Published online Feb 15, 2016. doi: 10.4251/wjgo.v8.i2.147
Peer-review started: May 1, 2015
First decision: July 6, 2015
Revised: November 24, 2015
Accepted: December 13, 2015
Article in press: December 15, 2015
Published online: February 15, 2016
Processing time: 279 Days and 22.9 Hours
Helicobacter pylori (H. pylori) infection is highly prevalent in human, affecting nearly half of the world’s population; however, infection remains asymptomatic in majority of population. During its co-existence with humans, H. pylori has evolved various strategies to maintain a mild gastritis and limit the immune response of host. On the other side, presence of H. pylori is also associated with increased risk for the development of various gastric pathologies including gastric cancer (GC). A complex combination of host genetics, environmental agents, and bacterial virulence factors are considered to determine the susceptibility as well as the severity of outcome in a subset of individuals. GC is one of the most common cancers and considered as the third most common cause of cancer related death worldwide. Many studies had proved H. pylori as an important risk factor in the development of non-cardia GC. Although both H. pylori infection and GC are showing decreasing trends in the developed world, they still remain a major threat to human population in the developing countries. The current review attempts to highlight recent progress in the field of research on H. pylori induced GC and aims to provide brief insight into H. pylori pathogenesis, the role of major virulence factors of H. pylori that modulates the host environment and transform the normal gastric epithelium to neoplastic one. This review also emphasizes on the mechanistic understanding of how colonization and various virulence attributes of H. pylori as well as the host innate and adaptive immune responses modulate the diverse signaling pathways that leads to different disease outcomes including GC.
Core tip: Although the incidence and mortality of gastric cancer (GC) is declining in recent decades but it still remains a major threat in developing countries as compared to developed one. Among various etiological agents, Helicobacter pylori (H. pylori) play a detrimental role in development of GC. Through this review we focus on the recent progress in the field of research on H. pylori induced GC and providing the brief insight into H. pylori pathogenesis, the role of major virulence factors of H. pylori that modulates the host environment and transform the normal gastric epithelium to neoplastic one.
- Citation: Khatoon J, Rai RP, Prasad KN. Role of Helicobacter pylori in gastric cancer: Updates. World J Gastrointest Oncol 2016; 8(2): 147-158
- URL: https://www.wjgnet.com/1948-5204/full/v8/i2/147.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i2.147
In 1984 Marshall and Warren[1] identified Helicobacter pylori (H. pylori) from gastric biopsy culture. In 1994, H. pylori was recognized as definite carcinogen by International agency for research on cancer. H. pylori induced gastric cancer (GC) is accountable for 5.5% of global cancer burden[2].
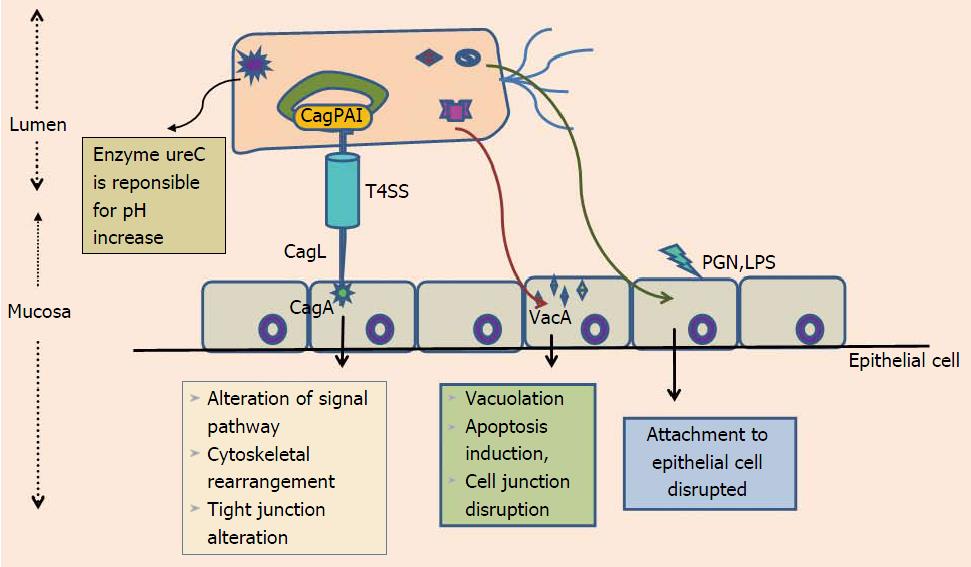
H. pylori is spiral shaped, gram-negative, microaerophilic, flagellated human pathogen that successfully colonizes gastric mucosa of majority of individuals[3]. Epidemiologically, the H. pylori infection is exists all over the world, but colonization rates vary considerably; high in developing compared to the developed world[4]. H. pylori acquisition thought to occurs in early childhood. Fecal-oral or oral-oral were considered as possible route of H. pylori transmission[4,5]. H. pylori urease is among the various virulence factors that aids in colonizing the highly acidic environment of stomach via breakdown of urea into ammonia, generating hospitable locale for its colonization[6] (Figure 1). Among the majority of H. pylori infected individuals only a small percentage of colonized individuals develop severe clinical disease such as GC. Determing factors responsible for variation in clinical outcomes of H. pylori infection are still not well studied. For a longer period of time association between H. pylori and GC was debatable. A study from Japan on 1526 patients gives a clear evidence that H. pylori infection is significantly associated with risk of developing GC[6]. Proof that H. pylori has an influence on early stages of gastric carcinogenesis is demonstrated by randomized prospective studies which shows association between H. pylori eradication and reduction of premalignant tumors[7,8]. Research on experimentally challenged Mongolian gerbils, provide evidence concerning H. pylori eradication with attenuation of developmental process related to GC progression[9,10]. Together these studies authenticate that H. pylori plays a key role in development of GC and indicate that H. pylori eradication provide protection against H. pylori-induced GC. Interaction among environmental factors, host genetic polymorphism and bacterial virulence attributes collectively influence the clinical outcome of H. pylori infections[11].
This review aims to highlight recent progress in H. pylori pathogenesis, especially the bacterial and host factors that are involved in the host-pathogen interaction during persistent colonization. It also highlights the host immune response towards H. pylori colonization and its effect on diverse clinical outcomes, especially on advancement leading to GC.
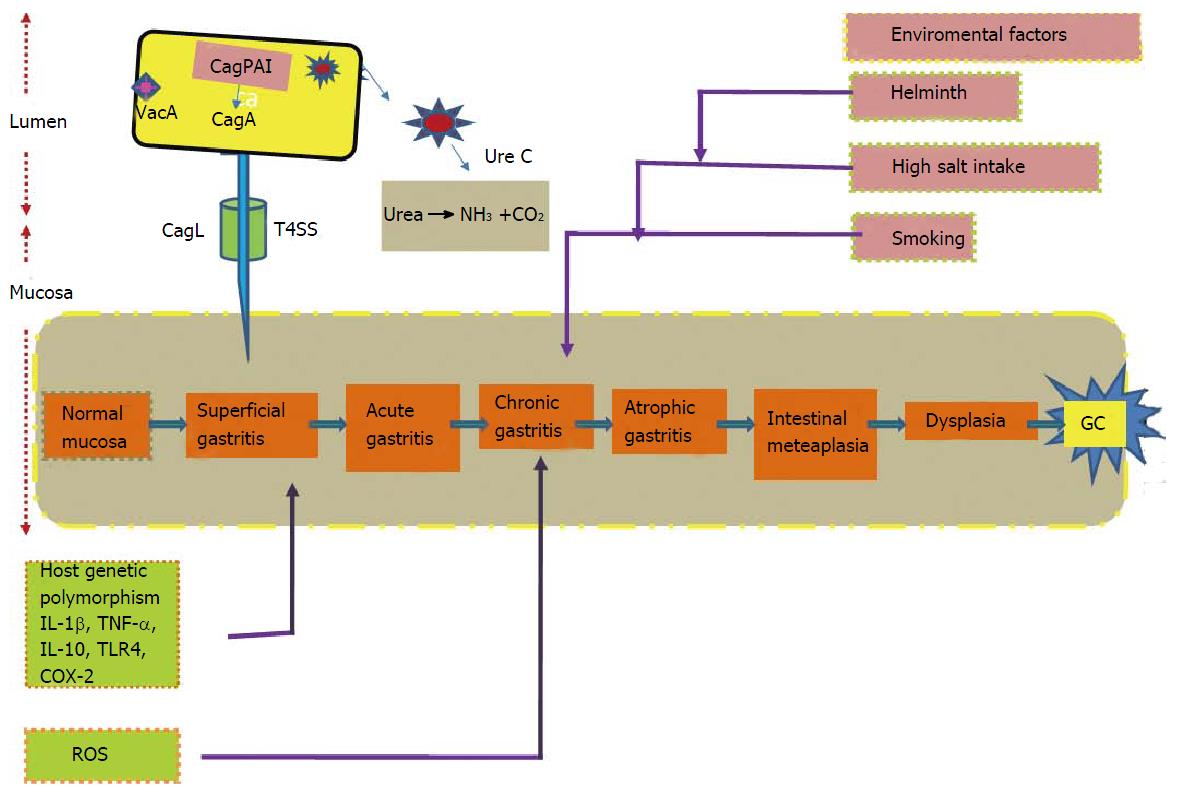
GC is a multifactorial disease. Correa’s model describes array of event beginning from chronic active gastritis, atrophic gastritis, intestinal metaplasia, dysplasia and eventually leads to GC[12] (Figure 2). Risk factors for the development of the GC include interaction among the pathogen, environmental and host-related factors[13]. World Health Organization recognized H. pylori as class I carcinogen in 1994. GC is identified as the fifth most common malignancy and third leading cause of cancer-related morbidity globally, constituting 9.7% of all cancer-related mortality[14]. Highest age-standardized mortality rate (ASMR) is predicated for Eastern Asia (28.1 per 100000 in men, 13.0 per 100000 in women), the lowest ASMR in North America (2.8 and 1.5 per 100000, respectively)[15]. Studies reported high mortality rates are from East Asia, Central and Eastern Europe, Central and South America[15]. Developing countries have high burden of GC compared to the developed world and GC accounts for approximately 70% of both new cases and deaths[16]. Categorizing on basis of gender, 466900 cases of males were reported from developing as compared to 173700 cases from developed countries and for females the corresponding disease load was 247000 and 102000 cases, respectively. GC is associated with age incidence; commonly occurs in age group of 55 to 80 years, rare among young individual. Frequency of GC rates are two fold higher in males than females[17].
Over past decades in western nations, GC has considerably declined. The possible reasons behind this reduction include fall in H. pylori prevalence accompanied by better hygienic practices and innovative medical diagnostic facility. Despite the decline in GC incidence in developed world, the scenario of developing world is diverse. GC incidence and mortality rate remain very high in the developing nations, particularly in regions of East Asia and South America[17]. It is expected that if appropriate measures are not implemented the number of estimated GC cases are likely to increase in future.
Majority of gastric malignant tumors are adenocarcinomas. Histologically Lauren categorized gastric adenocarcinoma in intestinal and diffuse subtypes. Intestinal type adenocarcinoma is event dependent, start from chronic atrophic gastritis to intestinal metaplasia to dysplasia and finally carcinoma. Intestinal type adenocarcinoma is more frequent in developing world, common in male, and associated with age incidence, whereas diffuse type occurs more often in younger patients having family history of cancers, more frequent in females, background of atrophic gastritis is not prerequisite condition for its occurrence[18,19]. Anatomical site of origin is another way of differentiation of gastric adenocarcinoma. Tumors arising in the cardia region of the stomach are said to be proximal, and those from body and antrum (non-cardia region) as distal. Histological subtypes represent etiological and epidemiological differences between the two tumor sub sites. Globally GC incidence is declining. However, studies show rise in incidence of cardia carcinoma which may be partly due to more accurate reporting and fall in incidence of distal cancers[20].
Colonization of the stomach by H. pylori causes development of gastritis. H. pylori is truly an “opportunistic” bacterium that uses various well defined virulence factors as tool for attachment and persistent colonization of human gastric mucosa. The possible transmission route is fecal-oral, but contaminated food or water are also reported[21,22]. The most likely sources are person-to-person contact in families and/or exposure to a common source of infection such as contaminated water or food as supported by majority of data[23]. This notion is supported by studies of children in custodial care where the prevalence of infection is higher than expected and from studies of crowded families in which there is at least one infected child[24].
Before attachment of H. pylori to gastric epithelium, it has to first cross the thick mucus layer by adhering to the mucosal surface. This is aided by the presence of unipolar sheathed flagella, which allows H. pylori to quickly move from inhospitable low pH of gastric lumen to surface epithelium where pH is high and favorable for its successful colonization despites efforts made by the host to get rid of this bacterium. Non-motile mutant H. pylori strains fail to colonize the stomach of gnotobiotic piglets[25,26]. In majority of infected individuals colonization results in development of inflammatory and immune responses against H. pylori, but in some subjects H. pylori infection becomes chronic and leads to induction of gastric inflammation which can eventually lead to destruction of normal gastric glands and their replacement by intestinal-type epithelium resulting in atrophy of gastric mucosa.
The risk for atrophic gastritis depends on pattern as well as extent of distribution of chronic active inflammation. The individuals with lower acid output show a higher tendency towards atrophy[27]. Reduction in gland size and level of intestinal metaplasia were associated with rise in GC risk by 5- to 90-folds depending on the extent and severity of atrophy[28].
Increased odds ratios were evident from case-control studies that aimed to seriously study the signs of earlier H. pylori infection in GC patients and controls for development of non-cardia GC in presence of H. pylori infection[29]. This fact is supported by data from animal models including Mongolian gerbil model, in which H. pylori infection induces atrophic gastritis and GC[30-32]. A small number of subjects for research purposes were deliberately infected with pathogenic H. pylori strain and individuals developed acute inflammation of gastric mucosa with neutrophilic infiltration[33,34]. Volunteers after several decades when exposed repeatedly to intragastric pH-electrodes contaminated with H. pylori developed conditions called “epidemic hypochlorhydria”[34]. Such hypochlorhydric gastritis can either resolve spontaneously or change into chronic gastritis.
H. pylori infection results in three possible outcomes. First is corpus-predominant gastritis beginning from atrophic gastritis to hypochlorhydria and finally to GC. Second type results in a pangastritis having slightest impact on the host gastric acid production. Duodenal ulcer is third outcome, where an antrum-predominant gastritis leads to hyperchlorhydria. There arises controversy that infections of H. pylori can predispose to two equally exclusive situations. The possible explanation why some people are more expected to develop GC phenotype when compared with others may be due to disparity among individual host response to H. pylori infections (Figure 1). Initial evidence for the importance of host genetic polymorphisms was reflected in the study where a rise in incidence of atrophic gastritis and hypochlorhydria was evident from relatives of H. pylori induced GC patients than controls[35].
Pro-inflammatory cytokine like interleukin-1beta (IL-1β) act as a powerful negative regulator of acid secretion. IL-1β gene is now considered as a potential contender for host genetic polymorphisms that may elevates GC risk. Individuals possessing IL-1β gene cluster polymorphisms have 2–3-folds increased risk of non-cardia cancer[36,37] (Figure 2). Elevated levels of TNF-α in gastric mucosa of H. pylori infected individuals were evident from numerous studies. However, down regulation of anti-inflammatory cytokine IL-10, that suppresses the level of pro-inflammatory cytokines including IL-1β, TNF-α and interferon-γ (IFN-γ) is also reported[36].
The risk associated with GC development in H. pylori infected individuals upsurges 27-folds in individuals with three or four polymorphisms[38]. This evidently illustrates that interaction between host genetics and environment plays a key role in progression of GC, by regulating hosts adaptive immune response resulting in transformation of normal gastric mucosa to neoplastic one.
Higher expression of chemokine IL-8 and polymorphism (promoter region) has been reported in studies and linked with increased risk for GC[39]. Study on Caucasian populations proved that relationship among functional polymorphism within Toll like receptor 4, risk of GC and decrease in production of anti-inflammatory cytokine IL-10[40]. These studies reflect that host genetic polymorphisms are capable of modulating the innate immune response which results, severe inflammation and premalignant lesions in H. pylori infected individuals (Figure 2). These studies raises a query that whether H. pylori strain characteristics are responsible for increasing cancer risk employed by host genotypes, needs to be studied further. Odds ratios for non-cardia GC were highest for individuals with elevated IL-1β expression, colonized by H. pylori vacAs1-type strains[41].
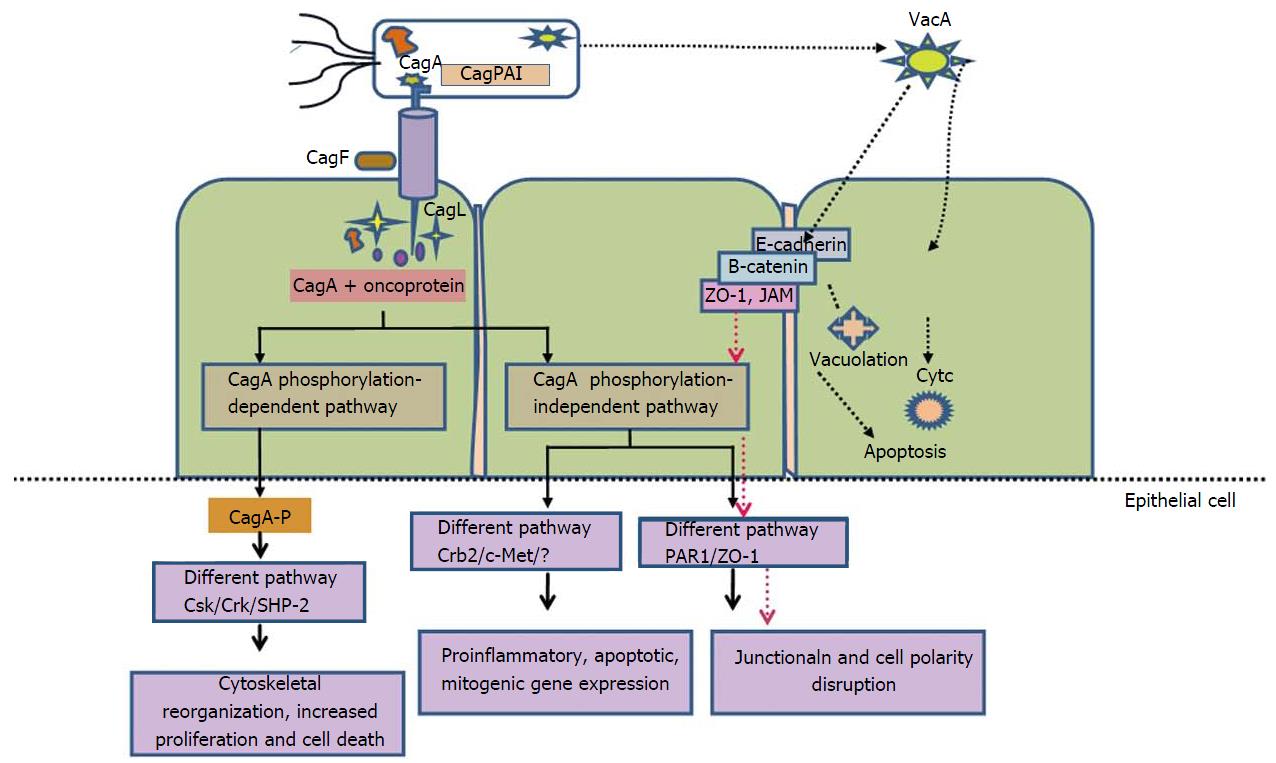
It is evident from case-control studies that H. pylori successfully form a vital equations with host by its ability to send and receive signals from its hosts[42,43]. Only certain H. pylori strains enhance the possibility of carcinogenesis because the equilibrium is likely different for each colonized individual. For example, individual infected with CagA strains leads to severe gastritis, which results in rise of proinflammatory cytokines levels that are responsible for both amplifying the mucosal inflammatory response as well as reducing the acid production. This creates a milieu encouraging growth of H. pylori that promote inflammation and continually produce oxidative stress, thus augmenting risk for transformation of normal mucosa to neoplastic through series of events (Figure 2).
H. pylori triggers numerous forms of proinflammatory cyclooxygenase (COX) enzymes. Production of endoperoxide from arachidonic acid is brought by COX enzymes. Enzymes prostaglandin synthases produces prostaglandins and various eicosanoids from endoperoxide[44]. Important role is played by prostaglandins in regulating physiologic processes for instance immunity and development. Two COX isoforms (COX-1 and COX-2) have been categorized on the basis of variances in expression characteristics and inhibition profiles for nonsteroidal anti-inflammatory drugs (NSAIDs). COX-2 expression is inducible while COX-1 is constitutively expressed in cells and tissues[45-47]. Expression of COX-2 can be stimulated by proinflammatory cytokines, growth factors such as TNF-α, IFN-γ and IL-1. COX-2 expression are raised in H. pylori infected human gastric mucosa, gastric premalignant and malignant lesions[47-49]. Inhibitors of COX (aspirin and NSAIDs) are associated with reduced risk of non-cardia GC[50]. Numerous studies demonstrate substantial role of COX-2-generated products involved in promoting neoplasia. Mechanisms like apoptosis inhibition, regulation of expression of cell surface adhesion, and production of promoting factors of neoplasia leads to malignancy[51,52] (Figure 2).
H. pylori have genetically heterogeneous genome. A number of H. pylori virulence factors are supposed to play an essential role in diverse clinical outcome of H. pylori infections. The Cag pathogenicity island (CagPAI) is a 40-kb region, consisting of 32 genes, flanked by 31-bp direct repeats. CagPAI is an island consisting of virulence genes, which are acquired by horizontal transfer. CagPAI encodes a type IV secretion system (T4SS) that is responsible for the entrance of a most remarkably investigated H. pylori virulence determinant effector protein CagA[53-55] (Figure 1). Positive association of CagA was found with peptic ulcer disease[56,57]. Due to its association with several gastroduodenal pathologies, initially CagA was considered as an indicator for presence of the entire CagPAI but as research speeded up, studies demonstrated that despite its presence, CagPAI intactness and clinical outcome varied.
More or less 70% of H. pylori strains from western world and nearly 100% of East Asian strains express virulent protein CagA[54,58,59]. Majority of H. pylori strains induces superficial gastritis but the risk for chronic gastritis, atrophic gastritis, metaplasia, and non-cardia GC with intact CagPAI is much higher compared to those that lacked it[56,57,60-66]. Among 32 genes of CagPAI, 18 genes are thought to code for structural parts of a T4SS, this system is responsible for exporting peptidoglycans and cagA into host gastric epithelial cells, via forming a pilus like assembly connecting bacterial and host epithelial membrane (Figure 1).
CagA is terminal gene product of the CagPAI. Classifying H. pylori strains on the basis of presence and absence of cagA into cagA-positive and cagA-negative strains. After the H. pylori attachment to epithelial cell, CagA is internalized through T4SS apparatus. After translocation, CagA is tyrosine phosphorylated at glutamate-proline-isoleucine-tyrosine-alanine (EPIYA) motif, i.e., EPIYA motif which is associated with cell morphological changes known as “the hummingbird phenotype,” which results in increased cellular migration[67-71].
Polymorphic region of CagA, has been identified within the carboxy-terminal and distinguished by different amino acid sequences. Till date, four distinct EPIYA motifs (EPIYA-A, -B, -C and D) are known[72,73]. EPIYA-A and -B motifs are present in strains all over the world, whereas EPIYA-C is specific to western world (Europe, North America, and Australia). Variation in number of EPIYA-C sites occurs, while majority of CagA proteins contain a single EPIYA-C site (A-B-C type). The level of phosphorylation of EPIYA-C sites is greater than EPIYA-A and EPIYA-B sites. Risk for development of GC is found to be associated with the number of cagA EPIYA-C in western strains[74]. EPIYA-D motif is exclusive to East Asian strains (from Japan, South Korea, and China), and strains possessing this motif produces higher level of IL-8 from gastric epithelial cells as compared to strains harboring western A-B-C-type CagA[72,75].
Kinase families of Abl and Src are responsible for phosphorylation of CagA into phospho-CagA. Interaction between phosphorylated CagA and various intracellular effectors, triggers an eukaryotic tyrosine phosphatase (SHP-2), which results in continuous stimulation of extracellular signal-regulated kinases 1 and 2 (ERK1/2), Crk adaptor[76] and C-terminal Src kinase in a tyrosine phosphorylation-dependent manner. In East Asian A-B-D types, negative response is induced by interactions of phospho-CagA with C-terminal Src kinase resulting down regulation of Src signaling[77] (Figure 3).
Experimental studies on cell lines revealed that CagA internalization give rise to “hummingbird phenotype”. These alterations are characterized by cell elongation and cell scattering[69,78]. Additional study also indicates that interplay among phosohorylated CagA, dephosphorylation of SHP-2 and down-regulation of focal adhesion kinase, causes cell elongation[69,79]. A different mechanism of cell elongation by phosphorylated CagA is by making a defect in cell retraction; yet the signaling molecules prerequisite for this phenotype remain vague[80]. Phosphorylated CagA obstructs the enzymatic activity of c-Src, which leads to tyrosine dephosphorylation of actin binding proteins such as cortactin, ezrin, and vinculin, ultimately results in cell elongation[81-83] (Figure 3).
Non-phosphorylated CagA have a different way of exerting effects within the cell. CagA translocation without phosphorylation leads to aberrant catenin activation, apical-junctional complex disruption and cellular polarity loss[84-89]. Relation between non-phosphorylated CagA and epithelial tight junction scaffolding proteins, zonula occludens 1 and junctional adhesion molecule A, results in imperfect association of tight junctions at located sites of bacterial attachment. Additional molecules includes E-cadherin, hepatocyte growth factor receptor c-Met, phospholipase C gamma (PL), adaptor protein Grb2, and kinase partitioning defective 1b/microtubule affinity-regulating kinase 2 (PAR1b/MARK2) resulting in mitogenic responses, interruption of cell-cell junctions and cell polarity destruction[84,87,88,90] (Figure 3). Recent study revealed that CagA directly binds to the cell polarity regulator such as PAR1b/MARK2. This binding prevents kinase PAR1b/MARK2 activity and deregulates the formation of mitotic spindle by cells which affects cell polarity[88,91].
Studies on transgenic mice revealed the correlation between CagA and oncogenesis by showing that CagA expression led to gastric epithelial cell proliferation and neoplastic changes. However, the following modifications were not detected in mice expressing phosphorylation-resistant CagA[52].
Presence of contradictory documentation on functionality of CagA as a bacterial oncoprotein in mammals exists besides solid proof provided by animal models. Pathological alterations described for transgenic CagA mice followed by absence of inflammation, which reflects disparity to what is seen in humans[52]. Although CagA act as oncoprotein, it remains to be explored why only few individuals inhabited by CagA-positive H. pylori develop GC. Recent study demonstrate that H. pylori prompts the presence of a host phospholipid, phosphatidylserine where CagA can explicitly interact and gain entry into the cells[92]. Focus of future research should be to define the exact mechanism of CagA internalization in gastric epithelial, factor responsible for regulation of this process and when during chronic infection CagA delivery in human epithelial cells.
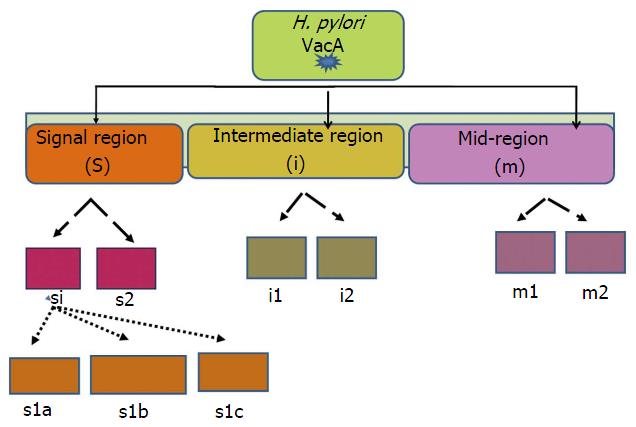
Another important H. pylori virulence gene is vacuolating cytotoxin (VacA), which encodes a bacterial toxin (VacA) that induces series of cascades leading apoptosis of epithelial cells via induction of cytoplasmic vacuoles (Figure 4). VacA is found throughout the H. pylori strains. The diverse polymorphic form of VacA are related with clinical outcomes[93]. Considerable genetic variations are found in: The s (signal) region with alleles s1a, slb, slc, or s2; the m (middle) region with m1 or m2 alleles; and the i (intermediate) region with type i1 or i2 alleles (Figure 4).
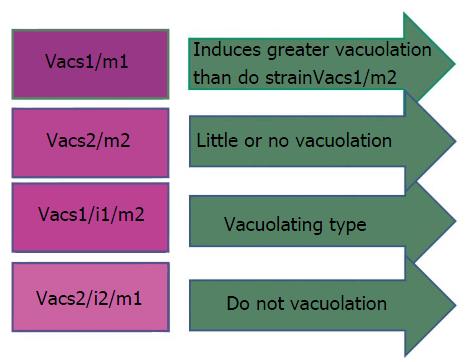
H. pylori strains having combination VacAs1/m1 or vacAs1/m1/i1 are associated with increased risk of progression to premalignant lesion and GC than vacA s2/m2 or vacAs2/m2/i2 strains[94] (Figure 5).
Besides H. pylori, the following other environmental factors are considered to contribute in the pathogenesis of GC.
The variations in GC incidence are due to environmental inputs, particular in dietary pattern. Previous accumulating studies have been indicated that downward trend in GC occurrence. This may be due to the advent of widespread refrigeration of foodstuff and reduction in dependency on food preservation. In addition, other studies have suggested that a preventive role of diet containing fresh vegetables and fruits. However, data from European prospective study failed to show an overall association between fresh fruits and vegetables intake and GC risk[95]. Recent studies being conducted on this field revealed that a significant association between total dietary vegetables contents (onion and garlic intake) and intestinal GC subtypes.
Additional studies are required for demonstration of positive association between H. pylori eradication and prevention of cancer. The controversy related to point of no return in case of atrophy and metaplasia is still debatable. Proposed studies on side effects and expenses of such preventive measures are required in future for proper management and treatment of GC, therefore GC prevention remains a key part of research on H. pylori.
H. pylori is not the only the culprit for the development of GC; other influential causes include host polymorphisms and environmental elements (Figure 2). High dietary salt intake was found to be uniformly been associated with an increased risk of GC[12,96]. Two studies, one study from Japan and other a case-control study from South Korea stated that H. pylori-infected subjects taking high-salt diet had an greater risk of GC than those with lower levels of salt[97,98]. Association between the frequency of H. pylori infection and amount of dietary salt intake is reported in another study[99].
Research on Mongolian gerbils had shown that the H. pylori presence and usage of a more salt containing diet applied concerted effects on development of precancerous satge[100,101]. Additional study on H. pylori-infected gerbils demonstrates that there is a positive association between level of severity of gastric inflammation and rate of proliferation of epithelial cells in gerbils consuming high-salt diet than those consuming a normal diet[100]. Similar studies on gerbils infected with H. pylori, when treated with carcinogen (N-methyl-N-nitroso urea) shows that higher frequency of GC related with animals consuming high-salt diet as compared to animals with a normal diet[101,102].
Mechanisms behind the high-salt diet increases the risk of development of GC in humans remains unclear. Among various explanations, one plausible hypothesis is that salt may lower the threshold for malignant transformation by altering the physiology of gastric epithelium thus allowing entry of carcinogens into gastric tissue and resulting in damage to gastric mucosa. Another possibility is that high salt intake might be regulating the gene expression in H. pylori. Two independent studies suggested that consumption of excess amount of salts in diet leads to higher expression of H. pylori virulence factors[103,104].
Many studies had proved the antioxidants present in food in green vegetables and fruits plays a preventive role against progression of GC[105]. There is scarcity on studies on association of H. pylori infection with nutritive elements in gastric carcinoma. A case-control study recommended that consistent excessive consumption of vitamin C and carotene might be able to curtail the casual for developing GC in subjects having infection of H. pylori[106].
A randomized study on population susceptible for GC development demonstrated that combination of vitamin C and carotene dietary supplements and H. pylori eradication increases the preneoplastic lesions regression at 6 years of follow-up; at another 6 years follow-up lacking dietary supplements, the protective role of vitamin C and carotene gradually end up[7]. These results were also validated by other studies[95,106]. A similar study from Hawaii proof that consumption of fresh vegetable among H. pylori infected individuals provided a little protection against GC occurrence[107]. On the contrary, other studies fail to provide a positive association between H. pylori infection and plasma vitamin C level, with risk of GC incidence[108]. Additional research is required to determine whether antioxidants are capable of providing protection against GC among H. pylori infected patients.
It is evident from various studies that cigarette smoking is associated with risk of developing GC in H. pylori infected subjects. In Japan, cigarette smoking and H. pylori infection together are considered as potential threat for developing GC[109]. Swedish and German population-based case control studies also demonstrated combination of cigarette smoking and infection by CagA positive H pylori strains increased the risk of developing GC (Figure 2). Los Angeles study also reported a tendency toward increased risk of GC in smokers[59,110]. On collectively analyzing studies, it emerges that there exists relationship between H. pylori infection and smoking with increased risk of developing GC.
H. pylori co-infection with helminths may have some impact in disease pathogenesis. Reduced Th1 response associated with higher levels of Th2 cytokines was reported in one study[111]. Another study on Colombian children from a coastal region having infection of both helminths and H. pylori, showed a higher Th2 associated IgG1 response[112]. Further studies are needed to assess the impact of H. pylori and helminthes co-infection in disease pathogenesis.
Gastric cancer remains a major threat to mankind. Improvement in living standards, increase awareness in sanitation and hygiene practices, reduction in intake of salted food products and advent of refrigeration in households resulted in measurable decline both in incidence of H. pylori infection and GC. Although both H. pylori infection and GC are showing decreasing trends in the developed world, they still remain a major threat to human population in the developing countries. Therefore, there is a need for improvement in early diagnosis, identification of risk factors, and development of preventive strategies and initiation of timely therapeutic interventions, especially focused for the developing countries. Further, it remains to be investigated why a small fraction of individuals colonized by H. pylori develop GC, and future research should focus on bacterial, host genetics, environmental and dietary factors.
There is need to formulate clear cut recommendation for screening and timely intervention of high risk population with family history of GC. Whether all high-risk areas should undergo routine screening of H. pylori infection is still questionable. Since the patients having atrophic gastritis or dysplasia in the gastric mucosa are at increased risk of developing GC, there is a need for special recommendations including endoscopic surveillance for such patients.
Jahanarah Khatoon and Ravi Prakash Rai gratefully acknowledge Indian Council of Medical Sciences, for junior research fellowship through Fellowship sanction No. 3/1/3/3 JRF-2011/HRD-87(32606) and 3/1/3/JRF-2012/HRD-162 (80220) respectively.
P- Reviewer: Guimaraes NM S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3262] [Article Influence: 79.6] [Reference Citation Analysis (1)] |
| 2. | Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1012] [Article Influence: 67.5] [Reference Citation Analysis (1)] |
| 3. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3181] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 4. | Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 5. | Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 397] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Vaux DL, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA. 1996;93:2239-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 664] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 7. | Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 254] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1044] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 9. | Nozaki K, Shimizu N, Inada K, Tsukamoto T, Inoue M, Kumagai T, Sugiyama A, Mizoshita T, Kaminishi M, Tatematsu M. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res. 2002;93:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Romero-Gallo J, Harris EJ, Krishna U, Washington MK, Perez-Perez GI, Peek RM. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab Invest. 2008;88:328-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest. 2001;107:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 247] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 13. | Schistosomes£¬liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1-241. [PubMed] |
| 14. | Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330-1344. [PubMed] |
| 15. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [PubMed] |
| 16. | Ang TL, Fock KM. Clinical epidemiology of gastric cancer. Singapore Med J. 2014;55:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 17. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [PubMed] |
| 18. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [PubMed] |
| 19. | McColl KE. Cancer of the gastric cardia. Best Pract Res Clin Gastroenterol. 2006;20:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 21. | Klein PD, Graham DY, Gaillour A, Opekun AR, Smith EO. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Gastrointestinal Physiology Working Group. Lancet. 1991;337:1503-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 316] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Hopkins RJ, Vial PA, Ferreccio C, Ovalle J, Prado P, Sotomayor V, Russell RG, Wasserman SS, Morris JG. Seroprevalence of Helicobacter pylori in Chile: vegetables may serve as one route of transmission. J Infect Dis. 1993;168:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 132] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Nurgalieva ZZ, Malaty HM, Graham DY, Almuchambetova R, Machmudova A, Kapsultanova D, Osato MS, Hollinger FB, Zhangabylov A. Helicobacter pylori infection in Kazakhstan: effect of water source and household hygiene. Am J Trop Med Hyg. 2002;67:201-206. [PubMed] |
| 24. | Malaty HM. Epidemiology of Helicobacter pylori infection. Best Pract Res Clin Gastroenterol. 2007;21:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Eaton KA, Morgan DR, Krakowka S. Campylobacter pylori virulence factors in gnotobiotic piglets. Infect Immun. 1989;57:1119-1125. [PubMed] |
| 26. | Scott D, Weeks D, Melchers K, Sachs G. The life and death of Helicobacter pylori. Gut. 1998;43 Suppl 1:S56-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 163] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 496] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 28. | Sipponen P, Kekki M, Haapakoski J, Ihamäki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 234] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Ekström AM, Held M, Hansson LE, Engstrand L, Nyrén O. Helicobacter pylori in gastric cancer established by CagA immunoblot as a marker of past infection. Gastroenterology. 2001;121:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 287] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255-4259. [PubMed] |
| 31. | Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology. 2005;128:1229-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 674] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 33. | Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 621] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 34. | Harford WV, Barnett C, Lee E, Perez-Perez G, Blaser MJ, Peterson WL. Acute gastritis with hypochlorhydria: report of 35 cases with long term follow up. Gut. 2000;47:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | El-Omar EM, Oien K, Murray LS, El-Nujumi A, Wirz A, Gillen D, Williams C, Fullarton G, McColl KE. Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of H. pylori. Gastroenterology. 2000;118:22-30. [PubMed] |
| 36. | El-Omar EM. Role of host genes in sporadic gastric cancer. Best Pract Res Clin Gastroenterol. 2006;20:675-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979-2990. [PubMed] |
| 38. | El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, Stanford JL, Mayne ST, Goedert J, Blot WJ. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 676] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 39. | Gewirtz AT, Yu Y, Krishna US, Israel DA, Lyons SL, Peek RM. Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. J Infect Dis. 2004;189:1914-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 40. | Weeks DL, Eskandari S, Scott DR, Sachs G. A H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonization. Science. 2000;287:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 334] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 41. | Figueiredo C, Machado JC, Pharoah P, Seruca R, Sousa S, Carvalho R, Capelinha AF, Quint W, Caldas C, van Doorn LJ. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 471] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 42. | Kirschner DE, Blaser MJ. The dynamics of Helicobacter pylori infection of the human stomach. J Theor Biol. 1995;176:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1343] [Article Influence: 58.4] [Reference Citation Analysis (1)] |
| 44. | Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 784] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 45. | Miyamoto T, Ogino N, Yamamoto S, Hayaishi O. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1976;251:2629-2636. [PubMed] |
| 46. | Williams CS, Smalley W, DuBois RN. Aspirin use and potential mechanisms for colorectal cancer prevention. J Clin Invest. 1997;100:1325-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Romano M, Ricci V, Memoli A, Tuccillo C, Di Popolo A, Sommi P, Acquaviva AM, Del Vecchio Blanco C, Bruni CB, Zarrilli R. Helicobacter pylori up-regulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J Biol Chem. 1998;273:28560-28563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Fu S, Ramanujam KS, Wong A, Fantry GT, Drachenberg CB, James SP, Meltzer SJ, Wilson KT. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 293] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 49. | Jüttner S, Cramer T, Wessler S, Walduck A, Gao F, Schmitz F, Wunder C, Weber M, Fischer SM, Schmidt WE. Helicobacter pylori stimulates host cyclooxygenase-2 gene transcription: critical importance of MEK/ERK-dependent activation of USF1/-2 and CREB transcription factors. Cell Microbiol. 2003;5:821-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Akre K, Ekström AM, Signorello LB, Hansson LE, Nyrén O. Aspirin and risk for gastric cancer: a population-based case-control study in Sweden. Br J Cancer. 2001;84:965-968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J. 2008;27:1671-1681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 52. | Ohnishi N, Yuasa H, Tanaka S, Sawa H, Miura M, Matsui A, Higashi H, Musashi M, Iwabuchi K, Suzuki M. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA. 2008;105:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 465] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 53. | Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, Bukanov NO, Drazek ES, Roe BA, Berg DE. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 400] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 54. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648-14653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1375] [Cited by in RCA: 1392] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 55. | Covacci A, Rappuoli R. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell. J Exp Med. 2000;191:587-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Cover TL, Dooley CP, Blaser MJ. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990;58:603-610. [PubMed] |
| 57. | Crabtree JE, Taylor JD, Wyatt JI, Heatley RV, Shallcross TM, Tompkins DS, Rathbone BJ. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 418] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 58. | Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1387] [Cited by in RCA: 1358] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 59. | Simán JH, Forsgren A, Berglund G, Florén CH. Tobacco smoking increases the risk for gastric adenocarcinoma among Helicobacter pylori-infected individuals. Scand J Gastroenterol. 2001;36:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Blaser MJ, Chyou PH, Nomura A. Age at establishment of Helicobacter pylori infection and gastric carcinoma, gastric ulcer, and duodenal ulcer risk. Cancer Res. 1995;55:562-565. [PubMed] |
| 61. | Crabtree JE, Wyatt JI, Sobala GM, Miller G, Tompkins DS, Primrose JN, Morgan AG. Systemic and mucosal humoral responses to Helicobacter pylori in gastric cancer. Gut. 1993;34:1339-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 156] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 62. | Queiroz DM, Mendes EN, Rocha GA, Oliveira AM, Oliveira CA, Magalhães PP, Moura SB, Cabral MM, Nogueira AM. cagA-positive Helicobacter pylori and risk for developing gastric carcinoma in Brazil. Int J Cancer. 1998;78:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Vorobjova T, Nilsson I, Kull K, Maaroos HI, Covacci A, Wadström T, Uibo R. CagA protein seropositivity in a random sample of adult population and gastric cancer patients in Estonia. Eur J Gastroenterol Hepatol. 1998;10:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 672] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 65. | Peek RM, Miller GG, Tham KT, Pérez-Pérez GI, Cover TL, Atherton JC, Dunn GD, Blaser MJ. Detection of Helicobacter pylori gene expression in human gastric mucosa. J Clin Microbiol. 1995;33:28-32. [PubMed] |
| 66. | Torres J, Pérez-Pérez GI, Leal-Herrera Y, Muñoz O. Infection with CagA+ Helicobacter pylori strains as a possible predictor of risk in the development of gastric adenocarcinoma in Mexico. Int J Cancer. 1998;78:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 67. | Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 373] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 68. | Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 954] [Cited by in RCA: 966] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 69. | Segal ED, Cha J, Lo J, Falkow S, Tompkins LS. Altered states: involvement of phosphorylated CagA in the induction of host cellular growth changes by Helicobacter pylori. Proc Natl Acad Sci USA. 1999;96:14559-14564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 592] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 70. | Stein M, Bagnoli F, Halenbeck R, Rappuoli R, Fantl WJ, Covacci A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol Microbiol. 2002;43:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 350] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 71. | Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 449] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 72. | Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 585] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 73. | Higashi H, Yokoyama K, Fujii Y, Ren S, Yuasa H, Saadat I, Murata-Kamiya N, Azuma T, Hatakeyama M. EPIYA motif is a membrane-targeting signal of Helicobacter pylori virulence factor CagA in mammalian cells. J Biol Chem. 2005;280:23130-23137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 74. | Basso D, Zambon CF, Letley DP, Stranges A, Marchet A, Rhead JL, Schiavon S, Guariso G, Ceroti M, Nitti D. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135:91-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 294] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 75. | Argent RH, Hale JL, El-Omar EM, Atherton JC. Differences in Helicobacter pylori CagA tyrosine phosphorylation motif patterns between western and East Asian strains, and influences on interleukin-8 secretion. J Med Microbiol. 2008;57:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 76. | Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683-686. [PubMed] |
| 77. | Tsutsumi R, Higashi H, Higuchi M, Okada M, Hatakeyama M. Attenuation of Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J Biol Chem. 2003;278:3664-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 78. | Moese S, Selbach M, Kwok T, Brinkmann V, König W, Meyer TF, Backert S. Helicobacter pylori induces AGS cell motility and elongation via independent signaling pathways. Infect Immun. 2004;72:3646-3649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Tsutsumi R, Takahashi A, Azuma T, Higashi H, Hatakeyama M. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol Cell Biol. 2006;26:261-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 80. | Bourzac KM, Botham CM, Guillemin K. Helicobacter pylori CagA induces AGS cell elongation through a cell retraction defect that is independent of Cdc42, Rac1, and Arp2/3. Infect Immun. 2007;75:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Moese S, Selbach M, Brinkmann V, Karlas A, Haimovich B, Backert S, Meyer TF. The Helicobacter pylori CagA protein disrupts matrix adhesion of gastric epithelial cells by dephosphorylation of vinculin. Cell Microbiol. 2007;9:1148-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 82. | Selbach M, Moese S, Backert S, Jungblut PR, Meyer TF. The Helicobacter pylori CagA protein induces tyrosine dephosphorylation of ezrin. Proteomics. 2004;4:2961-2968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | Selbach M, Moese S, Hurwitz R, Hauck CR, Meyer TF, Backert S. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 2003;22:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 84. | Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 584] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 85. | Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci USA. 2005;102:16339-16344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 208] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 86. | Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA. 2005;102:10646-10651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 413] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 87. | Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM, Azuma T. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617-4626. [PubMed] |
| 88. | Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 396] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 89. | Suzuki M, Mimuro H, Suzuki T, Park M, Yamamoto T, Sasakawa C. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J Exp Med. 2005;202:1235-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 90. | Churin Y, Al-Ghoul L, Kepp O, Meyer TF, Birchmeier W, Naumann M. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol. 2003;161:249-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 293] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 91. | Lu H, Murata-Kamiya N, Saito Y, Hatakeyama M. Role of partitioning-defective 1/microtubule affinity-regulating kinases in the morphogenetic activity of Helicobacter pylori CagA. J Biol Chem. 2009;284:23024-23036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | Murata-Kamiya N, Kikuchi K, Hayashi T, Higashi H, Hatakeyama M. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host Microbe. 2010;7:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 93. | Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 411] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 94. | Wroblewski LE, Peek RM. Helicobacter pylori in gastric carcinogenesis: mechanisms. Gastroenterol Clin North Am. 2013;42:285-298. [PubMed] |
| 95. | Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 483] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 96. | Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 2005;96:1-6. [PubMed] |
| 97. | Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol. 2003;13:162-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 98. | Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, Tanizaki Y, Doi Y, Tanaka K, Oishi Y. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 99. | Beevers DG, Lip GY, Blann AD. Salt intake and Helicobacter pylori infection. J Hypertens. 2004;22:1475-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Gamboa-Dominguez A, Ubbelohde T, Saqui-Salces M, Romano-Mazzoti L, Cervantes M, Domínguez-Fonseca C, de la Luz Estreber M, Ruíz-Palacios GM. Salt and stress synergize H. pylori-induced gastric lesions, cell proliferation, and p21 expression in Mongolian gerbils. Dig Dis Sci. 2007;52:1517-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Kato S, Tsukamoto T, Mizoshita T, Tanaka H, Kumagai T, Ota H, Katsuyama T, Asaka M, Tatematsu M. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006;119:1558-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 102. | Peek RM, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 446] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 103. | Gancz H, Jones KR, Merrell DS. Sodium chloride affects Helicobacter pylori growth and gene expression. J Bacteriol. 2008;190:4100-4105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709-4715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 105. | Stanner SA, Hughes J, Kelly CN, Buttriss J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Public Health Nutr. 2004;7:407-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 106. | Ekström AM, Serafini M, Nyrén O, Hansson LE, Ye W, Wolk A. Dietary antioxidant intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: a population-based case-control study in Sweden. Int J Cancer. 2000;87:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 107. | Epplein M, Nomura AM, Hankin JH, Blaser MJ, Perez-Perez G, Stemmermann GN, Wilkens LR, Kolonel LN. Association of Helicobacter pylori infection and diet on the risk of gastric cancer: a case-control study in Hawaii. Cancer Causes Control. 2008;19:869-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 108. | Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, Friesen M, Tjønneland A, Olsen A, Overvad K. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis. 2006;27:2250-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 109. | Shikata K, Doi Y, Yonemoto K, Arima H, Ninomiya T, Kubo M, Tanizaki Y, Matsumoto T, Iida M, Kiyohara Y. Population-based prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: the Hisayama Study. Am J Epidemiol. 2008;168:1409-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 110. | Brenner H, Arndt V, Bode G, Stegmaier C, Ziegler H, Stümer T. Risk of gastric cancer among smokers infected with Helicobacter pylori. Int J Cancer. 2002;98:446-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 359] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 112. | Whary MT, Sundina N, Bravo LE, Correa P, Quinones F, Caro F, Fox JG. Intestinal helminthiasis in Colombian children promotes a Th2 response to Helicobacter pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2005;14:1464-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |