Published online Nov 15, 2015. doi: 10.4251/wjgo.v7.i11.285
Peer-review started: May 9, 2015
First decision: July 17, 2015
Revised: July 28, 2015
Accepted: September 10, 2015
Article in press: September 16, 2015
Published online: November 15, 2015
Processing time: 196 Days and 10.2 Hours
The effect of chemotherapy on peritoneal carcinomatosis (PC) of gastric cancer remains unclear. Recently, the intraperitoneal (IP) administration of taxanes [e.g., paclitaxel (PTX) and docetaxel (DOC)] during the perioperative period has shown promising results. Herein, we summarized the rationale and methodology for using IP chemotherapy with taxanes and reviewed the clinical results. IP administered taxanes remain in the IP space at an extremely high concentration for 48-72 h. The drug directly infiltrates peritoneal metastatic nodules from the surface and then produces antitumor effects, making it ideal for IP chemotherapy. There are two types of perioperative IP chemotherapy with taxanes: neoadjuvant intraperitoneal and systemic chemotherapy and sequential perioperative intraperitoneal chemotherapy (SPIC). In SPIC, patients receive neoadjuvant IP chemotherapy and the same regimen of IP chemotherapy after cytoreductive surgery (CRS) until disease progression. Usually, a taxane dissolved in 500-1000 mL of saline at ordinary temperature is administered through an IP access port on an outpatient basis. According to phase I studies, the recommended doses (RD) are as follows: IP DOC, 45-60 mg/m2; IP PTX [without intravenous (IV) PTX], 80 mg/m2; and IP PTX (with IV PTX), 20 mg/m2. Phase II studies have reported a median survival time of 14.4-24.6 mo with a 1-year overall survival of 67%-78%. A phase III study comparing S-1 in combination with IP and IV PTX to S-1 with IV cisplatin started in 2011. The prognosis of patients who underwent CRS was better than that of those who did not; however, this was partly due to selection bias. Although several phase II studies have shown promising results, a randomized controlled study is needed to validate the effectiveness of IP chemotherapy with taxanes for PC of gastric cancer.
Core tip: Herein, we provided an overview on the recent advances in intraperitoneal (IP) chemotherapy using taxanes (e.g., paclitaxel and docetaxel) for peritoneal carcinomatosis of gastric cancer. In particular, we focus on the rationale of IP chemotherapy with taxanes, treatment methodology, and results of current clinical studies. Intraperitoneally administered taxanes remain in the IP cavity for a long time, and they directly infiltrate the peritoneal metastatic nodule from the surface. Therefore, the repeated intra-abdominal administration of taxanes through an IP access port is needed to increase the antitumor effect of IP chemotherapy.
- Citation: Yamaguchi H, Kitayama J, Ishigami H, Kazama S, Nozawa H, Kawai K, Hata K, Kiyomatsu T, Tanaka T, Tanaka J, Nishikawa T, Otani K, Yasuda K, Ishihara S, Sunami E, Watanabe T. Breakthrough therapy for peritoneal carcinomatosis of gastric cancer: Intraperitoneal chemotherapy with taxanes. World J Gastrointest Oncol 2015; 7(11): 285-291
- URL: https://www.wjgnet.com/1948-5204/full/v7/i11/285.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v7.i11.285
Gastric cancer is the fourth most common cancer worldwide, and it is the second leading cause of cancer-related deaths[1]. Gastric cancer may disseminate along the inside surface of the peritoneal cavity, leading to peritoneal carcinomatosis (PC). PC is the most frequent mode of metastasis and recurrence in patients with gastric cancer. According to the national registry database of Japan, PC accounted for 51% of deaths in 355 patients with non-curable primary gastric cancer[2]. The same database also revealed that PC was the most frequent cause of death in 13002 patients who underwent gastrectomy for primary gastric cancer[2]. Yoo et al[3] reported that in 508 patients who underwent radical gastrectomy for gastric cancer, the first recurrence site was the peritoneum (43.9%) and then a local site (32.5%) followed by the liver (16.9%).
Despite recent advances in chemotherapy regimens for gastric cancer, the effect of systemic chemotherapy on PC remains unclear. Clinical trials on methotrexate + 5-fluorouracil (5-FU), FOLFOX-4, and continuous 5-FU for PC of gastric cancer showed that the median survival time (MST) was 5.2-10.6 mo, and the 1-year overall survival (OS) was 16.2%-40.7%[4-7].
In alternative treatment modalities, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been used for treating PC of gastric cancer. Reportedly, the MST and 1-year survival were 9.2-11.5 mo and 35.5%-48.1% respectively[8-11]. However, CRS + HIPEC should be performed in specialized facilities, because these demanding procedures are associated with a high mortality and morbidity[12].
The intraperitoneal (IP) administration of anticancer drugs is a reasonable method for treating PC, because an IP administered cytotoxic drug acts directly on the peritoneal metastatic nodules at a high concentration. In HIPEC procedures, mitomycin C (MMC) and/or cisplatin (CDDP) dissolved in heated saline at 42 °C-43 °C are usually administered into the peritoneal cavity[13].
Recently, the IP administration of taxanes such as paclitaxel (PTX) or docetaxel (DOC) without heating them at the ordinary temperature during the perioperative period in gastric cancer patients with PC has been performed mainly in Japan. Several clinical trials using IP chemotherapy with taxanes have shown promising results[14-18].
Based on the literature published in the last decade, we summarized the rationale for using IP chemotherapy with taxanes, methodology used for IP chemotherapy, and clinical results of IP chemotherapy in gastric cancer patients with PC.
Taxanes such as PTX and DOC produce cytotoxic effects by inducing excessive polymerization of tubulin and dysfunctional microtubules, which leads to mitotic arrest and cell death[19,20]. PTX and DOC are water insoluble, and for clinical use, they are solubilized with Cremophor EL (Taxol®; Bristol-Myers Squibb Co.) and Polysorbate 80 (Taxotere®; Aventis Pharma SA), respectively.
Since taxanes are hydrophobic, high-weight molecular materials, IP administered taxanes are gradually drained from the peritoneum through lymphatic stomata that open directly into the pleural space[21,22]. In contrast, hydrophilic, low-weight molecular materials such as MMC or CDDP are rapidly absorbed through the peritoneal mesothelial layer and into the capillary vessels.
The area under the curve ratios of the intra-abdominal space to the plasma after IP administration of the drug are about 1000 for PTX, 207-552 for DOC, 10-24 for MMC, and 12-21 for CDDP[23-28]. The prolonged retention of IP administered taxanes within the IP space allows the taxanes to directly penetrate into peritoneal disseminated tumors[23,29-31], which leads to the destruction of peripheral microvessels of tumor nodules[32]. However, the depth of infiltration from the surface of the peritoneal disseminated nodules after the one time IP administration of a taxane is limited[33,34]. In a previous study, we showed that the distance of PTX infiltration reached approximately 100-200 μm from the surface of the tumor[35]. Therefore, to improve the antitumor effects of taxanes against PC, repeated IP administration is necessary.
From the perspective of pharmacokinetics and tissue penetration, taxanes are ideal drugs for IP chemotherapy. Moreover, even if taxanes are repeatedly administered intraperitoneally, they rarely cause adhesion of organs in the peritoneal cavity because of their antiproliferative effect. Thus, the distribution of IP administered taxanes across the intra-abdominal space is not hampered by drug-induced peritonitis.
There are two types of perioperative IP chemotherapy with taxanes for treating PC of gastric cancer: neoadjuvant intraperitoneal and systemic chemotherapy (NIPS)[36] and sequential perioperative intraperitoneal chemotherapy (SPIC)[37]. In NIPS, patients receive 1-6 courses of IP chemotherapy with a taxane as a neoadjuvant therapy; however, they do not receive IP chemotherapy after CRS[17,38,39]. In SPIC, patients receive several courses of IP chemotherapy preoperatively, and they receive the same regimen of IP chemotherapy after CRS until disease progression[14-16].
In most reported studies, a peritoneal access port system was used for IP chemotherapy. However, this device was not used when patients received a single IP administration during staging laparoscopy[28,39], or if patients received IP administration two times via a catheter as neoadjuvant chemotherapy[17]. A peritoneal access port is implanted into the subcutaneous space of the lower abdomen, and a catheter is placed usually in the pelvic cavity. Taxane dissolved in 500-1000 mL of saline at the ordinary temperature is administered through the peritoneal access port. Thus, using this method, taxanes can be repeatedly administered on an outpatient basis.
Complications associated with the port system occurred in 20.6% of 131 patients at our institution[40]. Inflow obstruction and infection were the main complications that occurred in 7.6% and 6.9% of patients, respectively. The median period of IP chemotherapy using the peritoneal port system was 12.9 mo (range, 0.8-61.5 mo). Compared to previous studies on ovarian cancer[41], the course of IP chemotherapy performed at our institution was much longer, but the complication rate was lower.
The use of a peritoneal port system can facilitate IP administration and reduce the patients’ burden of receiving IP chemotherapy. Moreover, the device can provide another benefit to patients, because the peritoneal lavage sample, which is essential for evaluating the effect of IP chemotherapy on PC, can be obtained noninvasively through the peritoneal access port.
The findings from six phase I studies on IP chemotherapy with taxanes are summarized in Table 1. PTX was used for intraperitoneally administering agents in three studies, and DOC was used in the other three studies. PTX or DOC was IP administered without other anticancer drugs in two studies[26,42], DOC was IP administered with S-1 in two[16,43], PTX was IP administered with S-1 in one[44], and intravenous (IV) PTX and S-1 was administered in one[45].
| Ref. | n | Intraperitoneally administered taxanes | Initial dose(mg/m2) | MTD(mg/m2) | RD(mg/m2) | DLT |
| Kodera et al[42] | 4 | PTX | 60 | - | - | - |
| Fushida et al[26] | 24 | DOC | 25 | 60 | 45 | Abdominal pain and |
| diarrhea | ||||||
| Ishigami et al[45] | 9 | PTX | 20 | 30 | 20 | Febrile neutropenia and diarrhea |
| Fujiwara et al[43] | 12 | DOC | 40 | - | 60 | - |
| Kurita et al[44] | 18 | PTX | 40 | 90 | 80 | Leukocytopenia |
| Fushida et al[16] | 12 | DOC | 35 | 50 | 45 | Febrile neutropenia and diarrhea |
The recommended dose (RD) of DOC IP administration was 45-60 mg/m2. The RD of PTX IP administration was 80 mg/m2 when PTX was not IV administered, and it was 20 mg/m2 when PTX was IV administered. Although the RD of 20 mg/m2 in our phase I study was relatively low because we used a combination of IV PTX, the IP PTX concentration remained extremely high for > 72 h.
Dose-limiting toxicities of these phase I studies included grade 3 febrile neutropenia, leukopenia, and diarrhea for the PTX IP regimen; and grade 3 febrile neutropenia, abdominal pain, and diarrhea for the DOC IP regimen.
The findings of six phase II studies on IP chemotherapy with taxanes are summarized in Table 2. PTX was used for IP administered agents in three studies[14,15,39], and DOC was used in the other three studies[16,17,38]. The overall response rate among these phase II studies ranged from 55%-71%. The MSTs and 1-year OS were 14.4-24.6 mo and 67%-78%, respectively. The main toxicities were hematologic (e.g., anemia, neutropenia, and leukopenia), and the non-hematological toxic effects were relatively mild. Regarding CRS, gastrectomy with D2 dissection was usually performed. In addition to D2 gastrectomy, peritonectomy was performed only by Yonemura et al[38]. Post-operative complications, ranging 9%-22%, were reported in four studies[16,17,38,39]. Surgery-related mortality was found in one patient, and the cause of death was sepsis from an abdominal abscess[38].
| Ref. | n | Method | Intraperitoneally administered agents | MST (mo) | 1-yr OS (%) | 2-yr OS (%) | 5-yr OS (%) |
| Yonemura et al[38] | 61 | NIPS | DOC (40 mg) + CBDCA (150 mg) | 14.4 | 67 | ||
| Ishigami et al[14] | 40 | SPIC | PTX (20 mg/m2) | 22.6 | 78 | ||
| Fujiwara et al[17] | 18 | NIPS | DOC (40-60 mg/m2) | 24.6 | 76 | 54 | |
| Imano et al[39] | 35 | NIPS | PTX (80 mg/m2) | 21.3 | 69 | 46 | 14 |
| Yamaguchi et al[15] | 35 | SPIC | PTX (20 mg/m2) | 17.6 | 77 | 45 | |
| Fushida et al[16] | 27 | SPIC | DOC (35-50 mg/m2) | 16.2 | 70 | 33 |
In three of six phase II studies, patients received 1-6 courses of NIPS. The MSTs of patients who underwent CRS after NIPS were 20.4-29.8 mo. In the other phase II studies, patients received SPIC. In 2010, we reported on a phase II study on SPIC in 40 gastric cancer patients with PC, which included six cytology positive (CY1) and macroscopically negative (P0) patients[14]. Sixteen patients underwent CRS. According to recently updated survival data, the MST was 23.6 mo and the 1-, 2-, and 5-year OS were 78%, 50%, and 18%, respectively.
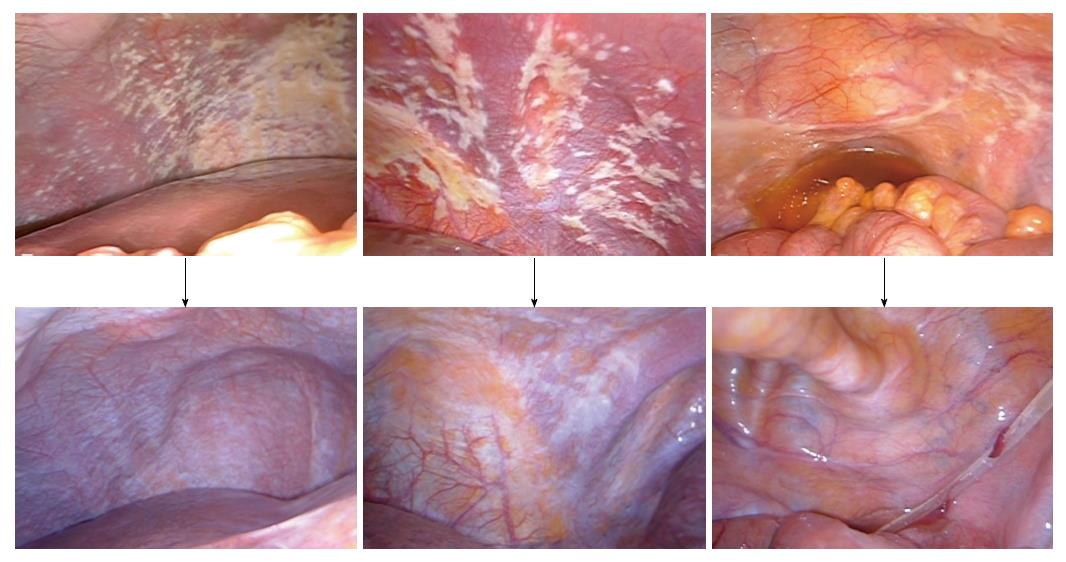
We performed another phase II study with the same regimen in 35 gastric cancer patients with PC[15]. However, in this study, CY1P0 patients were excluded, because they may have a better prognosis compared to macroscopic PC (P1) patients. CRS was performed in 21 patients. Patients with peritoneal cancer index (PCI) scores ≥ 20 had a lower survival rate than those with PCI scores < 20. According to recently updated data, the MST was 18.0 mo, and the 1-, 2-, and 4-year OS were 77%, 42%, and 10%, respectively. The findings from staging laparoscopy and second-look laparoscopy are shown from a representative case (Figure 1).
Fushida et al[16] performed a phase I/II study on SPIC with IP DOC in 27 patients. Fourteen patients underwent CRS and received postoperative IP chemotherapy. The 1- and 2-year OS of patients who underwent CRS were 92.8% and 62.5%, respectively.
In Japan, a randomized, multicenter, phase III trial (the PHOENIX-GC trial, UMIN000005930) compared S-1 in combination with IV and IP PTX to S-1 with IV CDDP in 180 gastric cancer patients with P1. This study began in 2011, and the final analysis will be obtained in November 2015.
If PC can be controlled by IP chemotherapy with a taxane, gastrectomy as CRS is considered to be a reasonable treatment. Because IP chemotherapy as a localized therapy for peritoneal cavity may not have intensive antitumor effects on primary gastric tumors and metastatic lymph nodes. Other than the aforementioned phase II studies, two studies have reported on the treatment results of IP chemotherapy combined with CRS.
Kitayama et al[18] treated 64 gastric cancer patients with PC who had malignant ascites with IP and IV PTX combined with S-1. CRS without peritonectomy was performed in 34 patients. After CRS, chemotherapy with the same regimen was continued (i.e., SPIC). The MST of these patients and the 1-year OS were 26.4 mo and 82%, respectively. Those of the 30 patients who did not undergo gastrectomy were 12.1 mo and 26%, respectively.
Yonemura et al[46] performed NIPS with IP DOC and CDDP combined with S-1 in 96 patients. After two cycles of NIPS, 82 patients underwent CRS (gastrectomy with D2 dissection and peritonectomy). Complete cytoreduction was achieved in 58 patients. The MST and 1-year OS of patients who underwent CRS was 14.4 mo and 61%, respectively. The MST of patients who underwent complete cytoreduction and those who did not undergo CRS were 21.1 mo and 9 mo, respectively.
In these reports, the prognosis of patients who underwent CRS was better than that of those who did not. However, this survival difference was partly due to a strong selection bias since CRS was performed only in good responders. A randomized controlled study will need to be performed in order to determine the significance of CRS.
It is important whether IP chemotherapy with taxanes is needed after CRS. Yonemura et al[46] reported that 22 of 61 patients who received NIPS with complete CRS had recurrence in the peritoneum. Fujiwara et al[17] suggested that IP chemotherapy may have been needed in their patients, because 8 of 14 patients who had curative surgery following NIPS died from peritoneal recurrence. It is reasonable to consider that IP chemotherapy with a taxane should be continued as long as possible even after CRS to suppress the development of microscopic cancer cells that may still exist in the whole peritoneal cavity. Therefore, we consider that SPIC is better suited for treating PC of gastric cancer.
Another important issue is how the criteria for performing CRS are determined. If patients do not respond to IP chemotherapy, CRS should not be performed. We have performed CRS in patients who have met the following criteria: (1) no distant metastasis, except in the peritoneum; (2) a negative peritoneal lavage cytology; and (3) a second-look laparoscopy reveals that the peritoneal metastatic nodules are reduced. To select eligible patients for CRS more precisely, novel and useful biomarkers that reflect a good response to IP chemotherapy are needed.
Phase III studies on IP chemotherapy with taxanes have been reported in the gynecological field, especially for PC of ovarian cancer. IP PTX with systemic chemotherapy for PC of ovarian cancer showed a significant survival benefit[47]. Based on the findings from these phase III studies[47-49], the National Cancer Institute has recommended IP chemotherapy in patients with optimally debulked ovarian cancer[50].
Regarding the treatment of PC from gastric cancer, there are promising findings from several phase II studies with IP chemotherapy using taxanes. However, it is difficult to draw any definitive conclusions about the overall clinical usefulness of this treatment method until we obtain the findings from the PHOENIX-GC phase III trials.
In conclusion, IP administered taxanes remain in the IP cavity for a long period, and they produce antitumor effects by infiltrating peritoneal metastatic nodules from the surface. In addition, repeated IP administration of taxanes through an IP access port before and after CRS seems necessary for improving the effect of IP chemotherapy. Lastly, IP chemotherapy with taxanes for PC from gastric cancer is safe and feasible. Although several phase II clinical studies have shown promising results, further randomized phase III clinical trials are needed to validate IP chemotherapy with taxanes for gastric PC.
P- Reviewer: Arsenijevic T S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11836] [Article Influence: 845.4] [Reference Citation Analysis (4)] |
| 2. | Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 555] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 4. | Yamao T, Shimada Y, Shirao K, Ohtsu A, Ikeda N, Hyodo I, Saito H, Iwase H, Tsuji Y, Tamura T. Phase II study of sequential methotrexate and 5-fluorouracil chemotherapy against peritoneally disseminated gastric cancer with malignant ascites: a report from the Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group, JCOG 9603 Trial. Jpn J Clin Oncol. 2004;34:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Oh SY, Kwon HC, Lee S, Lee DM, Yoo HS, Kim SH, Jang JS, Kim MC, Jeong JS, Kim HJ. A Phase II study of oxaliplatin with low-dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) for gastric cancer patients with malignant ascites. Jpn J Clin Oncol. 2007;37:930-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Shirao K, Boku N, Yamada Y, Yamaguchi K, T . D, Takiuchi H, Nasu J, Nakamura K, Fukuda H, Ohtsu A. Randomized phase III study of 5-fluorouracil continuous infusion (5FUci) versus methotrexate and 5-FU sequential therapy (MF) in gastric cancer with peritoneal metastasis (JCOG0106). J Clin Oncol. 2009;27 Suppl:abstr 4545. |
| 7. | Imazawa M, Kojima T, Boku N, Onozawa Y, Hironaka S, Fukutomi A, Yasui H, Yamazaki K, Taku K. Efficacy of sequential methotrexate and 5-fluorouracil (MTX/5FU) in improving oral intake in patients with advanced gastric cancer with severe peritoneal dissemination. Gastric Cancer. 2009;12:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Glehen O, Schreiber V, Cotte E, Sayag-Beaujard AC, Osinsky D, Freyer G, François Y, Vignal J, Gilly FN. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg. 2004;139:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 493] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 11. | Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F, Elias D. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 12. | Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E, Schiller D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | González-Moreno S, González-Bayón LA, Ortega-Pérez G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol. 2010;2:68-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 176] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119:3354-3358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Fushida S, Kinoshita J, Kaji M, Hirono Y, Goda F, Yagi Y, Oyama K, Sudo Y, Watanabe Y, Fujimura T. Phase I/II study of intraperitoneal docetaxel plus S-1 for the gastric cancer patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2013;71:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Fujiwara Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Mori M, Doki Y. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol. 2012;105:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Kitayama J, Ishigami H, Yamaguchi H, Yamashita H, Emoto S, Kaisaki S, Watanabe T. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol. 2014;21:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82:1247-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 717] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991;83:288-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 496] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | Flessner MF, Fenstermacher JD, Blasberg RG, Dedrick RL. Peritoneal absorption of macromolecules studied by quantitative autoradiography. Am J Physiol. 1985;248:H26-H32. [PubMed] |
| 22. | Wang ZB, Li M, Li JC. Recent advances in the research of lymphatic stomata. Anat Rec (Hoboken). 2010;293:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Ceelen WP, Flessner MF. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat Rev Clin Oncol. 2010;7:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Yan TD, Cao CQ, Munkholm-Larsen S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J Gastrointest Oncol. 2010;2:109-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 26. | Fushida S, Kinoshita J, Yagi Y, Funaki H, Kinami S, Ninomiya I, Fujimura T, Nishimura G, Kayahara M, Ohta T. Dual anti-cancer effects of weekly intraperitoneal docetaxel in treatment of advanced gastric cancer patients with peritoneal carcinomatosis: a feasibility and pharmacokinetic study. Oncol Rep. 2008;19:1305-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Yonemura Y, Elnemr A, Endou Y, Hirano M, Mizumoto A, Takao N, Ichinose M, Miura M, Li Y. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol. 2010;2:85-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 92] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Imano M, Peng YF, Itoh T, Nishikawa M, Satou T, Yasuda A, Inoue K, Kato H, Shinkai M, Tsubaki M. A preliminary study of single intraperitoneal administration of paclitaxel followed by sequential systemic chemotherapy with S-1 plus paclitaxel for advanced gastric cancer with peritoneal metastasis. Anticancer Res. 2012;32:4071-4075. [PubMed] |
| 29. | Yonemura Y, Endou Y, Bando E, Kuno K, Kawamura T, Kimura M, Shimada T, Miyamoto K, Sasaki T, Sugarbaker PH. Effect of intraperitoneal administration of docetaxel on peritoneal dissemination of gastric cancer. Cancer Lett. 2004;210:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Soma D, Kitayama J, Ishigami H, Kaisaki S, Nagawa H. Different tissue distribution of paclitaxel with intravenous and intraperitoneal administration. J Surg Res. 2009;155:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Soma D, Kitayama J, Konno T, Ishihara K, Yamada J, Kamei T, Ishigami H, Kaisaki S, Nagawa H. Intraperitoneal administration of paclitaxel solubilized with poly(2-methacryloxyethyl phosphorylcholine-co n-butyl methacrylate) for peritoneal dissemination of gastric cancer. Cancer Sci. 2009;100:1979-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Kitayama J, Emoto S, Yamaguchi H, Ishigami H, Watanabe T. Intraperitoneal paclitaxel induces regression of peritoneal metastasis partly by destruction of peripheral microvessels. Cancer Chemother Pharmacol. 2014;73:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Kuh HJ, Jang SH, Wientjes MG, Weaver JR, Au JL. Determinants of paclitaxel penetration and accumulation in human solid tumor. J Pharmacol Exp Ther. 1999;290:871-880. [PubMed] |
| 34. | Kyle AH, Huxham LA, Yeoman DM, Minchinton AI. Limited tissue penetration of taxanes: a mechanism for resistance in solid tumors. Clin Cancer Res. 2007;13:2804-2810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Kamei T, Kitayama J, Yamaguchi H, Soma D, Emoto S, Konno T, Ishihara K, Ishigami H, Kaisaki S, Nagawa H. Spatial distribution of intraperitoneally administrated paclitaxel nanoparticles solubilized with poly (2-methacryloxyethyl phosphorylcholine-co n-butyl methacrylate) in peritoneal metastatic nodules. Cancer Sci. 2011;102:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Yonemura Y, Endou Y, Sasaki T, Hirano M, Mizumoto A, Matsuda T, Takao N, Ichinose M, Miura M, Li Y. Surgical treatment for peritoneal carcinomatosis from gastric cancer. Eur J Surg Oncol. 2010;36:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Cashin PH, Graf W, Nygren P, Mahteme H. Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: prognosis and treatment of recurrences in a cohort study. Eur J Surg Oncol. 2012;38:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Yonemura Y, Bandou E, Sawa T, Yoshimitsu Y, Endou Y, Sasaki T, Sugarbaker PH. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. Eur J Surg Oncol. 2006;32:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Imano M, Yasuda A, Itoh T, Satou T, Peng YF, Kato H, Shinkai M, Tsubaki M, Chiba Y, Yasuda T. Phase II study of single intraperitoneal chemotherapy followed by systemic chemotherapy for gastric cancer with peritoneal metastasis. J Gastrointest Surg. 2012;16:2190-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Emoto S, Ishigami H, Hidemura A, Yamaguchi H, Yamashita H, Kitayama J, Watanabe T. Complications and management of an implanted intraperitoneal access port system for intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Jpn J Clin Oncol. 2012;42:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Walker JL, Armstrong DK, Huang HQ, Fowler J, Webster K, Burger RA, Clarke-Pearson D. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. Gynecol Oncol. 2006;100:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 42. | Kodera Y, Ito Y, Ito S, Ohashi N, Mochizuki Y, Yamamura Y, Koike M, Fujiwara M, Nakanishi H, Nakao A. Intraperitoneal paclitaxel: a possible impact of regional delivery for prevention of peritoneal carcinomatosis in patients with gastric carcinoma. Hepatogastroenterology. 2007;54:960-963. [PubMed] |
| 43. | Fujiwara Y, Nishida T, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Yamamoto K, Moon JH, Mori M, Doki Y. Feasibility study of S-1 and intraperitoneal docetaxel combination chemotherapy for gastric cancer with peritoneal dissemination. Anticancer Res. 2010;30:1335-1339. [PubMed] |
| 44. | Kurita N, Shimada M, Iwata T, Nishioka M, Morimoto S, Yoshikawa K, Higashijima J, Miyatani T, Nakao T. Intraperitoneal infusion of paclitaxel with S-1 for peritoneal metastasis of advanced gastric cancer: phase I study. J Med Invest. 2011;58:134-139. [PubMed] |
| 45. | Ishigami H, Kitayama J, Otani K, Kamei T, Soma D, Miyato H, Yamashita H, Hidemura A, Kaisaki S, Nagawa H. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology. 2009;76:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Yonemura Y, Elnemr A, Endou Y, Ishibashi H, Mizumoto A, Miura M, Li Y. Effects of neoadjuvant intraperitoneal/systemic chemotherapy (bidirectional chemotherapy) for the treatment of patients with peritoneal metastasis from gastric cancer. Int J Surg Oncol. 2012;2012:148420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2030] [Cited by in RCA: 1949] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 48. | Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 870] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 49. | Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, Wadler S, Sickel J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001-1007. [PubMed] |
| 50. | National Cancer Institute. Clinical Announcement: Intraperitoneal chemotherapy for ovarian cancer. [accessed 2006 Jan 5]. Available from: http://ctep.cancer.gov/highlights/clin_annc_010506.pdf. |