Published online Nov 15, 2015. doi: 10.4251/wjgo.v7.i11.369
Peer-review started: March 10, 2015
First decision: May 19, 2015
Revised: August 1, 2015
Accepted: September 10, 2015
Article in press: September 16, 2015
Published online: November 15, 2015
Processing time: 258 Days and 5.7 Hours
St. John’s Wort (SJW) is an old herb which has long been consumed widely for its anti-inflammatory, antiviral, and anti-depressive properties. Here we present a detailed clinical evaluation of three cases (two colon and one duodenal adenocarcinoma) with remarkable and intensive lymphoplasmocytic host reaction, at the basal part of tumor, intensive fibrosis, giant cells, plasma cell increase in lymph nodes and few giant cells in germinal centers in resection specimens. The observation of similar host reaction in those tumors having otherwise usual appearance was interesting. None of the cases received neoadjuvant chemoradiotherapy or additional treatment before surgery but only SJW. These cases are presented to increase the awareness about such cases. Further research is needed to reveal the possible effect of SJW, which has long been consumed for different treatment purposes, on human tumors.
Core tip: St. John’s Wort (SJW) is a well known herb that was used in treatment of many diseases during centuries. In this article we offer a perspective about the anti-tumoral effect of SJW with possible mechanisms and pathological data in three gastrointestinal cancer cases, where usage of SJW was identified in history questioning because of tumor regression and intensive inflammatory host reaction following pathological examination.
- Citation: Karaarslan S, Cokmert S, Cokmez A. Does St. John’s Wort cause regression in gastrointestinal system adenocarcinomas? World J Gastrointest Oncol 2015; 7(11): 369-374
- URL: https://www.wjgnet.com/1948-5204/full/v7/i11/369.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v7.i11.369
St. John’s Wort (SJW) is a substance widely used for its anti-inflammatory, antiviral, antidepressant and anticancer effects[1-3]. It contains two active compounds: Firstly, hyperforin is responsible for anti-depressant activity and has supplied to be also a good inhibitor of leukocyte elastase, exerting forceful inhibition of in vitro tumor cell chemoinvasion and reduction of neovascularization and metastasis formation in vivo[4]. Secondly, hypericin is responsible for photocytotoxic effects in vivo and in vitro. The in vivo and in vitro photodynamic activities of hypericin as a photosensitizer mainly to induce a very potent anti-tumoral effect[5]. Also, the anti-retroviral feature of hypericin has beeen demonstrated in vitro and in animal models[6].
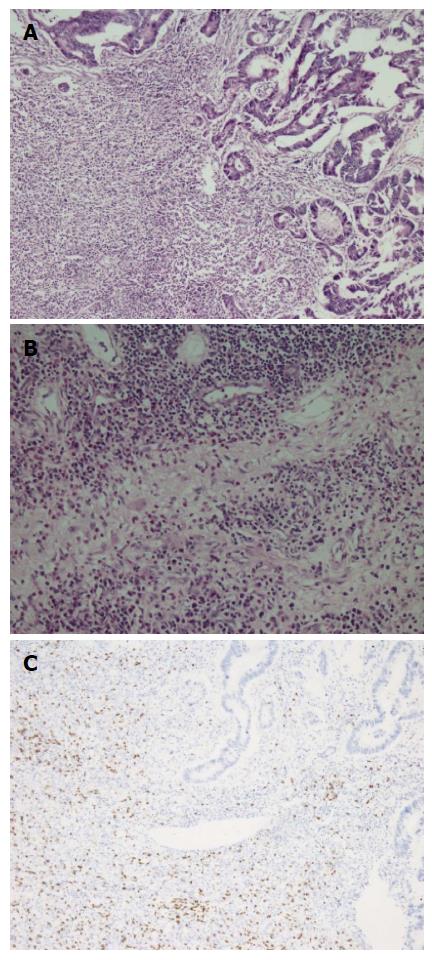
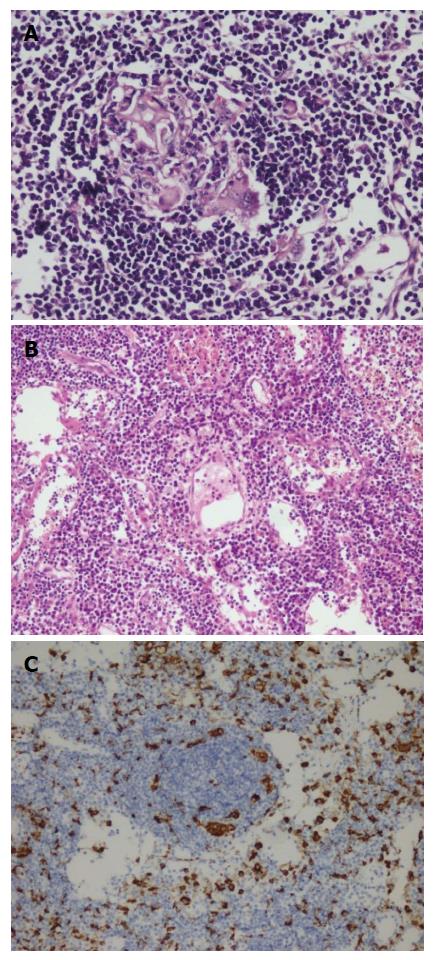
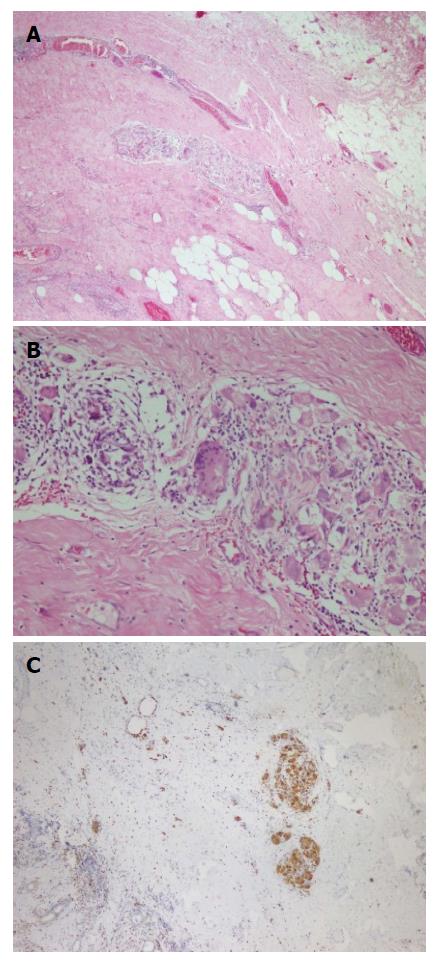
A fifty-nine years old male patient has undergone colonoscopy for anemia evaluation, which revealed a tumoral mass in the cecum. The histological diagnosis of the biopsy was adenocarcinoma and no distant metastasis was detected in further clinic radiological investigation. Right hemicolectomy was performed and a pathological examination of surgical material revealed a cecal ulcero-vegetative mass which was 7 cm × 6 cm × 5 cm in size. The tumor invaded through muscularis propria to subserosal fat tissue and was consistent with a moderately differentiated adenocarcinoma. Notably, it showed fibrosis and inflammatory cell infiltration in the transitional zone between deep intestinal layers and normal mucosa, which was easily detectable even under low magnification (Figure 1A). Under higher magnifications, inflammatory cell infiltration was rich in plasma cells and lymphocytes. scattered eosinophils, polymorphonuclear leucocytes and few giant cells were also noted focally (Figure 1B). The inflammatory reaction and fibrosis were surrounding the tumor, as if they were trying to prevent the penetration of the tumor into deep tissue. Most of these lymphocytes were T lymphocytes and showed cytotoxic T cell (CD8+) phenotype on immunohistochemical examination (Figure 1C). CD20 and CD4 stains were almost negative. Plasma cells were stained positive with CD138 and polytypic with kappa/lambda. Two of 18 lymph nodes dissected from mesentery showed few tumor cells located in sub-capsular sinuses while no gross metastasis was detected. Notably, germinal centers of some lymph nodes had giant cells and increased number of plasma cells in inter-follicular areas (Figure 2A and B). Giant cells were CD68 positive on immunohistochemical examination (Figure 2C). These features were suggestive of changes developed secondary to neoadjuvant chemotherapy/radiotherapy, but the patient’s past medical history did not reveal such treatment. His detailed medical history was taken and when he was also asked for the usage of some alternative treatments, he mentioned usage of SJW for other complaints such as diabetes, dyspepsia. He has been consuming SJW tea in the morning for five years, then he had used SJW oil regularly (one teaspoon in the morning) for two years and he has been using it regularly (one teaspoon in the morning and evening) for the last three years. Medical records of the patient revealed that he had chemotherapy for six months after surgery (FOLFOX-4 protocole once every 14 d) and no recurrence or metastasis were detected during two years of follow up.
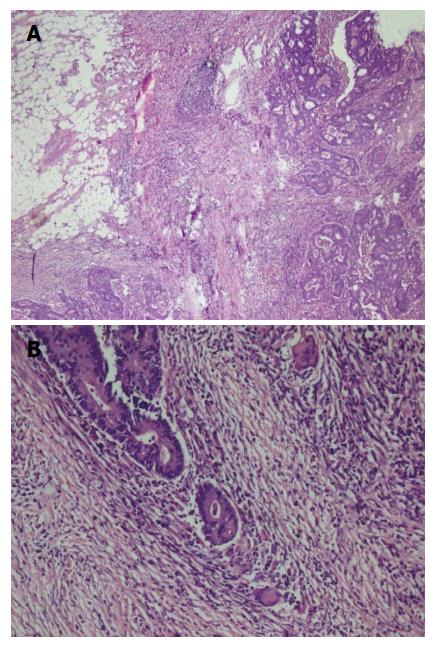
A fifty-eight years old female patient has undergone colonoscopy for anemia evaluation, which revealed a tumoral mass in the transverse colon. No distant metastasis was detected and the patient had undergone colectomy. On macroscopic examination of colectomy specimen, an ulcerovegetative tumor infiltrating all layers of intestinal wall was detected, measuring 3.5 cm × 2.5 cm × 2 cm in size. Microscopic examination revealed moderately differentiated adenocarcinoma with mixed inflammatory cell infiltration rich in lymphoplasmocytes on the background (Figure 3). Eosinophils were also prominent with a few giant cells. Fourteen lymph nodes, dissected from mesentery, were reactive. However, one of the lymph nodes had an increased number of plasma cells and giant cells in germinal center of the follicle. Immunohistochemical characteristics were similar to that of the first case. Based on the experience of the morphology of the first case, the patient was also asked for usage of alternative treatments. To our surprise she has also mentioned usage of SJW oil (one teaspoon in the morning on an empty stomach) for 1.5 mo. Her medical records revealed that she has refused chemotherapy and followed-up without treatment. No recurrence or metastases were detected during the first six months of follow-up period.
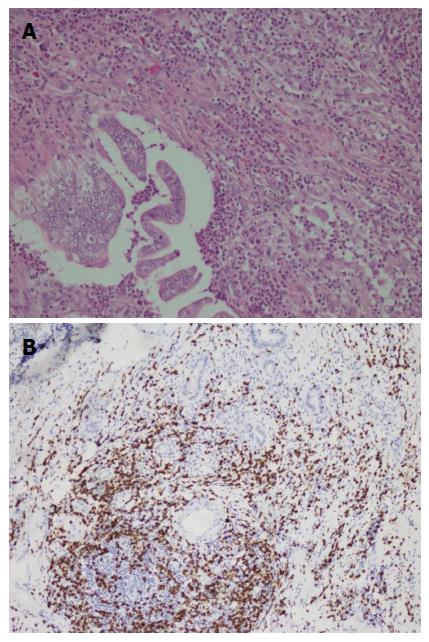
A duodenal mass was detected in a 73 years old male patient with the complaints of abdominal pain and weight loss. The biopsy was reported as adenocarcinoma. Since there was no distant metastasis, surgery was recommended. Although, he initially refused surgery he agreed to an operation three months later. On his second admission to hospital it was seen that the tumor size had somewhat reduced during this three months period. When a detailed medical history was taken, it was also revealed there was daily use of SJW oil of one teaspoon for the last three months. On macroscopic examination, an ulcero-vegetative ampullary tumor was observed measuring 3.8 cm × 2.5 cm × 2.5 cm in size, involving all layers of duodenum and infiltrating the pancreas. Areas showing the characteristics of moderately differentiated adenocarcinoma and mixed inflammatory cell infiltration rich in PNLs were observed. Similar to the previous two cases, eosinophils were also present and most prominent in the basilar parts of these areas (Figure 4A). The most common lymphocytic component was again CD8 positive T cells immunohistochemically (Figure 4B). Giant cells were seen in all layers, being more prominent in the areas in the vicinity of serosal surfaces (Figure 5A and B). These cells were stained with CD68 immunohistochemically (Figure 5C). Additionally, extensive perineural infiltration and intra-lymphatic tumoral thrombi were present. Four of 12 lymph nodes dissected from surrounding adipose tissue showed metastasis. The patient died due to anastomosis leakage and bleeding complications after surgery.
Hypericum perforatum, known as SJW, is a plant of the genus Hypericum and a herb with antidepressant feature and effective anti-inflammatory characteristics as an arachidonic acide/5-lipoxygenase inhibitor and COX-1 inhibitor[7]. In many countries, its drug form is available and sold out as an over the counter drug without prescription. It is most commonly used for the treatment of depression. Hyperforin is responsible for anti-depressant activity. The hyperforin constituent of SJW is TRPC6 receptor agonist and therefore, it causes noncompetitive reuptake inhibition of monoamines (especially, dopamine, norepinephrine, and serotonin), gamma-aminobutyric acid and glutamate[8]. Hyperforin inhibits reuptake of these neurotransmitters by increasing intra-cellular sodium ion amounts. Furthermore, SJW is known to downregulate the β1 adrenoceptor and upregulate postsynaptic 5-HT1A and 5-HT2A receptors which are serotonin receptor[9]. A 2008 Cochrane review of 29 clinical trials inferred that it was superior to placebo in cases with major depression[10]. With respect to the National Center for Complementary and Integrative Health of the National Institutes of Health, it “may help some types of depression, though the evidence is not definitive”[11]. Hyperforin is also an anti-inflammatory complex with anti-angiogenic, antibiotic, and neurotrophic estates[12]. Moreover, it prevents neutrophil activation of matrix metalloproteinase-9 (MMP9) mobility and recruitment. Anti-proliferative and anti-metastatic feature has also been associated to down-regulation of NF-jB and its regulated molecules for example survivin and MMP9[13].
Hypericin is a photosensitive compound synthesized by SJW, and possesses properties suitable for photodynamic therapy (PDT). PDT is a carcinoma treatment methodology abusing non-toxic photosensitizer specifically localized in tumor tissue and its targeted activation with light. Thus, it leads to reactive oxygen kinds production and causes photochemically caused cell death[14]. The response to PDT depends on the photosensitizer’s features, the illumination circumstances and the oxygenation conditions of the tissue[15]. It was also observed that hypericin blocks cell cycle at G2/M control point in colon cancer cell culture[16] Another colon cancer cell culture study showed re-localisation of apoptosis-inducing factor on the nucleus after hypericin treatment. Thus the anti-tumor effect of hypericins likely resulted from its apoptosis stimulating effect and its anti-proliferative effect by decreasing Ras protein[17].
Besides its many benefits there are also some studies in the literature showing its undesired adverse effects. Development of hepatotoxicity, cirrhosis and alteration of dosage properties and bioavailability of some drugs are some of its important adverse effects[18]. SJW has been displayed to cause a lot of drug interactions. Its effects are due to cytochrom P4503A enzyme activation and P-glycoprotein. This drug metabolizing enzyme induction effects in the raised metabolism of some drugs, such as indinavir, cyclosporine and oral contraceptives leading to reduced plasma density and possible clinical impact[19]. The main constituent thought to be responsible is hyperforin.In an other study it has been shown that the amount of intestinal and hepatic cytochrome P4503A and intestinal P-glycoprotein are increased by the short term usage of SJW in humans and rats[20]. Bone marrow necrosis, orofacial dystonia and radiation recall dermatitis are reported as less often adverse effects[21-23].
In an experimental study by Martarelli et al[24], on hormone independent human prostate cancer cells, it was shown that Hypericum perforatum extract decreased tumor cell proliferation by inhibiting serotonin reuptake and showed cytotoxic effects. In addition, it decreased frequency of local lymph node metastasis when compared to the control group[24]. There are experimental studies on the effects of SJW on colon, bladder and prostate carcinomas. In an experimental study by Dongre et al[25], the effect of Hypericum hookerianum on carcinomas was evaluated and it was found that serum neutrophil, lymphocyte, eosinophil, hemoglobin and erythrocyte values were closer to normal range when compared to control group[25]. In our cases, neutrophils and histiocytes-giant cells were more prominent early in the course (2nd and 3rd cases), while plasma cells, histiocytes and lymphocytes (cytotoxic CD8+) took over during chronic usage (1st case). Similar to the study by Dongre et al[25], morphological properties of our 2nd and 3rd cases may be due to acute effects (15 d) of Hypericum. In our case with long term SJW use, extensive host reaction and tendency to form barrier against tumor were remarkable and we interpreted it as a morphological sign of its anti-tumor response. Although the exact mechanism of these events is unknown, it may be a result of a chain of events triggered immunologically.
The aim of this presentation is not recommending SJW as a substitute for cancer treatment. The observations presented herein reflect the histological findings of only three cases and not enough to make a precise conclusion on its effects. We don’t know yet either whether all cases using SJW present similar morphology or whether any other substances also induce a similar tumor-host reaction. We present these cases only to share our observations and draw attention to its possible effects on human tumor-host interaction. Further dedicated research is needed to unveil these questions.
The authors thank Professor Dr. Basak Doganavsargil, MD, for critical reading the manuscript.
The authors present a detailed clinical evaluation of three intestinal adenocarcinoma cases which used St. John’s Wort (SJW).
Patients have undergone colonoscopy for anemia, abdominal pain and weight loss evaluation, which revealed a tumoral mass in the colon and duodenum.
Biopsy and resection materials of all three cases were evaluated morphologically and immunohistochemically. Inflammatory cell population was rich in plasma cells and lymphocytes. In patients that used SJW in early stages polymorphonuclear leucocytes were significant. In patient those who used SWJ for long periods fibrosis and lymphoplasmositic cell infiltration was remarkable. Lymphocytes stained predominantly CD8 positive phenotype immnuhistochemically. Plasma cells were found to be kappa/lambda polytypic nature.
Case revealed that he had chemotherapy for six months after surgery (FOLFOX-4 1 protocole once every 14 d).
The aim in this study is not about to recommend usage of SJW. The authors only want to indicate their awareness of SJW usage after pathologic examination. The authors thing that these pathologic features might flash the benefits of SJW that had been discussed for ages.
This manuscript reports the clinico-pathological findings of three adenocarcinoma cases treated with SJW.
P- Reviewer: Chiba T, Merino G S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Tian R, Koyabu N, Morimoto S, Shoyama Y, Ohtani H, Sawada Y. Functional induction and de-induction of P-glycoprotein by St. John’s wort and its ingredients in a human colon adenocarcinoma cell line. Drug Metab Dispos. 2005;33:547-554. [PubMed] |
| 2. | Šemeláková M, Mikeš J, Jendželovský R, Fedoročko P. The pro-apoptotic and anti-invasive effects of hypericin-mediated photodynamic therapy are enhanced by hyperforin or aristoforin in HT-29 colon adenocarcinoma cells. J Photochem Photobiol B. 2012;117:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Lavie G, Valentine F, Levin B, Mazur Y, Gallo G, Lavie D, Weiner D, Meruelo D. Studies of the mechanisms of action of the antiretroviral agents hypericin and pseudohypericin. Proc Natl Acad Sci USA. 1989;86:5963-5967. [PubMed] |
| 4. | Hostanska K, Reichling J, Bommer S, Weber M, Saller R. Hyperforin a constituent of St John’s wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cell lines. Eur J Pharm Biopharm. 2003;56:121-132. [PubMed] |
| 5. | Agostinis P, Vantieghem A, Merlevede W, de Witte PA. Hypericin in cancer treatment: more light on the way. Int J Biochem Cell Biol. 2002;34:221-241. [PubMed] |
| 6. | Lavie G, Mazur Y, Lavie D, Prince AM, Pascual D, Liebes L, Levin B, Meruelo D. Hypericin as an inactivator of infectious viruses in blood components. Transfusion. 1995;35:392-400. [PubMed] |
| 7. | Wölfle U, Seelinger G, Schempp CM. Topical application of St. John’s wort (Hypericum perforatum). Planta Med. 2014;80:109-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Pharmacology. Hyperforin. Drugbank. University of Alberta. Retrieved 5, December 2013. . |
| 9. | Nathan PJ. Hypericum perforatum (St John’s Wort): a non-selective reuptake inhibitor? A review of the recent advances in its pharmacology. J Psychopharmacol. 2001;15:47-54. [PubMed] |
| 10. | Linde K, Berner MM, Kriston L. St John’s wort for major depression. Cochrane Database Syst Rev. 2008;CD000448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3573] [Cited by in RCA: 2754] [Article Influence: 1377.0] [Reference Citation Analysis (0)] |
| 11. | National Institute for Complementary and Integrative Health: St. John’s Wort. Retrieved 2015-02-05. . |
| 12. | Zanoli P. Role of hyperforin in the pharmacological activities of St. John’s Wort. CNS Drug Rev. 2004;10:203-218. [PubMed] |
| 13. | Butterweck V. Mechanism of action of St John’s wort in depression: what is known? CNS Drugs. 2003;17:539-562. [PubMed] |
| 14. | Barathan M, Mariappan V, Shankar EM, Abdullah BJ, Goh KL, Vadivelu J. Hypericin-photodynamic therapy leads to interleukin-6 secretion by HepG2 cells and their apoptosis via recruitment of BH3 interacting-domain death agonist and caspases. Cell Death Dis. 2013;4:e697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Kleemann B, Loos B, Scriba TJ, Lang D, Davids LM. St John’s Wort (Hypericum perforatum L.) photomedicine: hypericin-photodynamic therapy induces metastatic melanoma cell death. PLoS One. 2014;9:e103762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Sacková V, Fedorocko P, Szilárdiová B, Mikes J, Kleban J. Hypericin-induced photocytotoxicity is connected with G2/M arrest in HT-29 and S-phase arrest in U937 cells. Photochem Photobiol. 2006;82:1285-1291. [PubMed] |
| 17. | Sačková V, Kuliková L, Kello M, Uhrinová I, Fedoročko P. Enhanced antiproliferative and apoptotic response of HT-29 adenocarcinoma cells to combination of photoactivated hypericin and farnesyltransferase inhibitor manumycin A. Int J Mol Sci. 2011;12:8388-8405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Lampri ES, Ioachim E, Harissis H, Balasi E, Mitselou A, Malamou-Mitsi V. Pleomorphic hepatocellular carcinoma following consumption of hypericum perforatum in alcoholic cirrhosis. World J Gastroenterol. 2014;20:2113-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Henderson L, Yue QY, Bergquist C, Gerden B, Arlett P. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol. 2002;54:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Dürr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ, Fattinger K. St John’s Wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598-604. [PubMed] |
| 21. | Demiroglu YZ, Yeter TT, Boga C, Ozdogu H, Kizilkilic E, Bal N, Tuncer I, Arslan H. Bone marrow necrosis: a rare complication of herbal treatment with Hypericum perforatum (St. John’s wort). Acta Medica (Hradec Kralove). 2005;48:91-94. [PubMed] |
| 22. | Milton JC, Abdulla A. Prolonged oro-facial dystonia in a 58 year old female following therapy with bupropion and St John’s Wort. Br J Clin Pharmacol. 2007;64:717-718. [PubMed] |
| 23. | Putnik K, Stadler P, Schäfer C, Koelbl O. Enhanced radiation sensitivity and radiation recall dermatitis (RRD) after hypericin therapy -- case report and review of literature. Radiat Oncol. 2006;1:32. [PubMed] |
| 24. | Martarelli D, Martarelli B, Pediconi D, Nabissi MI, Perfumi M, Pompei P. Hypericum perforatum methanolic extract inhibits growth of human prostatic carcinoma cell line orthotopically implanted in nude mice. Cancer Lett. 2004;210:27-33. [PubMed] |
| 25. | Dongre SH, Badami S, Natesan S, H RC. Antitumor Activity of the Methanol Extract of Hypericum hookerianum Stem Against Ehrlich Ascites Carcinoma in Swiss Albino Mice. J Pharmacol Sci. 2007;103:354-359. [PubMed] |