosa after successful eradication in individuals at extremely high risk for GC. Further research that uses functional imaging may provide new insights into the mechanisms of regeneration and carcinogenesis in the gastric mucosa post-eradication and may allow for the development of useful biomarkers.
Core tip: Since the indication for eradication therapy for Helicobacter pylori became widespread, the number of gastric cancer (GC) cases that emerge after eradication has continued to increase. However, the underlying mechanism of restoration/carcinogenesis in the gastric mucosa after eradication therapy has not been fully elucidated. We previously demonstrated that, instead of conventional endoscopy, Congo-red chromoendoscopy might be more precise in its ability to identify the multi-focal distribution of functionally irreversible mucosa that may be present in individuals with high risk for GC even after successful eradication. Further research using functional imaging may provide new insights to address the mechanism of gastric regeneration/carcinogenesis, and subsequently, may allow for the development of biomarkers for GC in high risk individuals during post-eradication surveillance.
- Citation: Uno K, Iijima K, Shimosegawa T. Gastric cancer development after the successful eradication of Helicobacter pylori. World J Gastrointest Oncol 2016; 8(3): 271-281
- URL: https://www.wjgnet.com/1948-5204/full/v8/i3/271.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i3.271
Gastric cancer (GC) is considered a major cause of cancer-related deaths worldwide[1], and cancer prevention based on the knowledge of gastric carcinogenesis has been considered the most useful strategy. It has been shown that GC is closely associated with chronic inflammation due to persistent infection with Helicobacter pylori (H. pylori), a class I gastric carcinogen. The development of GC occurs through a multi-step sequence from chronic gastritis, chronic atrophic gastritis, intestinal metaplasia (IM), dysplasia, and finally, GC after an extended period of time[2-4]. In a cohort study of 4655 healthy asymptomatic individuals with a 7.7 ± 0.9-year surveillance, the risk of GC was increased in a stepwise fashion from patients with H. pylori-positive chronic gastritis to those with H. pylori-positive chronic atrophic gastritis, and finally, to those with atrophic metaplastic gastritis (HR = 7.1; 95%CI: 1-53.3 vs HR = 14.9; 95%CI: 2-107.7 vs HR = 61.9; 95%CI: 5.6-682.6, respectively)[5].
In fact, clinical outcomes are determined by a complex interaction of multiple factors, such as H. pylori virulence factors, genetic susceptibility of the host, and environmental factors[6,7]. The bacterial virulence factors, such as cytotoxin-associated gene A antigen (CagA) and vacuolating cytotoxin, play an important role in H. pylori-associated gastric disorders[8,9]. Notably, the CagA gene, which is part of the cag pathogenicity island, produces one of the most pivotal virulence factors of many carcinogenic signaling pathways[10,11]. Moreover, the epithelial cells and inflammatory cells in the gastric mucosa are in direct contact with the outer environment. Therefore, the immune receptors on these cells, such as toll-like receptors and nucleotide-binding oligomerization domain-containing protein, recognize bacterial components of H. pylori and other oral microbes that reach the gut[12,13]. Actually, the long-term colonization by H. pylori was reported to cause an environment of chronic inflammation together with reductions in acidity and in the level of antioxidant enzymes in the gastric juice. This results in the synergistic overgrowth of ingested outer bacteria in the intra-gastric environment, which sustains the inflammation[12,14]. As a result, these reactions can produce large amounts of inflammatory mediators, such as reactive oxygen/nitrogen species and inflammatory cytokines, which may facilitate the aberrant methylation of the cytosine ring preceding guanine (i.e., CpG dinucleotide) islands in the promoters of DNA or microRNAs (miRNAs)[15,16]. A theory of the field effect of carcinogenesis, which states that pathologically non-cancerous tissue that surrounds the tumor may also have genetic/epigenetic alterations, is currently accepted as a reliable mechanism of gastric carcinogenesis[17]. For example, aberrant genetic methylation was reported to accumulate during the sequential development of GC[18,19], and further quantitative methylation analyses of both driver and passenger genes showed that patients with GC had higher methylation levels in the background normal-appearing tissues than those without GC; additionally, patients with multiple gastric tumors had significantly higher methylation levels than those with a single gastric tumor[20-22]. Thereafter, some epigenetic alterations were shown to be restored by the elimination of H. pylori[23-31].
Accordingly, it is strongly believed that successful eradication of H. pylori may be an effective approach for the prevention of GC, and the numbers of subjects in whom H. pylori has been eradicated have continued to increase. However, it was also demonstrated that eradication therapy had only limited effects on the inhibition of GC development. Sporadic cases of GC that emerge after successful eradication have been observed in a clinical setting. These cases strongly suggest two possibilities for the development of sequential carcinogenesis after eradication: A point-of-no-return, where irreversible alterations have already occurred in spite of the eradication or another cause of carcinogenesis such as bile reflux or other microorganisms. Here, we review the results of clinical and laboratory studies on the preventive effect of H. pylori eradication on gastric carcinogenesis to establish future surveillance strategies.
Although some studies have found that the H. pylori eradication gradually led to regression or complete resolution of chronic gastritis[32-34], it is still unclear whether eradication therapy might truly prevent the development of premalignant lesions/GC in patients with chronic gastritis and several grades of inflammation, atrophy and metaplasia.
In a randomized control trial (RCT) that included a total of 435 H. pylori-infected subjects with/without eradication followed by an endoscopic biopsy after 5 years of surveillance, Leung et al[35] showed that eradication was protective against the progression of premalignant gastric lesions in 52.9% of H. pylori-positive subjects. On the contrary, in a population-based RCT of 1630 healthy H. pylori-carriers in China, Wong et al[36] showed that the incidence of GC was similar between participants who had received eradication treatment and those who received placebo during an observation period of 7.5 years (7/817 vs 11/813, P = 0.33, respectively). However, eradication therapy significantly decreased the incidence of GC only in a limited group of 988 H. pylori-carriers who did not have premalignant lesions when they entered the study. A well-designed meta-analysis of 6 RCTs that were conducted primarily in Japan and China, demonstrated a significant decline of 1.6% (51/3294) in the occurrence of GC in patients who had received eradication therapy compared with 2.4% (76/3203) of all patients who did not receive eradication therapy during a period longer than 4 years [relative risk (RR) = 0.66; 95%CI: 0.46-0.95]. However, eradication therapy did not demonstrate a significant benefit in terms of the prevention of GC incidence in any of the subjects with or without premalignant lesions at base-line[37,38]. These data not only provided evidence that successful eradication might reduce the incidence of GC in asymptomatic H. pylori-infected Asian individuals but also suggested the possibility of a point-of-no-return, beyond which eradication may be of no use for cancer prevention. Actually, some discrepancies exist about whether eradication therapy can reduce the risk of GC occurrence in subjects with pathologically defined premalignant changes. Such discrepancies may be caused by several factors, as follows: Selection of the study population, including age, dietary habits, smoking, alcohol abuse, and genetic factors that may influence the susceptibilities of the host; H. pylori strain; intra-gastric conditions with/without premalignant lesions at the time of eradication therapy; and methodological differences during surveillance, including biopsy protocols and the interval/duration of surveillance. A more recent study identified several risk factors for the failure of eradication therapy to prevent GC in a Chinese community-based randomized trial, and thus the sequential data from the long-term surveillance of these patients should provide promising data on the preventive effect of eradication on the incidence of GC[39].
Previous studies have demonstrated that eradication did not completely eliminate the risk of secondary cancer occurrence after curative endoscopic resection (ER) of the primary GC, although it was found that eradication might suppress or delay the development of cancer[40]. Based on the theory of field cancerization, the remaining gastric mucosa after ER is thought to harbor endoscopically invisible micro-lesions with malignant potential[41,42].
Uemura et al[43] first reported that eradication after ER significantly decreased the occurrence of metachronous GC[44]. Similarly, an RCT in Japan also showed that eradication after ER had a protective effect with respect to the development of secondary GC during a 3-year follow-up period. In contrast, a RCT in South Korea revealed that eradication after ER for gastric neoplasms, including GC and low- or high-grade dysplasia, did not reduce the incidence of metachronous GC[45]. A Japanese retrospective study used longer-term follow-up data of 1.1 to 11.1 years (median 3.0 years) for 268 H. pylori–positive patients who had undergone ER for GC, which may or may not have been followed by successful eradication. This study showed that eradication did not reduce the incidence of metachronous GC more than 5 years after ER (8.5% vs 14.3%, respectively, P = 0.262, log-rank test) and that the existence of mucosal atrophy at the time of entry into the study, and not H. pylori-infection status, was an independent risk factor for the incidence of metachronous GC[46]. Accordingly, the preventive effect of successful eradication on metachronous GC remains controversial, possibly because of selection bias, underpowered numbers and residual confounding factors that may have been present in the studies. However, in an up-to-date meta-analysis of 13 relevant studies including 3 prospective trials, Yoon SB et al[47] demonstrated a preventive effect of eradication on the incidence of metachronous GC after ER. In detail, compared with the patients who did not receive eradication therapy, the pooled odds ratio in those who received eradication therapy was 0.42 (95%CI: 0.32-0.56). The pooled analysis of 6237 patients in all 13 studies demonstrated a significantly lower incidence of metachronous GC in the patients who received eradication therapy compared with those who did not [4.7% (111/2382) vs 6.8% (263/3855, 6.8%), respectively]. From now on, it will be difficult to conduct further well-designed and practical studies with long-term surveillance due to the ethical considerations that result from the fact that Japanese and Korean clinical guidelines have already recommended H. pylori eradication in cases of chronic gastritis as well as in cases of post-ER gastric background mucosa[48,49]. Instead, regular surveillance endoscopy after eradication is recommended as the most practical method because GC occurrence may be uninterrupted even after successful eradication. Therefore, the development of useful biomarkers for GC that emerges after successful eradication will be urgently needed to detect high risk groups among such a large number of individuals who have received eradication therapy.
Previous systematic reviews and meta-analyses have demonstrated the impact of H. pylori eradication on the gastric histology of precancerous lesions (e.g., atrophy and IM) according to the (updated) Sydney System, during a surveillance period of less than 10 years[50-52]. Through an examination of one RCT and seven observational studies, Rokkas et al[50] found that eradication had beneficial effects on gastric atrophy, but not on IM, in both the antrum and corpus. After the inclusion of three RCTs and nine observational studies, Wang et al[51] showed that gastric atrophy in the corpus only recovered after eradication, but atrophy in the antrum and IM of the corpus and antrum did not recover. On the contrary, some studies incorporated longer-term follow-up of the histological changes after successful eradication based on the updated Sydney System. These previous studies revealed that, after atrophy of any site within the gastric mucosa and IM in the lesser curvature of the corpus were gradually restored, scores at both 5 and 10 years after eradication were significantly decreased compared with those at base-line[52-54].
Accordingly, controversial results have been obtained from limited surveillance periods and studies with various methodological flaws, and therefore, additional RCTs with a longer observational period are required.
Genetic and epigenetic alterations have been implicated mainly in the activation of oncogenes and/or the inactivation of tumor suppressor genes that are involved in cell-cycle regulation, cell-cell adhesion, DNA repair, and telomerase activation during gastric carcinogenesis.
The effect of eradication treatment on genetic abnormalities remains unclear. Ohara et al[55] showed that eradication might not have led to changes in chromosomal structures, such as loss of heterozygosity and microsatellite instability, in 13 patients with H. pylori-positive GC, 12 patients with H. pylori-negative GC and 8 patients with GC detected after eradication. On the contrary, Zhu et al[56] revealed that increased expression of human telomerase, including ribonucleoprotein/human telomerase reverse transcriptase and human telomerase RNA, in the H. pylori-infected mucosa was significantly diminished after successful eradication.
Recent interest in oncology research has shifted from genetics toward epigenetics. Actually, 90% of heritable alterations in cancer cells have been reported to be epigenetic in nature[57,58]. Additionally, the most important difference between genetic and epigenetic alterations is that epigenetic changes are reversible by therapeutic interventions. Previous studies demonstrated that the medical elimination of H. pylori infection might reverse the epigenetic alterations and thus restore the histological phenotype of the gastric mucosal tissue, in spite of some discrepancies.
Gene promoter methylation: Gene promoter methylation is one of the major epigenetic alterations that is responsible for gene silencing[19]. In particular, the hyper-methylation of CpG-dinucleotide islands within promoter regions of genes, such as CDH1, APC, COX2, CHFR, DAPK, DCC, GSTP1, MAGE, MLH1, p16, PTEN, p14, RASSF1A, RUNX3, SNCG, THBS1, TIMP-3, and TSLC1, have been reported to be associated with aberrant transcriptional gene silencing and consequent loss of protein expression during the multistep development of gastric carcinogenesis. It is well-known that dense methylation of CpG islands through the accumulation of “non-core” and “scattered” DNA methylation causes permanent overall deregulation of carcinogenic signal transduction[59-68]. These phenomena are strongly supported by the epigenetic field defect hypothesis, but previous studies have shown complex changes in DNA methylation in non-cancerous mucosa after eradication; these changes are possibly due to the complicated results of reversible and irreversible changes of aberrant methylation in the gastric mucosa with various grades of inflammation/atrophy/metaplasia.
Originally, two studies from groups in Hong-Kong found that eradication reversed CDH1 promoter methylation and caused a reduction of inflammatory cell infiltration in the non-cancerous gastric mucosa[25,26]. The first RCT involved 81 patients with biopsy-proven H. pylori-positive chronic active gastritis who were treated with/without eradication therapy. In this study, Chan et al[25] demonstrated that the methylation status of CDH1 as well as inflammatory cell infiltration at 6 wk after eradication therapy were significantly reversed only in patients without IM. In the second RCT, Leung et al[26] showed that eradication significantly reduced the methylation density of the CDH1 promoter in biopsy specimens that were obtained from the antrum and corpus of 28 middle-aged H. pylori-infected subjects without GC.
Thereafter, interest in other target genes has increased. Perri et al[27] suggested that gene-specific alterations in the gastric mucosa after successful eradication resulted from promoter methylation of CDH1, APC, COX2, MLH1, and p16. This conclusion was based on an examination of three paired gastric biopsy specimens obtained from the antrum, angles, and corpus of 57 H. pylori-positive dyspeptic outpatients before and 1 year after eradication. Significant differences were observed in the mean numbers of methylated genes, which were 0, 1.1 ± 0.9, and 1.6 ± 0.9 among the 12 H. pylori-/IM- patients, 28 H. pylori+/IM- patients, and 17 H. pylori+/IM+ patients, respectively. In the 17 patients who received eradication therapy out of 45 total H. pylori-positive patients, a significant reversal of DNA methylation in both CDH1 and p16, and to a lesser extent, in APC and COX2, was observed after eradication. Sepulveda et al[28] revealed that hyper-methylation of CpG islands within the promoter of the gene that encodes the DNA repair protein MGMT was significantly reduced at 6-8 wk after successful eradication in 19 patients with H. pylori-gastritis (from 70% to 48% of cases, P < 0.039); an increase in the expression of MGMT protein was only seen on the mucosal surface. Using data from real-time methylation-specific PCR for FLNc and THBD and the updated Sydney System, Nakajima et al[29] examined specimens from 35 post-ER patients and 11 H. pylori-negative healthy volunteers who underwent successful eradication. They showed that the methylation levels were gradually decreased together with a decrease in inflammatory cell infiltration throughout the 1 year after eradication, but that the methylation levels in post-ER patients were persistently higher than those in healthy individuals. Shin et al[30] investigated DNA methylation of the tumor-suppressor genes LOX, APC and MOS over a time course of 26.0 mo in non-cancerous gastric mucosa after eradication in 221 subjects. They showed that successful eradication significantly decreased the methylation levels within the LOX promoter, but not in APC, while a reduction in the MOS methylation level after eradication was significant only in subjects without IM at base-line. In addition, hypo-methylation of CpG islands is also considered to be another major mechanism of DNA methylation at an early phase during H. pylori-associated gastric carcinogenesis, because many genes that are susceptible to aberrant hyper-methylation were reported to overlap with the target genes of global hypo-methylation[69-71]. Using an immunohistochemical evaluation of 5-methylcytosine, a marker of global hypo-methylation, as well as Ki67 and p53, Compare et al[31] assessed the changes in global hypo-methylation during a 10-year follow-up period after successful eradication in 10 patients with pre-neoplastic gastric lesions (i.e., atrophy and IM). They demonstrated that global DNA methylation was negatively correlated with a gradual decrease in proliferative/apoptotic markers, depending on the histological grade of the multi-step transformation into GC, but independently of the Cag-A status and treatment response to eradication therapy.
miRNA: MiRNAs, which are non-coding RNA sequences that are 18- to 25-nucleotides in length, are transcribed without translation into proteins and are involved in post-transcriptional gene silencing[72,73]. Tissue-specific expression of miRNAs has been shown to be associated with apoptosis, proliferation, differentiation, metastasis, angiogenesis, and immune responses in inflammatory-associated carcinogenesis[74,75]. With regard to gastric carcinogenesis, several studies have reported different phenotypes of miRNA expression in the normal mucosa, H. pylori-induced precancerous lesions and GC[76-80], but the changes in miRNA expression after successful eradication are still not completely understood.
Matsushima et al[79] revealed that the down-regulation of 14 miRNAs in 4 H. pylori-positive individuals was increased at 4 wk after successful eradication, although the expression of oncogenic miRNAs was significantly diminished only in non-metaplastic mucosa, but not in cases of IM, at 1 year after the eradication. Specifically, hsa-miR-21, hsa-miR-25, hsa-miR-93, hsamiR-194 and hsa-miR-196 were found to be overexpressed in GC in comparison with non-cancerous gastric tissues, and eradication therapy decreased the expression levels of these miRNAs only in cases of atrophic gastritis without metaplastic changes. Moreover, in a case-control study using endoscopic biopsy specimens from 20 patients who were treated with ER for GC and 14 sex- and age-matched non-cancer controls, Shiotani et al[81] revealed that, although the expression of oncogenic miRNAs (miR-17/92 and the miR-106b-93-25 cluster, miR-21, miR-194, and miR-196) was significantly higher in the gastric mucosa of the post-ER group than in the controls, none of these miRNAs that were expressed in cases of IM were significantly changed after eradication in any of the groups. This indicated that eradication therapy might be effective in the restoration of the expression of oncogenic miRNAs only in patients with atrophic gastritis and not in those with IM. On the contrary, in a more recent multi-center prospective cohort study of 782 patients who were treated with ER for GC followed by eradication, Asada et al[82] demonstrated that the risk of metachronous GC after ER might be predicted via an assessment of epigenetic field defects using the methylation levels of three genes (miR-124a-3, EMX1 and NKX6-1) by quantitative methylation-specific PCR. In the post-ER background gastric mucosa, which might be highly associated with metaplastic changes, the highest quartile of the miR-124a-3 methylation level had a significant univariate (HR = 2.2; 95%CI: 1.1-4.4; P = 0.03) and a multivariate-adjusted (HR = 2.3; 95%CI: 1.0-5.1; P = 0.04) for the incidence of metachronous GC[82]. However, questions remain about whether those parameters are simultaneously expressed in the metaplastic lesions as well as in the non-metaplastic mucosa, both of which are categorized as background gastric mucosa with malignant potential; questions also remain as to whether some uniform phenotypes of IM with those parameters might develop into GC after eradication. Considering that the metaplastic mucosa is already regarded as a clinically malignant predictor, future biological predictors will provide valuable ways to overcome these clinical issues.
Furthermore, miRNAs have been reported to exist in cell-free stable forms in the plasma and serum, and therefore, serum miRNAs may serve as novel biomarkers during non-invasive surveillance for large numbers of individuals.
Clinical and laboratory studies have found only limited usefulness of H. pylori eradication in the regression of DNA methylation and miRNA expression in the non-cancerous gastric mucosa, which seems to be dependent on the gene type and histological grade of the atrophy and metaplasia. In other words, successful eradication might reverse the malignant potential of pathologically non-cancerous gastric mucosa prior to the point-of-no-return.
The first limitation of the aforementioned studies is gene-specific restoration after eradication. Instead of genome-wide studies, the investigators targeted only a single gene or a few genes, whose role in gastric carcinogenesis had not yet been clarified[17,30]. The second limitation may have originated from differences in the sensitivities of the histology and molecular biology techniques, such as methylation-specific PCR, that were used to detect the abnormalities. In addition, the distribution of metaplastic/dysplastic lesions is uneven throughout the entire gastric mucosa, which results in sampling error by targeted biopsy, although the distribution as well as the phenotype of the IM were suggested to provide a higher predictive value of the cancer risk. Cassaro et al[83] showed that IM on the lesser curvature from the cardia to the pylorus, or throughout the entire stomach, was associated with a higher risk of GC than focal/antrum-predominant IM. Among the three phenotypes of IM [i.e., complete IM (type I) or incomplete IM (types II, type III)], type III IM was reported to harbor more genetic/epigenetic changes, which are similar to those that were found in cases of gastric dysplasia[84,85]. However, the distribution of molecular and morphological alterations in the gastric mucosal tissue after successful eradication are likely to be more complex than those that were present prior to eradication, where the multi-focal distribution of epigenetic events is related to the severity of inflammation or metaplastic changes. As previous studies have demonstrated the gradual and irregular occurrence of areas of gastric-acid secretion up to 2 years after successful eradication, the areas that are restored after eradication might be irregular/atypical[86-88]. These phenomena may result in increased sampling errors by random biopsies under a conventional endoscopic inspection, especially those in the post-eradicated gastric mucosa. Since the updated Sydney System was originally established to assist pathologists in the formal recording of their findings in a clinical setting, but not in a research setting, a suitable scoring system to guide targeted biopsies of the gastric mucosa after successful eradication is needed[89].
Functional reversibility of the gastric mucosa after successful eradication will provide another important clue, similar to what was provided by the reversibility of the macroscopic/microscopic morphology. The reversibility might be caused by the reduced production of inflammatory cytokines that have inhibitory effects on gastric acid secretion or by the increased expression of H+/K+-ATPase, intrinsic factor, and M3 muscarinic receptor mRNA in the gastric mucosa, but is likely not caused by the numbers of parietal cells[90,91].
Ji et al[92] used in vivo functional imaging as well as tissue morphological imaging by confocal laser endomicroscopy (CLE) and fluorescein tracers to investigate the reversibility of mucosal barrier defects after eradication in 42 H. pylori-positive patients. They demonstrated that gastric IM was associated with an impaired para-cellular barrier, which did not recover after eradication. Essentially, the para-cellular permeability in the setting of IM, despite H. pylori infection, was significantly higher than that in non-IM tissue with H. pylori infection, which was in turn higher than that in non-IM tissue without H. pylori infection. Six months after successful eradication in 14 patients, the para-cellular barrier dysfunction of the non-IM mucosa was significantly improved, as shown by electron microscopy and CLE, in spite of a lack of significant changes in IM. The irreversible mucosal barrier dysfunction of IM may cause constant trans-epithelial penetration of various intra-gastric substances, which may promote an immune response and the further development of cancer. Accordingly, the CLE findings may be useful for the depiction of the functional and morphological irreversibility of the disrupted para-cellular barrier of IM as a reasonable cause of carcinogenesis in patients with IM, although CLE has point-sampling characteristics.
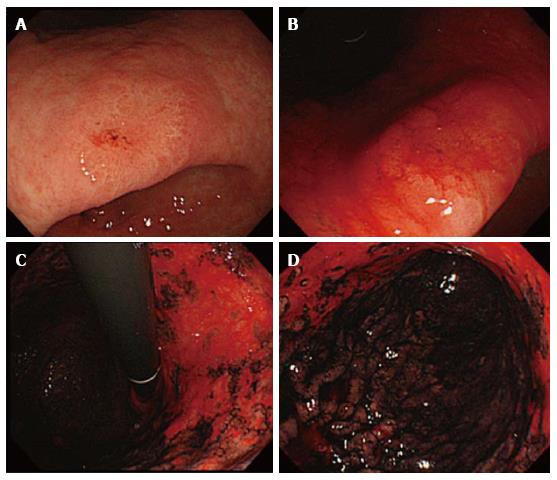
Congo-red chromoendoscopy is another functional test that is used to visualize the gastric areas that are capable of gastric acid secretion; this technique involves a pH-dependent color reaction, which may be performed during a conventional endoscopic inspection (Figure 1)[93,94]. Instead of CLE and an endoscopic biopsy, this method provides a comprehensive view that distinguishes endoscopically normal-appearing gastric mucosa based on functional characteristics. Additionally, its reproducibility may be ideal for the measurement of functional and morphological changes in the mucosa during long-term surveillance after eradication.
Based on findings by Congo-red chromoendoscopy, Iijima et al[88] found an expansion of the acid-secreting mucosa during a mean follow-up of 62 mo after eradication in 24 H. pylori-positive patients. Subsequently, after an investigation of the histological features using a Ki67 labeling index in the biopsy samples that were obtained from both acid-secreting and non-acid-secreting areas, they showed that functionally irreversible gastric mucosa after eradication was associated with extensive IM and sustained hyper-proliferation. This indicates the importance of increased malignant potential in the genesis of GC. Specifically, during the surveillance periods, the entire area of the greater curvature became occupied exclusively by acid-secreting mucosa, whereas the expansion of the acid-secreting area in the lesser curvature was limited. With the exception of the score for neutrophil infiltration, the mean inflammation score in the non-acid-secreting area was significantly higher than in the acid secreting area (1.3 ± 0.5 vs 0.8 ± 0.5, respectively). Marked atrophic/metaplastic changes with high numbers of Ki67-positive cells were observed in non-acid-secreting areas, whereas none to mild atrophic/metaplastic changes with a low number of Ki67-positive cells were found in acid-secreting areas. Accordingly, this study showed that even long after successful eradication, the histological degrees of residual inflammation infiltration, atrophy, IM, and epithelial proliferation in the functionally irreversible non-acid-secreting areas were significantly higher than those in the functionally recovered area. Another study that used Congo-red chromoendoscopy in a total of 19 GC lesions that emerged after more than 2 years post-successful eradication in 14 consecutive patients demonstrated that GCs were observed exclusively in non-acid-secreting areas with sustained hyper-proliferation and accumulation of p53 protein[95]. Accordingly, the non-acid secreting areas that were observed by Congo-red chromoendoscopy might truly be at a high risk for gastric carcinogenesis after eradication. Surprisingly, these studies also demonstrated that histological evaluation of targeted biopsy specimens from both acid-secreting and non-acid-secreting areas based on Congo-red chromoendoscopic findings revealed striking differences in their histological manifestations, even if these areas were separated by only a few centimeters. Therefore, Congo-red chromoendoscopy enables us to identify high-risk areas in a site-specific manner and to determine the distribution of functionally and morphologically irreversible mucosa after successful eradication. With careful consideration of the balance between the risks and benefits, Congo-red chromoendoscopy can provide important clues to gastric carcinogenesis that occurs after successful eradication[96]. Further research on biopsy specimens guided by functional imaging, such as Congo-red chromoendoscopy, may be a promising approach in the clarification of molecular-biological events in the functional and morphological restoration and carcinogenesis after successful eradication.
In conclusion, we reviewed the limited effect of the H. pylori eradication on the reduction of GC incidence, although the indication for eradication therapy is clinically widespread. No useful biomarkers for individuals who are at a high risk for GC that emerges after successful eradication have been identified because previous studies by conventional methods have not yet fully elucidated the underlying mechanisms of mucosal restoration as well as of gastric carcinogenesis after eradication. In accordance with a proof-of-concept of the epigenetic field defect of gastric cancerization, we propose the usefulness of Congo-red chromoendoscopy to differentiate functionally irreversible mucosal areas that are at an extremely high risk for gastric carcinogenesis. In addition, another study that used CLE with tracers identified the functional irreversibility of the metaplastic mucosa in the setting of post-eradication carcinogenesis. Therefore, future research that focuses on functional aspects may provide new insights that address both the mechanisms of mucosal regeneration and cancer development after successful eradication.
P- Reviewer: Misra SP S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4227] [Cited by in RCA: 4049] [Article Influence: 144.6] [Reference Citation Analysis (2)] |
| 2. | Danesh J. Helicobacter pylori infection and gastric cancer: systematic review of the epidemiological studies. Aliment Pharmacol Ther. 1999;13:851-856. [PubMed] |
| 3. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [PubMed] |
| 4. | Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37-S43. [PubMed] |
| 5. | Ohata H, Kitauchi S, Yoshimura N, Mugitani K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138-143. [PubMed] |
| 6. | Graham DY, Go MF. Helicobacter pylori: current status. Gastroenterology. 1993;105:279-282. [PubMed] |
| 7. | Cadamuro AC, Rossi AF, Maniezzo NM, Silva AE. Helicobacter pylori infection: host immune response, implications on gene expression and microRNAs. World J Gastroenterol. 2014;20:1424-1437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (2)] |
| 8. | Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 9. | Wen S, Moss SF. Helicobacter pylori virulence factors in gastric carcinogenesis. Cancer Lett. 2009;282:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Higashi H, Nakaya A, Tsutsumi R, Yokoyama K, Fujii Y, Ishikawa S, Higuchi M, Takahashi A, Kurashima Y, Teishikata Y. Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation. J Biol Chem. 2004;279:17205-17216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 11. | Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: The master key hypothesis. Helicobacter. 2010;15:163-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Uno K, Kato K, Atsumi T, Suzuki T, Yoshitake J, Morita H, Ohara S, Kotake Y, Shimosegawa T, Yoshimura T. Toll-like receptor (TLR) 2 induced through TLR4 signaling initiated by Helicobacter pylori cooperatively amplifies iNOS induction in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1004-G1012. [PubMed] |
| 13. | Uno K, Kato K, Shimosegawa T. Novel role of toll-like receptors in Helicobacter pylori - induced gastric malignancy. World J Gastroenterol. 2014;20:5244-5251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Kim KK, Kim HB. Protein interaction network related to Helicobacter pylori infection response. World J Gastroenterol. 2009;15:4518-4528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Kang GH, Lee S, Kim JS, Jung HY. Profile of aberrant CpG island methylation along the multistep pathway of gastric carcinogenesis. Lab Invest. 2003;83:635-641. [PubMed] |
| 16. | Hmadcha A, Bedoya FJ, Sobrino F, Pintado E. Methylation-dependent gene silencing induced by interleukin 1beta via nitric oxide production. J Exp Med. 1999;190:1595-1604. [PubMed] |
| 17. | Ushijima T. Epigenetic field for cancerization. J Biochem Mol Biol. 2007;40:142-150. [PubMed] |
| 18. | Kang GH, Shim YH, Jung HY, Kim WH, Ro JY, Rhyu MG. CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res. 2001;61:2847-2851. [PubMed] |
| 19. | Nardone G, Rocco A, Malfertheiner P. Review article: helicobacter pylori and molecular events in precancerous gastric lesions. Aliment Pharmacol Ther. 2004;20:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989-995. [PubMed] |
| 21. | Kang GH, Lee S, Cho NY, Gandamihardja T, Long TI, Weisenberger DJ, Campan M, Laird PW. DNA methylation profiles of gastric carcinoma characterized by quantitative DNA methylation analysis. Lab Invest. 2008;88:161-170. [PubMed] |
| 22. | Nakajima T, Maekita T, Oda I, Gotoda T, Yamamoto S, Umemura S, Ichinose M, Sugimura T, Ushijima T, Saito D. Higher methylation levels in gastric mucosae significantly correlate with higher risk of gastric cancers. Cancer Epidemiol Biomarkers Prev. 2006;15:2317-2321. [PubMed] |
| 23. | Tahara E. Molecular mechanism of human stomach carcinogenesis implicated in Helicobacter pylori infection. Exp Toxicol Pathol. 1998;50:375-378. [PubMed] |
| 24. | Verma M, Srivastava S. Epigenetics in cancer: implications for early detection and prevention. Lancet Oncol. 2002;3:755-763. [PubMed] |
| 25. | Chan AO, Peng JZ, Lam SK, Lai KC, Yuen MF, Cheung HK, Kwong YL, Rashid A, Chan CK, Wong BC. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut. 2006;55:463-468. [PubMed] |
| 26. | Leung WK, Man EP, Yu J, Go MY, To KF, Yamaoka Y, Cheng VY, Ng EK, Sung JJ. Effects of Helicobacter pylori eradication on methylation status of E-cadherin gene in noncancerous stomach. Clin Cancer Res. 2006;12:3216-3221. [PubMed] |
| 27. | Perri F, Cotugno R, Piepoli A, Merla A, Quitadamo M, Gentile A, Pilotto A, Annese V, Andriulli A. Aberrant DNA methylation in non-neoplastic gastric mucosa of H. Pylori infected patients and effect of eradication. Am J Gastroenterol. 2007;102:1361-1371. [PubMed] |
| 28. | Sepulveda AR, Yao Y, Yan W, Park DI, Kim JJ, Gooding W, Abudayyeh S, Graham DY. CpG methylation and reduced expression of O6-methylguanine DNA methyltransferase is associated with Helicobacter pylori infection. Gastroenterology. 2010;138:1836-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Nakajima T, Enomoto S, Yamashita S, Ando T, Nakanishi Y, Nakazawa K, Oda I, Gotoda T, Ushijima T. Persistence of a component of DNA methylation in gastric mucosae after Helicobacter pylori eradication. J Gastroenterol. 2010;45:37-44. [PubMed] |
| 30. | Shin CM, Kim N, Lee HS, Park JH, Ahn S, Kang GH, Kim JM, Kim JS, Lee DH, Jung HC. Changes in aberrant DNA methylation after Helicobacter pylori eradication: a long-term follow-up study. Int J Cancer. 2013;133:2034-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Compare D, Rocco A, Liguori E, D’Armiento FP, Persico G, Masone S, Coppola-Bottazzi E, Suriani R, Romano M, Nardone G. Global DNA hypomethylation is an early event in Helicobacter pylori-related gastric carcinogenesis. J Clin Pathol. 2011;64:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, Ro JY. Epstein-barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002;160:787-794. [PubMed] |
| 33. | Byun DS, Lee MG, Chae KS, Ryu BG, Chi SG. Frequent epigenetic inactivation of RASSF1A by aberrant promoter hypermethylation in human gastric adenocarcinoma. Cancer Res. 2001;61:7034-7038. [PubMed] |
| 34. | Kang YH, Lee HS, Kim WH. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest. 2002;82:285-291. [PubMed] |
| 35. | Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, Lau JY, Sung JJ. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut. 2004;53:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 318] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 36. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [PubMed] |
| 37. | Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, Liu WD, Hu Y, Han ZX, Crystal-Mansour S. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 353] [Article Influence: 27.2] [Reference Citation Analysis (1)] |
| 38. | Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 442] [Article Influence: 40.2] [Reference Citation Analysis (1)] |
| 39. | Pan KF, Zhang L, Gerhard M, Ma JL, Liu WD, Ulm K, Wang JX, Zhang L, Zhang Y, Bajbouj M. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut. 2016;65:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 40. | Asaka M, Kato M, Graham DY. Prevention of gastric cancer by Helicobacter pylori eradication. Intern Med. 2010;49:633-636. [PubMed] |
| 41. | Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005;37:990-993. [PubMed] |
| 42. | Peng J, Wang Y. Epidemiology, pathology and clinical management of multiple gastric cancers: a mini-review. Surg Oncol. 2010;19:e110-e114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Haruma K, Sumii K, Kajiyama G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639-642. [PubMed] |
| 44. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 935] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 45. | Choi J, Kim SG, Yoon H, Im JP, Kim JS, Kim WH, Jung HC. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2014;12:793-800.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 46. | Maehata Y, Nakamura S, Fujisawa K, Esaki M, Moriyama T, Asano K, Fuyuno Y, Yamaguchi K, Egashira I, Kim H. Long-term effect of Helicobacter pylori eradication on the development of metachronous gastric cancer after endoscopic resection of early gastric cancer. Gastrointest Endosc. 2012;75:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 47. | Yoon SB, Park JM, Lim CH, Cho YK, Choi MG. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: a meta-analysis. Helicobacter. 2014;19:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 48. | Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, Uemura N, Murakami K, Satoh K, Sugano K. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter. 2010;15:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 49. | Kim SG, Jung HK, Lee HL, Jang JY, Lee H, Kim CG, Shin WG, Shin ES, Lee YC. [Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition]. Korean J Gastroenterol. 2013;62:3-26. [PubMed] |
| 50. | Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. The long-term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta-analysis. Helicobacter. 2007;12 Suppl 2:32-38. [PubMed] |
| 51. | Wang J, Xu L, Shi R, Huang X, Li SW, Huang Z, Zhang G. Gastric atrophy and intestinal metaplasia before and after Helicobacter pylori eradication: a meta-analysis. Digestion. 2011;83:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 52. | Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536-1540. [PubMed] |
| 53. | Kodama M, Murakami K, Okimoto T, Sato R, Uchida M, Abe T, Shiota S, Nakagawa Y, Mizukami K, Fujioka T. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol. 2012;47:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 54. | Ito M, Haruma K, Kamada T, Mihara M, Kim S, Kitadai Y, Sumii M, Tanaka S, Yoshihara M, Chayama K. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther. 2002;16:1449-1456. [PubMed] |
| 55. | Ohara T, Kasanuki J, Ohara H, Kanoh Y, Suzuki H, Hashimoto H, Chiba T, Morishita T, Hibi T. Analysis of the differences in structural chromosomal aberrations of the gastric mucosa between H. pylori positive and negative gastric cancer patients: involvement of H. pylori in the onset of gastric cancer and examination of the mechanism in gastric carcinogenesis following H. pylori eradication. Oncol Rep. 2006;16:1333-1342. [PubMed] |
| 56. | Zhu Y, Shu X, Chen J, Xie Y, Xu P, Huang DQ, Lu NH. Effect of Helicobacter pylori eradication on oncogenes and cell proliferation. Eur J Clin Invest. 2008;38:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Choi IS, Wu TT. Epigenetic alterations in gastric carcinogenesis. Cell Res. 2005;15:247-254. [PubMed] |
| 58. | Vogiatzi P, Vindigni C, Roviello F, Renieri A, Giordano A. Deciphering the underlying genetic and epigenetic events leading to gastric carcinogenesis. J Cell Physiol. 2007;211:287-295. [PubMed] |
| 59. | Shimazu T, Asada K, Charvat H, Kusano C, Otake Y, Kakugawa Y, Watanabe H, Gotoda T, Ushijima T, Tsugane S. Association of gastric cancer risk factors with DNA methylation levels in gastric mucosa of healthy Japanese: a cross-sectional study. Carcinogenesis. 2015;36:1291-1298. [PubMed] |
| 60. | Tsuchiya T, Tamura G, Sato K, Endoh Y, Sakata K, Jin Z, Motoyama T, Usuba O, Kimura W, Nishizuka S. Distinct methylation patterns of two APC gene promoters in normal and cancerous gastric epithelia. Oncogene. 2000;19:3642-3646. [PubMed] |
| 61. | Kikuchi T, Itoh F, Toyota M, Suzuki H, Yamamoto H, Fujita M, Hosokawa M, Imai K. Aberrant methylation and histone deacetylation of cyclooxygenase 2 in gastric cancer. Int J Cancer. 2002;97:272-277. [PubMed] |
| 62. | Iida S, Akiyama Y, Nakajima T, Ichikawa W, Nihei Z, Sugihara K, Yuasa Y. Alterations and hypermethylation of the p14(ARF) gene in gastric cancer. Int J Cancer. 2000;87:654-658. [PubMed] |
| 63. | Lee JH, Byun DS, Lee MG, Ryu BK, Kang MJ, Chae KS, Lee KY, Kim HJ, Park H, Chi SG. Frequent epigenetic inactivation of hSRBC in gastric cancer and its implication in attenuated p53 response to stresses. Int J Cancer. 2008;122:1573-1584. [PubMed] |
| 64. | Leung SY, Yuen ST, Chung LP, Chu KM, Chan AS, Ho JC. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 1999;59:159-164. [PubMed] |
| 65. | Kang SH, Choi HH, Kim SG, Jong HS, Kim NK, Kim SJ, Bang YJ. Transcriptional inactivation of the tissue inhibitor of metalloproteinase-3 gene by dna hypermethylation of the 5’-CpG island in human gastric cancer cell lines. Int J Cancer. 2000;86:632-635. [PubMed] |
| 66. | Park TJ, Han SU, Cho YK, Paik WK, Kim YB, Lim IK. Methylation of O(6)-methylguanine-DNA methyltransferase gene is associated significantly with K-ras mutation, lymph node invasion, tumor staging, and disease free survival in patients with gastric carcinoma. Cancer. 2001;92:2760-2768. [PubMed] |
| 67. | Honda T, Tamura G, Waki T, Kawata S, Terashima M, Nishizuka S, Motoyama T. Demethylation of MAGE promoters during gastric cancer progression. Br J Cancer. 2004;90:838-843. [PubMed] |
| 68. | Li QL, Ito K, Sakakura C, Fukamachi H, Inoue Ki, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113-124. [PubMed] |
| 69. | Gonzalez-Zulueta M, Bender CM, Yang AS, Nguyen T, Beart RW, Van Tornout JM, Jones PA. Methylation of the 5’ CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995;55:4531-4535. [PubMed] |
| 70. | Little M, Wainwright B. Methylation and p16: suppressing the suppressor. Nat Med. 1995;1:633-634. [PubMed] |
| 71. | Yoshida T, Yamashita S, Takamura-Enya T, Niwa T, Ando T, Enomoto S, Maekita T, Nakazawa K, Tatematsu M, Ichinose M. Alu and Satα hypomethylation in Helicobacter pylori-infected gastric mucosae. Int J Cancer. 2011;128:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2577] [Cited by in RCA: 2719] [Article Influence: 159.9] [Reference Citation Analysis (0)] |
| 73. | Beilharz TH, Humphreys DT, Clancy JL, Thermann R, Martin DI, Hentze MW, Preiss T. microRNA-mediated messenger RNA deadenylation contributes to translational repression in mammalian cells. PLoS One. 2009;4:e6783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 74. | Zhou P, Xu W, Peng X, Luo Z, Xing Q, Chen X, Hou C, Liang W, Zhou J, Wu X. Large-scale screens of miRNA-mRNA interactions unveiled that the 3’UTR of a gene is targeted by multiple miRNAs. PLoS One. 2013;8:e68204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7723] [Cited by in RCA: 7369] [Article Influence: 368.5] [Reference Citation Analysis (0)] |
| 76. | Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Müller A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic Gastritis and Colitis. J Immunol. 2011;187:3578-3586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 77. | Isomoto H, Matsushima K, Inoue N, Hayashi T, Nakayama T, Kunizaki M, Hidaka S, Nakayama M, Hisatsune J, Nakashima M. Interweaving microRNAs and proinflammatory cytokines in gastric mucosa with reference to H. pylori infection. J Clin Immunol. 2012;32:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Teng GG, Wang WH, Dai Y, Wang SJ, Chu YX, Li J. Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PLoS One. 2013;8:e56709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 79. | Matsushima K, Isomoto H, Inoue N, Nakayama T, Hayashi T, Nakayama M, Nakao K, Hirayama T, Kohno S. MicroRNA signatures in Helicobacter pylori-infected gastric mucosa. Int J Cancer. 2011;128:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 80. | Chen Q, Wang H, Liu Y, Song Y, Lai L, Han Q, Cao X, Wang Q. Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1β production in macrophages by targeting STAT3. PLoS One. 2012;7:e42971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 208] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 81. | Shiotani A, Uedo N, Iishi H, Murao T, Kanzaki T, Kimura Y, Kamada T, Kusunoki H, Inoue K, Haruma K. H. pylori eradication did not improve dysregulation of specific oncogenic miRNAs in intestinal metaplastic glands. J Gastroenterol. 2012;47:988-998. [PubMed] |
| 82. | Asada K, Nakajima T, Shimazu T, Yamamichi N, Maekita T, Yokoi C, Oda I, Ando T, Yoshida T, Nanjo S. Demonstration of the usefulness of epigenetic cancer risk prediction by a multicentre prospective cohort study. Gut. 2015;64:388-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 83. | Cassaro M, Rugge M, Gutierrez O, Leandro G, Graham DY, Genta RM. Topographic patterns of intestinal metaplasia and gastric cancer. Am J Gastroenterol. 2000;95:1431-1438. [PubMed] |
| 84. | Nardone G, Staibano S, Rocco A, Mezza E, D’armiento FP, Insabato L, Coppola A, Salvatore G, Lucariello A, Figura N. Effect of Helicobacter pylori infection and its eradication on cell proliferation, DNA status, and oncogene expression in patients with chronic gastritis. Gut. 1999;44:789-799. [PubMed] |
| 85. | Ochiai A, Yamauchi Y, Hirohashi S. p53 mutations in the non-neoplastic mucosa of the human stomach showing intestinal metaplasia. Int J Cancer. 1996;69:28-33. [PubMed] |
| 86. | Wallace JL, Cucala M, Mugridge K, Parente L. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol. 1991;261:G559-G564. [PubMed] |
| 87. | Sekine H, Iijima K, Koike T, Abe Y, Imatani A, Kato K, Ohara S, Shimosegawa T. Regional differences in the recovery of gastric acid secretion after Helicobacter pylori eradication: evaluations with Congo red chromoendoscopy. Gastrointest Endosc. 2006;64:678-685. [PubMed] |
| 88. | Iijima K, Koike T, Sekine H, Abe Y, Asanuma K, Ara N, Uno K, Imatani A, Ohara S, Shimosegawa T. Sustained epithelial proliferation in a functionally irreversible fundic mucosa after Helicobacter pylori eradication. J Gastroenterol. 2009;44:47-55. [PubMed] |
| 89. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 90. | Osawa H, Kita H, Ohnishi H, Hoshino H, Mutoh H, Ishino Y, Watanabe E, Satoh K, Sugano K. Helicobacter pylori eradication induces marked increase in H+/K+-adenosine triphosphatase expression without altering parietal cell number in human gastric mucosa. Gut. 2006;55:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Furuta T, Baba S, Takashima M, Shirai N, Xiao F, Futami H, Arai H, Hanai H, Kaneko E. H+/K+-adenosine triphosphatase mRNA in gastric fundic gland mucosa in patients infected with Helicobacter pylori. Scand J Gastroenterol. 1999;34:384-390. [PubMed] |
| 92. | Ji R, Zuo XL, Yu T, Gu XM, Li Z, Zhou CJ, Li YQ. Mucosal barrier defects in gastric intestinal metaplasia: in vivo evaluation by confocal endomicroscopy. Gastrointest Endosc. 2012;75:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Tatsuta M, Iishi H, Ichii M, Noguchi S, Okuda S, Taniguchi H. Chromoendoscopic observations on extension and development of fundal gastritis and intestinal metaplasia. Gastroenterology. 1985;88:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Tatsuta M, Okuda S. Location, healing, and recurrence of gastric ulcers in relation to fundal gastritis. Gastroenterology. 1975;69:897-902. [PubMed] |
| 95. | Iijima K, Abe Y, Koike T, Uno K, Endo H, Hatta W, Asano N, Asanuma K, Imatani A, Shimosegawa T. Gastric cancers emerging after H. pylori eradication arise exclusively from non-acid-secreting areas. Tohoku J Exp Med. 2012;226:45-53. [PubMed] |
| 96. | Shaw D, Blair V, Framp A, Harawira P, McLeod M, Guilford P, Parry S, Charlton A, Martin I. Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy? Gut. 2005;54:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |