INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies and a leading cause of cancer deaths for both men and women in Western countries[1]. The five-year survival rate remains poor despite significant advances in diagnosis and therapy. CRC results from an interaction among several factors, including lifestyle, family history and diet[2,3]. Since Lacassagne’s work in 1955 demonstrating that estrogen administration increases the incidence of mammary cancer in mice[4], many studies have shown the involvement of sex hormones in the risk and development of many types of cancer, including breast cancer and CRC. The incidence of CRC is slightly lower in women compared to men of a similar age[5], and epidemiologic studies and results of the Women’s Health Initiative clinical trial show that the risk is reduced in women who take hormone replacement therapy[6]. Furthermore, reduced serum levels of estradiol are associated with downregulated estrogen receptor (ER) expression in the colonic mucosa and a significantly increased risk of CRC[3,7].
ERα and ERβ are the two known subtypes through which estrogens exert their effects on various tissues. Experimental data show differential expression of these receptors, with very low levels of ERα either in normal or pathologic colonic mucosa (adenoma and carcinoma)[8], and high ERβ expression in healthy colonic mucosa, which decreases with the progression of CRC[8-11]. This has led to the proposal that ERβ functions as a tumor suppressor, protecting cells against malignant transformation, and is responsible for the protective effect of estradiol on CRC[12,13].
There is evidence that some polyphenols produced by plants have estrogen-like activity. It has been demonstrated that these phytoestrogens, with molecular structures similar to steroids, could be critical modulators of the human hormonal system and exert hormonal actions on target tissues[14,15]. Phytoestrogens have been widely studied for their potential therapeutic use in the prevention of different diseases and some carcinomas, given that they show some of the protective effects of estrogens in absence of the side effects associated with estrogen administration[16]. These effects may occur through binding to ERs or interacting with enzymes involved in sex steroid metabolism and biosynthesis[17]. Most phenolic compounds show a chemical structure similar to 17β-estradiol (17β-E2), suggesting they might compete for ER binding. However, phytoestrogens can produce estrogenic, anti-estrogenic and unique effects independent from estrogen binding recognition. These diverse actions of phenolic compounds are also tissue-specific, and thus are defined as selective estrogen receptor modulators[18].
Genistein is a phytoestrogen found in soy that may inhibit cancer progression by inducing apoptosis or inhibiting proliferation, the mechanisms by which are a subject of considerable interest[19]. A negative correlation was observed between the incidences of breast, prostate and colon cancer and the phytoestrogen-rich soy diet of some ethnic groups in Asia[20,21]. Recently, several studies have identified a dualistic mode of action by genistein in relation to cancer cell proliferation and cancer risk[22].
Whereas low concentrations of genistein have been shown to enhance the proliferation of breast cancer cells in vitro, high concentrations can inhibit their growth[23]. It is possible that the opposing effects of phytoestrogens depend on which ER isoform they interact with.
To better understand the influence of phytoestrogens on cancer development and progression, colon cancer cells were evaluated after exposure to genistein or quercetin, a flavonoid ubiquitously present in many fruits, vegetables, seeds, nuts, olive oil, tea and red wine[24] that also has potentially beneficial effects on cancer prevention[25-27]. The effect of these treatments on ERβ activation and expression, cell growth and cell viability, determined by staining with lysosomotropic acridine orange (AO) to detect lysosomal activation[28-30], were evaluated.
MATERIALS AND METHODS
Cell lines and chemicals
The human colon cancer HCT8 cell line[31,32] was obtained from the American Type Culture Collection (Rockville, MD, United States of America). Cells overexpressing human ERβ (HCT8-β8) were established via a stable transfection with the mammalian expression vector pCXN2-hERβ or a control pSV2neo vector (HCT8-pSV2neo)[33]. Genistein, quercetin and 17β-E2 (internal positive control) were purchased from Sigma-Aldrich (St. Louis, MO, United States). Solutions of 17β-E2 and phytoestrogens were dissolved in ethanol and then diluted in cell culture medium to the final concentrations.
Cell culture
Cells were cultured in RPMI 1640 medium (Lonza Group, Basel, Switzerland) supplemented with 10% fetal bovine serum (FBS) or FBS-stripped serum (SFBS; Biological Industries, Kibhutz Beit Haemek, Israel), without phenol red, with 1 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, 100 μg/mL penicillin, 100 μg/mL streptomycin and 280.25 μg/mL Geneticin (G418; Invitrogen of Thermo Fisher Scientific Inc., Waltham, MA, United States) at 37 °C with 5% CO2 humidified air. Confluent cell cultures were detached with a trypsin/ethylenediaminetetraacetic (EDTA) acid solution (Lonza Group) and plated at the desired density in the appropriate medium.
Cell proliferation analysis
For cell proliferation analysis, HCT8-β8- or HCT8-pSV2neo-expressing cells were plated on 6-well plates at a density of 5 × 103 cells/well. After 2 h, the medium was replaced with SFBS medium (phenol red-free medium supplemented with 10% SFBS, and penicillin-streptomycin) and stimulated with genistein or quercetin (5, 25, 50, 75, 100 μmol/L), or with 10 nmol/L 17β-E2 (cells without stimuli were used as a control). Cells were detached with trypsin/EDTA and the number was evaluated by a Bürker hemocytometer every 48 h for 8 d. Measurements for each dose at each time point were collected in triplicate and averaged.
AO staining
Following a 48 or 144 h treatment with quercetin, genistein or 17β-E2, HCT8-β8- or HCT8-pSV2neo-expressing cells were washed three times with phosphate buffered saline (PBS) to remove dead cells and serum proteins (cells without stimuli were used as a control). Cells were incubated in a 0.2% AO solution (in PBS, 2 mL/well) in the dark at room temperature for 10 min and washed three times with PBS. The cells were observed in phase contrast and under fluorescence (BP365/FT395/LP397 filter set) with an Axiovert 200 M microscope and images were acquired with Axiovision Software on an AxioCam HRC 12 megapixel camera (Carl Zeiss, Oberkochen, Germany). When stained with AO, DNA and mitochondria emit green fluorescence (530 nm) and lysosomes emit red fluorescence (650 nm) following excitation by ultraviolet (UV) light (365 nm).
Luciferase assay
HCT8-β8- or HCT8-pSV2neo-expressing were plated on 24-well plates at 2 × 104 cells/well in complete RPMI 1640 culture medium with 10% FBS and penicillin-streptomycin. Twenty-four hours later, the medium was replaced with phenol red-free medium supplemented with 10% SFBS and penicillin-streptomycin. A solution of Attractene Transfection Reagent (Qiagen, Venlo, Limburg, Netherlands) was used to transiently transfect cells with the pEREtkLUC (kindly supplied by Dr. MG Parker)[34] reporter plasmid (395 ng/well) and pERLNULL control plasmid (4 ng/well) (Promega, Madison, WI, United States), and cells were incubated in phenol- and FBS-free RPMI medium for 48 h. After a 24 h stimulation in the same medium with quercetin (50 μmol/L), genistein (50 μmol/L) or 17β-E2 (10 nmol/L) (or no stimulation for controls), whole cell extracts were obtained with the Luciferase Assay System (Promega) and luciferase activity was determined with a luminometer (LKB Instruments, Mount Waverly, Victoria, Australia). Luciferase activity was normalized to β-galactosidase activity measured by a β-gal Assay Kit (Invitrogen) and to total protein concentration. Measurements for each condition were collected in triplicate and averaged.
RNA isolation and real-time quantitative polymerase chain reaction
Total RNA was isolated from cultured cells after stimulation with quercetin (50 μmol/L), genistein (50 μmol/L) or 17β-E2 (10 nmol/L) (from triplicate plates) with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions and quantified by UV absorbance. Reverse transcription was performed using the Quantitect Reverse Transcription Kit followed by treatment with ribonuclease-free deoxyribonuclease I (Qiagen). Quantitative polymerase chain reaction (qPCR) was performed using the Kapa Probe Fast qPCR kit (Kapa Biosystems Inc., Wilmington, MA, United States) according to the manufacturer’s instructions. Briefly, reactions consisting of 2 μL cDNA, 10 μL KAPA PROBE FAST qPCR Master Mix, 2 μL gene specific primers (10 μmol/L), 1 μL TaqMan Probe (5 μmol/L), and 5 μL RNase-free H2O were heated at 95 °C for 5 min and amplified by 35 cycles of 95 °C for 10 s, and 60 °C for 30 s using a Rotor-Gene Q (Qiagen). The results obtained were normalized to a housekeeping gene (RPS18).
The following primers and corresponding TaqMan probes were used: ERβ: (forward) 5’-TCGCCAGTTATCACATCTGTATGCGG-3’, (reverse) 5’-GTGTCTCTCTGTTTACAGGTAAGGTGTG-3’, (probe) F/TCCCTGGTG/ZEN/TGAAGCAAGATCGCTAGAA/Q; RSP18: (forward) 5’-CTTCCACAGGAGGCCTAC-3’, (reverse) 5’-GATGGCAAAGGCTATTTTCCG-3’, (probe) F/TTCAGGGAT/ZEN/CACTAGAGACATGGCTGC/Q.
Statistical analysis
Statistical differences between groups were analyzed in Microsoft Excel (Microsoft, Redmond, WA, United States) using Student’s t-tests. Data are expressed as mean ± SD. Statistical differences for cell proliferation analysis between treated groups vs controls were analyzed in Excel using a parallelism test for linear regression.
RESULTS
Effects of genistein and quercetin on colon cancer cell proliferation
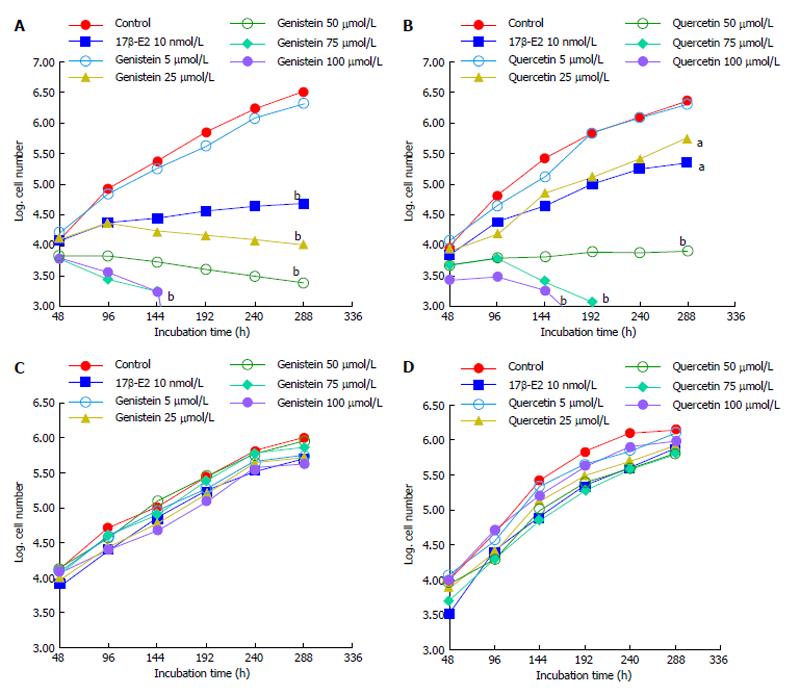
Cell counts of HCT8-β8- or HCT8-pSV2neo-expressing cells cultured with genistein, quercetin or 17β-E2 were performed every 48 h for up to 12 d to assess cell proliferation. Results show that both phytoestrogens dose-dependently significantly reduced the proliferation of HCT8-β8-expressing cells (Figure 1A and B). The inhibition of cell growth by genistein and quercetin was apparent at concentrations of 25 μmol/L, similar to the effects 10 nmol/L 17β-E2. However, higher concentrations of the phytoestrogens (75 and 100 μmol/L) prevented proliferation and reduced overall cell counts. In contrast, quercetin, genistein and 17β-E2 treatments had no effect on the proliferation of HCT8-pSV2neo-expressing cells (Figure 1C and D).
Figure 1 Effects of polyphenols on cell growth.
A: Growth of HCT8-â8-expressing cells in the presence of genistein and 17â-E2; B: Growth of HCT8-β8-expressing cells in the presence of quercetin and 17β-E2; C: Growth of HCT8-pSV2neo-expressing cells in the presence of genistein and 17β-E2; D: Growth of HCT8-pSV2neo-expressing cells in the presence of quercetin and 17β-E2. Values are the means of triplicates; aP < 0.05 vs control; bP < 0.01 vs control.
Effects of genistein and quercetin on colon cancer cell viability
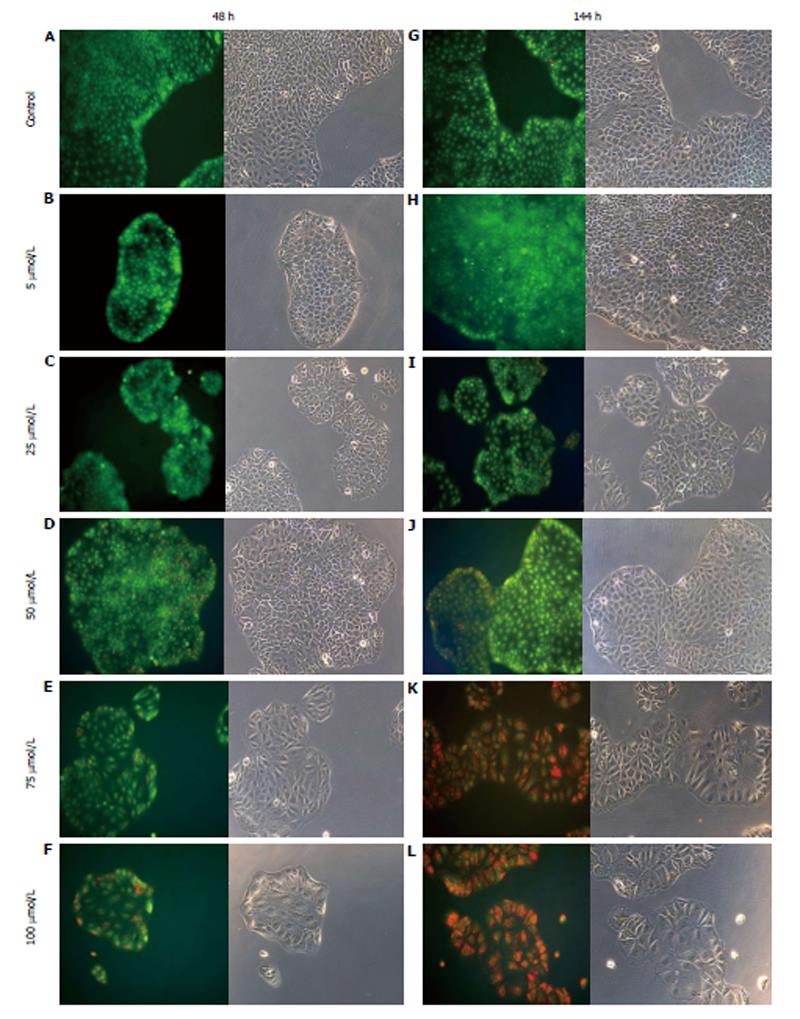
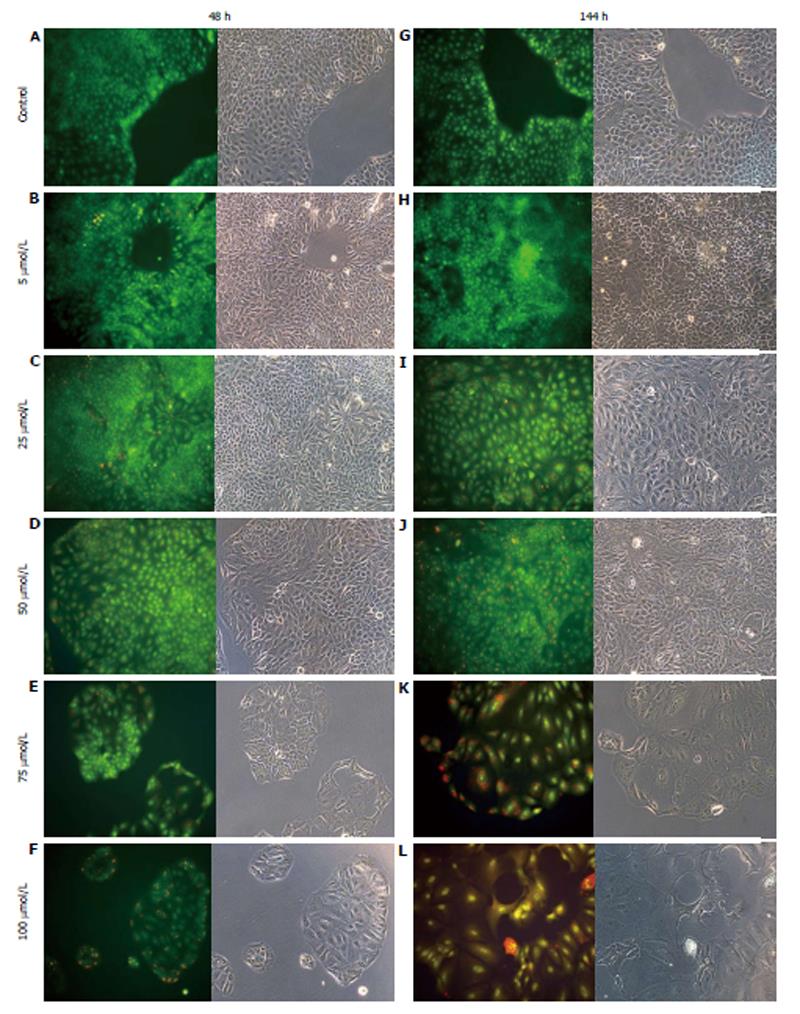
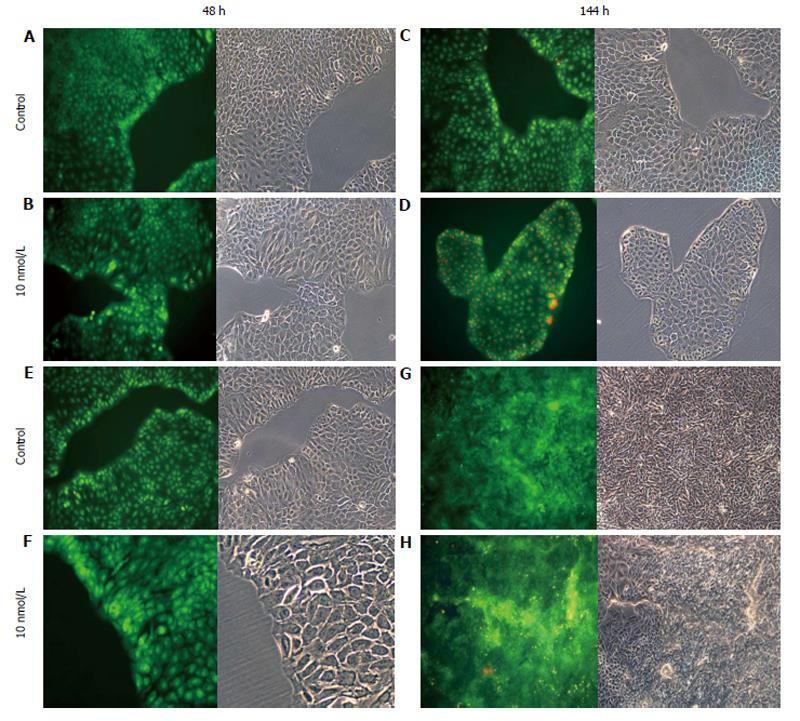
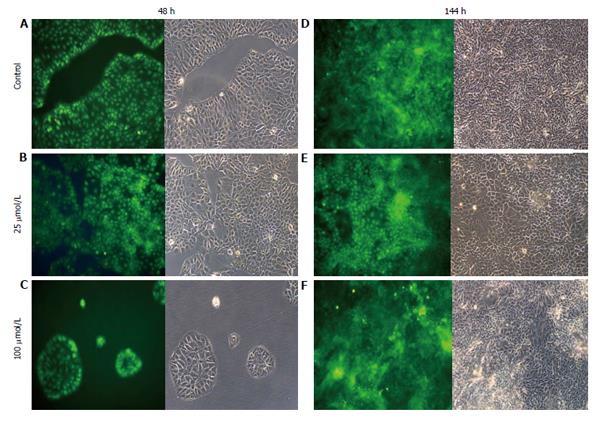
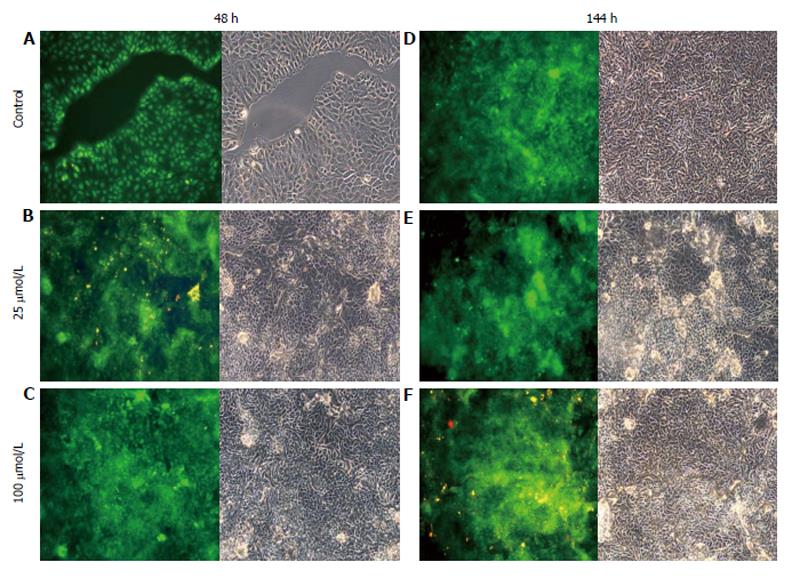
AO staining of HCT8-β8-expressing cells treated for 48 h with 5-25 μmol/L genistein (Figure 2B and C), 5-25 μmol/L quercetin (Figure 3B and C) or 10 nmol/L 17β-E2 (Figure 4B) revealed a homogenous green brilliant fluorescence, similar to the untreated control cells. However, red lysosomes became apparent with higher doses of both phytoestrogens (≥ 50 μmol/L) (Figures 2D-F, 3D-F), or extended exposure of concentrations ≥25 μmol/L (144 h; Figures 2I-L, 3I-L). There were some red-labeled lysosomes observed with 144-h treatment of 10 nmol/L of 17β-E2 (Figure 4D). Long-term treatment with high doses of phytoestrogens (≥ 75 μmol/L) revealed many cells with pale and homogeneous green fluorescence and many brilliant red-orange lysosomes (Figures 2K, L, and 3K, L), which indicate reduced viability and cellular stress. In contrast, HCT8-pSV2neo-expressing cells were largely unaffected by treatment with genistein (Figure 5), quercetin (Figure 6B), or 17β-E2 (Figure 4E-H), but rather exhibited strong, homogeneous green fluorescence with few lysosomes in all the treated samples after 48 and 144 h.
Figure 2 Treatment of HCT8-β8-expressing cells with genistein.
HCT8-β8-expressing cells were treated with various concentrations of genistein for 48 h (A-F) or 144 h (G-L) and stained with acridine orange. Nuclei and mitochondria appear green, whereas lysosomes appear red-orange under fluorescence, adjacent to corresponding phase contrast images (magnification × 20).
Figure 3 Treatment of HCT8-β8-expressing cells with quercetin.
HCT8- β8-expressing cells were treated with various concentrations of quercetin for 48 h (A-F) or 144 h (G-L) and stained with acridine orange. Nuclei and mitochondria appear green, whereas lysosomes appear red-orange under fluorescence, adjacent to corresponding phase contrast images (magnification, × 20).
Figure 4 Treatment of cells with 17β-E2.
A-D: HCT8-β8-expressing cells; or E-H: HCT8-pSV2neo-expressing cells were treated with 10 nmol/L 17β-E2 for 48 h (A, B, E, F) or 144 h (C, D, G, H) and stained with acridine orange. Nuclei and mitochondria appear green, whereas lysosomes appear red-orange under fluorescence, adjacent to corresponding phase contrast images (magnification × 20).
Figure 5 Treatment of HCT8-pSV2neo-expressing cells with genistein.
A-F: HCT8-pSV2neo-expressing cells were treated with 25 μmol/L (B and E) or 100 μmol/L (C and F) genistein for 48 h (A-C) or 144 h (D-F) and stained with acridine orange. Nuclei and mitochondria appear green, whereas lysosomes appear red-orange under fluorescence, adjacent to corresponding phase contrast images (magnification × 20).
Figure 6 Treatment of HCT8-pSV2neo-expressing cells with quercetin.
A-F: HCT8-pSV2neo-expressing cells were treated with 25 μmol/L (B and E) or 100 μmol/L (C and F) quercetin for 48 h (A-C) or 144 h (D-F) and stained with acridine orange. Nuclei and mitochondria appear green, whereas lysosomes appear red-orange under fluorescence, adjacent to corresponding phase contrast images (magnification × 20).
Effects of genistein and quercetin on ERβ transactivation
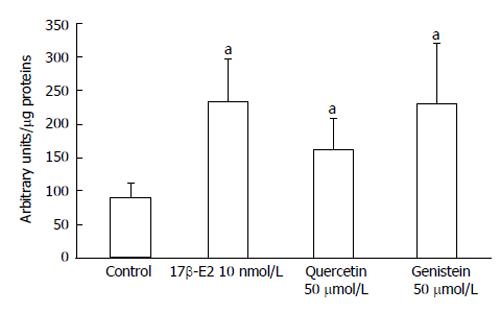
To determine if the anti-proliferative effects of genistein and quercetin occurred through activation of ERβ, ER-responsive luciferase activity was measured in HCT8-β8-expressing cells transiently transfected with the pEREtkLUC reporter plasmid. Luciferase activity was significantly increased (165%) following 24 h treatment with 10 nmol/L 17β-E2 (P < 0.05) (Figure 7). Similarly, treatment with 50 μmol/L genistein and 50 μmol/L quercetin produced an increase in luciferase activity of 158 and 81%, respectively (P < 0.05), compared to an untreated control. ER-responsive luciferase activity was not evaluated for HCT8-pSV2neo-expressing cells as neither of the two polyphenols produced anti-proliferative effects in this cell line.
Figure 7 Induction of EREtkLUC reporter gene activity.
Treatment of HCT8-â8-expressing cells with 17â-E2, genistein and quercetin induces EREtk expression observed as relative luciferase activity. Values are the mean ± SD of triplicates; aP < 0.05 vs control.
Effects of genistein and quercetin on ERβ transcription
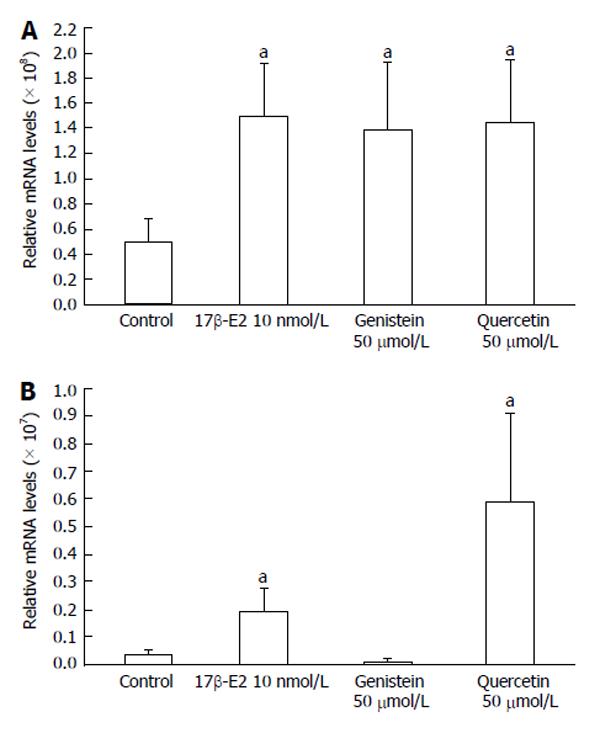
The expression of ERβ mRNA in HCT8-β8-expressing cells was significantly increased following a six-day treatment with 50 μmol/L genistein (1.39 × 108± 5.33 × 107), 50 μmol/L quercetin (1.45 × 108± 5.00 × 107) and 10 nmol/L 17β-E2 (1.49 × 108± 4.35 × 107), compared to untreated controls (5.00 × 107± 1.90 × 107) (all P < 0.05) (Figure 8A). Increases in ERβ mRNA levels were also observed in HCT8-pSV2neo-expressing cells treated with quercetin (5.88 × 106± 3.20 × 106) and 17β-E2 (1.91 × 106± 8.54 × 105) (P < 0.05) (Figure 8B), though the relative expression (3.97 × 105± 1.37 × 105) was much lower compared to HCT8-β8-expressing cells.
Figure 8 Expression of ERβ mRNA levels by quantitative real-time reverse transcription-polymerase chain reaction.
Induction of ERβ expression by 17β-E2, genistein and quercetin in A: HCT8-β8-expressing cells; B: HCT8-pSV2neo-expressing cells. The results are expressed relative to RPS18 mRNA levels. Values are the mean ± SD of quadruplicates; aP < 0.05 vs control.
DISCUSSION
Genistein, found in soybeans and their derivatives, and quercetin, one of the most abundant phytoestrogens in the Western diet[34], are two natural flavonoid molecules with molecular structures similar to 17β-E2, which is a substrate of ERβ. Consumption of phytoestrogen-rich foods is correlated with a reduced incidence of CRC[35,36]. Moreover, plasma concentrations of phytoestrogens are high in populations from China, Japan and countries of Southeast Asia, which are considered to have low risks for malignancy, particularly for hormone-sensitive cancers such as breast cancer, prostate cancer and CRC[20,37,38].
The possible antitumorogenic effects of phytoestrogens were tested in two CRC cell models, including a hormone-sensitive cell line of colon adenocarcinoma expressing very low levels of ERβ (HCT8-pSV2neo-expressing), and the same cell line with high levels of ERβ (HCT8-β8-expressing). The range of phytoestrogen concentrations used were based on epidemiologic and absorption human studies. Quercetin intake is reported to be approximately 16 mg/d[34], and a study by Hollman et al[39] found that 76% of orally administered quercetin aglycone is recovered in the ileostomy bags of subjects who underwent a colectomy, which can be considered a model compartment for the colon[40]. Therefore, an average 12 mg of quercetin reaches the colon daily, indicating that, depending on dietary intake, quercetin concentrations of 40-80 μmol/L in the colon are likely.
Dietary intakes of 39 and 47 mg of genistein/day for the adult Chinese and Japanese populations, respectively, have been reported[41-43], whereas the Western diet provides only 1-2 mg/d, with values of up to 3-12 mg of genistein/day for those following a vegetarian diet[44,45].
The results of the in vitro proliferation analyses show that even relatively low doses of phytoestrogens can reduce, and concentrations comparable to those found in Eastern diets can block, proliferation of HCT8-β8-expressing, but not HCT8-pSV2neo-expressing cancer cells. These data confirm results described in the literature regarding the behavior of the same phytoestrogens on different CRC cell lines, as well as in other hormone-sensitive cancer cells[34,46-48]. For example, genistein has an anti-proliferative effect on the estrogen-dependent human breast cancer MCF-7 cell line similar to that induced by 17β-E2[23], and the proliferation of prostate cancer cells is reduced by quercetin[24]. However, a study on the Caco-2 colon cancer cell line, which contains low levels of ERβ, showed that cell cycle gene expression and cell proliferation was reduced with 50 μmol/L of quercetin, resulting in cell cycle arrest[25,26].
The observed anti-proliferative effects of phytoestrogens on HCT8-β8-expressing cells were accompanied by activation of ERβ, as observed by luciferase activation. The results show that both genistein and quercetin increased luciferase activity, comparable to levels induced by 17β-E2. This activity likely depends directly on ERβ binding, which can then modulatate the expression of specific proteins directly involved in cell cycle regulation[49-55]. Furthermore, the concentrations of quercetin and genistein that inhibited cell growth but did not induce cell death were also found to increase ERβ mRNA levels. The basal level of ERβ in HCT8-β8-expressing cells perpetuated a large increase in mRNA after treatment with both phytoestrogens and 17β-E2. A proportionately larger increase was observed in HCT8-pSV2neo-expressing cells, though the relative levels were much lower.
Taken together, these data suggest that the inhibition of cell growth, activation of ERβ and the increased transcription of ERβ depend on the binding of phytoestrogens to ERβ, as these effects were absent or minimal in HCT8-pSV2neo-expressing cells, though future experiments with agents blocking the estrogen receptor will be necessary to confirm this. The data presented here are in agreement with observations from other hormone-sensitive cancers[25,56], and also demonstrate the protective role of ERβ that has been reported for estrogen-sensitive tissue such as breast, ovary, prostate and colorectal mucosa[57-61]. Furthermore, these results support the epidemiologic and experimental data which show the protective action of both the tested phytoestrogens at a concentration similar to the levels in colorectal mucosae that result from daily phytoestrogen intake in the Eastern diet, and indicate that dietary intake of phytoestrogens may protect against CRC by acting on tumoral cell growth and modulating gene transcription. In conclusion, our study indicates that the mechanism for antitumorogenic activity of phytoestrogens on CRC could involve regulation of ERβ expression.
COMMENTS
Background
Recent evidence suggests a close relationship between estrogen and colorectal cancer (CRC), one of the most common malignancies, such that reduction in circulating levels of estradiol increases the risk of developing cancer. Furthermore, regions with a high dietary intake of phytoestrogens, natural molecules with estrogen-like effects, have lower incidences of CRC. The expression of estrogen receptor β (ERβ), is high in healthy colorectal mucosa, and reduced in cancerous tissue. However, the mechanism regulating the effect of estrogen on the development of CRC is not well understood.
Research frontiers
Among the phytoestrogens examined for their antitumoral functions, the flavonoids genistein and quercetin are the most well studied. In this in vitro study, the authors evaluate these two phytoestrogens, which are common in food sources, and suggest that their anti-proliferative effects are through the activation and expression of ERβ.
Innovations and breakthroughs
Several in vivo studies have highlighted the protective antitumoral role of two phytoestrogens, quercetin and genistein, in different hormone-sensitive cancers and the protective role of ERβ on estrogen-sensitive tissues such as breast, ovary, prostate and colorectal mucosa. This in vitro study confirms epidemiologic and experimental data which show the protective action of these phytoestrogens against CRC, and demonstrate their effect on cancer cell growth and ERβ transcription. In particular, this study reveals that these effects occur at concentrations of quercetin that are equivalent to those obtained following a daily intake of 16 mg/d.
Applications
By studying the influence of phytoestrogens on the growth of colon cancer cells and their regulation of ERβ expression, this study suggests that similar results could also be found for other hormone-sensitive tissues. Furthermore, the results further suggest that an increase in the dietary consumption of foods rich in phytoestrogens could represent a future strategy for the prevention of CRC and other hormone-sensitive cancers.
Terminology
Estrogen receptors ERα and ERβ are activated by 17-estradiol. Phytoestrogens are a group of plant-derived compounds, including flavonoids, coumestans, lignans and stilbenes, with estrogenic properties. Genistein and quercetin are the most representative of the phytoestrogens that have been studied for their antitumorigenic properties.
Peer review
This study examines the biologic effects of two phytoestrogens on cell growth and expression of ERβ in colon cancer cell lines. The results indicate that quercetin and genistein exert their effects by activating and regulating the expression of ERβ. This study has significance for guiding future preventive therapies for colorectal cancer.