Published online Aug 15, 2014. doi: 10.4251/wjgo.v6.i8.275
Revised: April 8, 2014
Accepted: July 17, 2014
Published online: August 15, 2014
Processing time: 263 Days and 1.5 Hours
Esophageal carcinoma affects more than 450000 people worldwide and the incidence is rapidly increasing. In the United States and Europe, esophageal adenocarcinoma has superseded esophageal squamous cell carcinoma in its incidence. Esophageal cancer has a high mortality rates secondary to the late presentation of most patients at advanced stages. Endoscopic screening is recommended for patients with multiple risk factors for cancer in Barrett’s esophagus. These risk factors include chronic gastroesophageal reflux disease, hiatal hernia, advanced age, male sex, white race, cigarette smoking, and obesity. The annual risk of esophageal cancer is approximately 0.25% for patients without dysplasia and 6% for patients with high-grade dysplasia. Twenty percent of all esophageal adenocarcinoma in the United States is early stage with disease confined to the mucosa or submucosa. The significant morbidity and mortality of esophagectomy make endoscopic treatment an attractive option. The American Gastroenterological Association recommends endoscopic eradication therapy for patients with high-grade dysplasia. Endoscopic modalities for treatment of early esophageal adenocarcinoma include endoscopic resection techniques and endoscopic ablative techniques such as radiofrequency ablation, photodynamic therapy and cryoablation. Endoscopic therapy should be precluded to patients with no evidence of lymphovascular invasion. Local tumor recurrence is low after endoscopic therapy and is predicted by poor differentiation of tumor, positive lymph node and submucosal invasion. Surgical resection should be offered to patients with deep submucosal invasion.
Core tip: This review provides an up-to-date summary of the recent published studies on the use of endoscopic diagnosis and endoluminal management in patients with early esophageal adenocarcinoma, including endoscopic mucosal resection and local ablative techniques. Moreover, the review highlights the significance of this disease and the rising incidence of adenocarcinoma in the United States and western world.
- Citation: Hammoud GM, Hammad H, Ibdah JA. Endoscopic assessment and management of early esophageal adenocarcinoma. World J Gastrointest Oncol 2014; 6(8): 275-288
- URL: https://www.wjgnet.com/1948-5204/full/v6/i8/275.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v6.i8.275
The incidence of esophageal cancer has been increasing steadily in the United States and the western world, with a remarkable 7-fold increase in incidence in the last 30 years[1]. In fact, it has been the most rapidly increasing cancer in white male population[2]. Unfortunately, the overall 5-year survival for early esophageal adenocarcinoma (EAC) has not improved and remains lower than 15%[3].
According to the National Cancer Institute (NCI), it is estimated that 17990 new cases of esophageal cancer will be diagnosed in the United States in 2013, of which approximately 60% will be adenocarcinomas[4].
The other type of esophageal cancer, esophageal squamous cell cancer continues to be the predominant type of esophageal cancer worldwide, but its incidence has been decreasing in the western countries[5]. Although genetic factors play a role in the pathogenesis of esophageal adenocarcinoma[6]. The recent dramatic increase in the incidence of esophageal adenocarcinoma is likely related to increased prevalence of gastroesophageal reflux disease (GERD)[7], increased obesity[8,9] and Helicobacter pylori eradication[10,11].
Reflux injury to the lower esophagus resulting in Barrett’s esophagus (BE) seems to be the main precursor for EAC. This usually begins with inflammation (esophagitis), which could result after a period of time into intestinal metaplasia (BE) with increased risk to progress to dysplasia and eventually EAC[12]. In addition to acid reflux, bile acid reflux may also play an important role in the progression from Barrett esophagus to esophageal adenocarcinoma. Bile acids are synthesized from cholesterol and down-regulate caveolin-1 in esophageal epithelial cells through sterol responsive element-binding protein[13]. Caveolin-1 protects squamous epithelial cells. Moreover, bile acids increase reactive oxygen species production and cell proliferation via activation of PI-PLCgamma2, ERK2 MAP kinase, and NADPH oxidase NOX5-S, thereby contributing to the development of esophageal adenocarcinoma[14].
BE is two to three times more common in men than in women, and is more common in Caucasians. It is less common in African American and is extremely uncommon in Asians[15]. The risk of progression to adenocarcinoma in nondysplastic BE appears to be small. A recent population based study from the Denmark that followed 11028 patients with BE for a median of 5 years reported an annual risk of EAC of 0.12%[16].
The risk of progression to cancer increases in the presence of dysplasia and is up to 6% in patients with high grade dysplasia (HGD)[17].
Risk factors for progression of BE into cancer include low grade dysplasia (LGD), abnormal DNA ploidy and certain lectin binding patterns. Other biomarkers for progression include aberrant DNA methylation changes, expression of microRNAs, as well as overexpression or loss of expression of p53[18].
Endoscopic therapy with curative intent can only be undertaken when the risk of lymph node metastasis is negligible. It is estimated that the rate of lymph node spread is 0% in case of HGD and 1%-2% in case of intramucosal cancers (IMCs). The rate increases to 22% in case of submucosal invasion[19,20].
The diagnosis of BE is usually suspected on forward viewing upper endoscopy and is confirmed with histologic examination. Careful examination by high-resolution forward-viewing white-light endoscopy is recommended[21,22]. In a study by Gupta et al[23] post hoc analysis of an enriched study population and experienced endoscopists at tertiary referral centers. The authors showed that Longer time spent inspecting the BE segment (BIT) is associated with increased detection of HGD/EAC. Endoscopists who had an average BIT > 1 min per centimeter of BE detected more endoscopically suspicious lesions. Multiple random biopsies should be obtained from the four quadrants every 2 cm in non-dysplastic BE segments and every 1 cm if there is suspicion or history of dysplasia (Seattle protocol). Any visible nodule or lesion is usually suspicious for dysplasia or malignancy and should be sampled separately. Accurate description of the location, size and endoscopic appearance of the lesion is necessary for planning future therapy. Endoscopic description of lesions is usually done using the Paris classification of superficial neoplastic lesions (Table 1), which can help predict submucosal invasion in the digestive tract[24].
| Type | Lesion |
| 0-I | Protruding/polypoid |
| 0-Ip | Pedunculated |
| 0-Is | Sessile |
| 0-II | Non-protruding/non-excavated |
| 0-IIa | Slightly elevated |
| 0-IIb | Flat |
| 0-IIc | Slightly depressed |
| 0-III | Excavated |
When confirmed histologically, the current standard of care for BE surveillance involves careful inspection using high resolution white light endoscopy with random biopsies of the BE segment according to the Seattle protocol and targeted biopsies of any suspicious areas. Multiple studies have shown that the random biopsy protocol has low sensitivity for the detection of early neoplastic changes in BE and has low adherence among endoscopists (50%)[25,26]. Furthermore, a cost-utility analysis by Gordon et al[27] concluded that the endoscopic surveillance of patients with non-dysplastic BE is unlikely to be cost-effective for the majority of patients and depends heavily on progression rates between dysplasia grades unless new technologies improve the quality adjusted survival benefit from the surveillance[27].
Resorting to a “random” biopsy protocol reflects the difficulty to recognize early neoplastic changes in BE. One of the reasons for this is the fact that flat lesions (such as Paris 0-IIa and 0-IIb lesions, Table 1) are by far the most frequent macroscopic type of neoplastic lesion in BE, and these lesions are typically hard to detect using the standard while light endoscopy[28].
Therefore, there has been major development in image enhancement techniques to improve the detection of early neoplastic lesions in BE. These techniques include detection techniques “red flag” that cover a wide area and help detect a suspicious lesion, and characterization techniques that provide detailed information about a specific area.
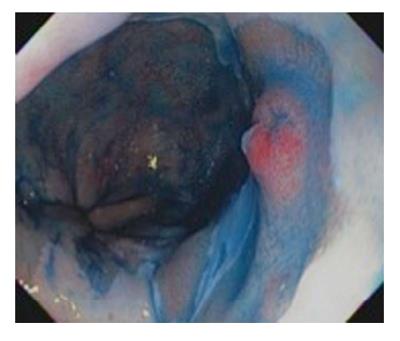
Dye-based Chromoendoscopy consists of spraying the Barrett’s mucosa with a dye to better evaluate the microarchitecture of the mucosa to detect early neoplastic changes. Methylene blue was used in the past for this purpose[29-31]; however, it had largely fallen out of favor due to many reasons including difficulty of use and concerns on mutagenesis[32,33]. Indigo carmine is a contrast stain that permeates into the mucosal surface pits and crevices which helps to accentuate any mucosal irregularities[34] (Figure 1). Since it is not absorbed by cells, it does not have safety concerns like methylene blue. A study of 80 patients with suspected BE using high magnification chromoendoscopy with indigo carmine. The yield of intestinal metaplasia (IM) on target biopsies was 97% and 100% for HGD. However, it was not able to distinguish LGD from non-dysplastic intestinal metaplasia[35].
Acetic acid has also been used and provides magnified aspect of the mucosal architecture to help differentiate neoplastic tissue[36]. Curvers et al[37] demonstrated that the addition of indigo carmine and acetic acid didn’t actually improve the diagnostic yield for early neoplastic lesions in BE compared to high resolution white light endoscopy. Dye-based chromoendoscopy can be labor intensive and is operator dependent and may prolong the procedure. Moreover, it has not been shown to consistently improve the detection of early neoplasia in BE.
This includes narrow band imaging (NBI) which uses optical filter to limit the white light illumination to narrow bands of light wavelengths (blue and green), which is predominantly absorbed by hemoglobin and can highlight the capillary network. This results in enhancement of the mucosal vascular and pit patterns and allows visualization of any subtle mucosal irregularities and alteration in vascular patterns concerning for early neoplastic changes[38]. Using pooled data from five studies, Curvers et al[39] showed promising results with NBI for detection of early neoplasia in BE with sensitivity of 97%, specificity of 94% and overall diagnostic accuracy of 96%. However, other studies showed a much lower accuracy (71%)[40].
Other virtual chromoendoscopy techniques include Pentax i-Scan and Fujinon intelligent color enhancement. These techniques use post-acquisition image computer reconstruction to enhance mucosal and vascular patterns.
At this time, there is little evidence that chromoendoscopy techniques (both dye-based and dye-less) provide improvements in the characterization and detection of early neoplasia in BE.
This technique uses fluorescence radiation following excitation of tissue using light of short wavelengths, which allows differentiation of neoplastic and normal tissue. Autofluorescence imaging (AFI) has been shown to significantly improve the detection of neoplasia in BE; however, the false positive rate is very high (up to 80%)[41]. AFI has also been studied in combination with high resolution endoscopy and NBI, so called Endoscopic Trimodal Imaging (ETMI). In a multicenter randomized trial, ETMI improved the targeted detection of early neoplastic lesions compared to standard video endoscopy. However, the overall histologic yield was not different[42].
Optical coherence tomography produces high quality cross-sectional images of the mucosa based on measuring the rate of backscattering of near-infrared light. This is usually achieved using a probe that is passed through the operative channel of the endoscope. Evans et al[43] developed a scoring system for optical coherence tomography (OCT) and reported a sensitivity of 83% and specificity of 75% in the detection of early neoplasia in BE.
Volumetric laser endomicroscopy, the second generation from of OCT, was shown to image the esophageal mucosa at a higher speed and obtain a better quality images[44]. The recent improvements in OCT technology make it a promising technique that can achieve the goal of wide field scanning (detection) as well as characterization of a specific area of concern.
Endoscopic ultrasound (EUS) may play a little role in the evaluation of patients with HGD or early adenocarcinoma and is not routinely recommended for evaluation of flat BE segments with HGD[45,46]. A systematic review by Young et al[47] showed that the diagnostic accuracy for EUS staging in early EAC was only 65%. A subsequent larger meta-analysis showed better accuracy for EUS in staging T1a and T1b lesions with the area under a receiver operating characteristic curve ≥ 0.93[48]. The use of high-frequency ultrasound catheter probe (miniprobe) can provide a significant better T staging than conventional radial EUS; however, the accuracy is low with both techniques (64% and 49% respectively)[49]. Nevertheless, the National Cancer Comprehensive Network recommends EUS staging prior to proceeding with mucosal resection in the setting of esophageal carcinoma.
This is an imaging technique that obtains real-time 1000-fold magnified view of the mucosa, and provides histological information of the target areas (so called virtual histology). Confocal laser endomicroscopy (CLE) could be performed using a dedicated CLE endoscope or miniprobes that can be used with regular large working channel endoscopes (probe-based CLE). A recent study showed that a combination of CLE and while light endoscopy increased the sensitivity for detection of early neoplastic changes compared to white light endoscopy (76% vs 34%)[50]. Disadvantages to this technique include that it is expensive, time consuming and requires intensive training.
This technique relies on the principle of light interaction with esophageal mucosa to generate a biochemical profile that reflects the cellular architecture. Early results appear to be promising for the real-time detection and diagnosis of esophageal adenocarcinoma with an accuracy of 96%[51]. More recently, Almond et al[52] used a novel probe-based endoscopic Raman spectroscopy in ex vivo esophageal tissue samples and showed sensitivity of 86% and specificity of 88% for detecting early neoplasia in BE.
The above mentioned enhanced imaging techniques are not widely used in clinical practice due to the limited diagnostic accuracy, high inter-observer variability and high cost. It is also unlikely that these techniques will replace standard high resolution white light endoscopy and random biopsies for surveillance in BE; however, they could play an important role in further characterization and grading of suspicious lesions detected during surveillance exams.
Neoplastic changes in BE can be classified as LGD, HGD, in situ (or intraepithelial) carcinoma, IMC and invasive carcinoma[53].
Mucosal lesions are further divided into M1 lesions (or in situ carcinoma) when the lesion is limited to the epithelial layer, M2 lesions when the lesion invades the lamina propria and M3 when the lesion invades into but not through the muscularis mucosa layer. Lesions that invade into the submucosal are labeled SM lesions. SM lesion can be further divided into SM1 lesions when the lesion invades into the upper one third of the submucosal, SM2 lesions when the lesion invades the middle third and SM3 lesions when the lesion invades the deep one third of the submucosal layer[54].
Pathologists should carefully evaluate biopsy or resection specimens of esophageal neoplasms to provide details about tumor depth of invasion, tumor differentiation (well, moderate and poorly differentiated), lymphovascular invasion and the presence of tumor invasion at the resection margin. Lymphovascular invasion and poorly differentiated histology increases the risk of lymph node metastasis and these patients should ideally be referred for surgical resection[55].
HGD is characterized by marked cytological atypia and distorted architecture. Architectural distortion changes include marked crypt crowding, crypt budding and branching. Cytologically, HGD shows cells with marked nuclear pleomorphism, increased N/C ratio, and an increased number of atypical mitoses, particularly in the upper levels of the crypts. Goblet and Paneth cells are usually scarce or absent. Adenomatous (intestinal) dysplasia is the most common subtype but non-adenomatous (foveolar) dysplasia has also been described[56].
Immunohistochemistry staining could help in the diagnosis of HGD. Promising markers include p53 and α-methylacyl coenzyme A racemase but these are not widely used yet[57,58]. Given the significant intraobserver and interobserver variability in the diagnosis of LGD and HGD in BE, most gastrointestinal (GI) societies recommend that a second experienced gastrointestinal pathologist confirm the diagnosis[59]. It is noteworthy that the Japanese and some European pathologists don’t use the term HGD and prefer to use the term in situ carcinoma for these lesions[60].
IMC invades through the basement membrane to the lamina propria and the muscularis mucosa. It is characterized by atypical cells or complex glands invading into the lamina propria. It is extremely important to differentiate between IMC (or T1a lesion) and carcinoma invading into the submucosa (T1b) as the distinction carries significant implications for the risk of lymph node metastasis and therapy. Such distinction is often difficult to make on biopsy specimens and larger resection specimens such as that resulting from endoscopic mucosal resection (EMR) are more helpful to distinguish between T1a and T1b lesions. In one study, 45% of patients had their final pathological stage changed after EMR compared to pre-EMR forceps biopsies[61]. It also known that most BE usually has double muscularis mucosa layer but this has no impact on the classification or the treatment of Barrett’s adenocarcinoma[62].
Several modalities have been used to stage esophageal adenocarcinoma. These include EUS, endoscopic mucosal resection with histological assessment and computed tomography/positron emission tomography (CT/PET). EUS and EMR are currently applied as staging tools for early esophageal adenocarcinoma. Early cancer is defined as T1sm1, as beyond this point metastases increases from 1% to 10% for T1sm2 based on a recent consensus[63]. Stage T1a malignancies include lesions confined to the mucosa: M1 (intraepithelial), M2 (lamina propria invasion), or M3 (muscularis mucosa invasion). Submucosal or T1b malignancies are classified into Sm1 (superficial submucosa invasion), Sm2 (invasion to center of submucosa), or Sm3 (invasion to deep submucosa). Mucosal (T1a) malignancies have extremely low risk of local lymph node progression while submucosal invasion (T1b) markedly increases the risk of lymph node metastases[64,65].
The clinical utility of EUS for staging patients with BE and high-grade dysplasia or intramucosal carcinoma prior to endoscopic therapy has a limited accuracy. The principal role of EUS in evaluating patients with Barrett’s-associated dysplasia is to identify patients who may be candidates for endoscopic ablative therapy such as endoscopic mucosal resection and/or photodynamic therapy. EUS has been shown to be superior to computed tomography or magnetic resonance imaging for preoperative staging in patients with high-grade dysplasia and carcinoma. EUS is considered the best tool for T and N staging of esophageal cancer, however, its performance in early Barrett’s neoplasia is suboptimal for tumor depth assessment. In a meta-analysis by Puli et al[66] the pooled sensitivity of EUS in T1 disease was (88.1%), T2 (82.3%), T3 (89.7%) and T4 (99.2%). EUS can identify nodal spread (N1) or deep tumor invasion (T3) for which it precludes surgical resection. The risk of nodal involvement in early esophageal cancer confined to the mucosa (T1a) ranges between 0% and 3%, and when the lesion extends into the submucosal layer (T1b) this risk approaches up to 30%-50%[67-69]. Tumor size (OR = 1.35 per centimeter, 95%CI: 1.07-1.71) and lymphovascular invasion (OR = 7.50, 95%CI: 3.30-17.07) were the strongest independent predictors of lymph node metastasis[70]. In a retrospective analysis of 135 with HGD (79%) or IMC (21%) who had staging by EUS. Pathologic lymph nodes or metastases were not found by EUS. There were no endosonographic abnormalities noted in any patient with non-nodular mucosa (0/79). However, abnormal EUS findings were present in 14% with nodular neoplasia (five IMC, three HGD)[71]. For patients with nodular neoplasia, endoscopic mucosal resection of the nodule with histological examination had greater utility than staging by EUS. The use of high frequency ultrasound catheter probe (HFP) have been studied in two large studies included 94 and 106 subjects[72,73]. Both studies revealed that HFP is significantly better for lesions localized in the tubular esophagus than the gastroesophageal junction. Moreover, the performance of HFP in assessing submucosal involvement is poor. At this time EUS and HFP staging technique is inadequate for predicting T1-2N0 disease in esophageal adenocarcinoma[74].
Endoscopic mucosa resection (EMR) has taken a central role in the staging and treatment modality for patients with early esophageal adenocarcinoma, as it allows the pathologist to provide tumor-staging information necessary for an appropriate clinical management decision process. In fact, it is the most accurate staging procedure to assess depth of invasion if full submucosa is provided in the specimen. By providing full thickness of the resected submucosa, pathologists are able to provide a clear histologic depth of the tumor (T staging) and evaluate for lymphovascular invasion. EMR provides better staging for visible lesions than do biopsies alone. Moreover, endoscopic mucosal resection may result in changing the histologic diagnosis in patients with BE with visible and flat neoplasia. In a multicenter study which evaluated 138 patients with BE-related neoplasia who undergone endoscopic eradication therapy showed EMR resulted in a change of the histologic diagnosis in 31.1% patients (upgrades 10.1%; downgrade 21%) with or without visible lesions[75]. At this time, EMR appears to be superior to biopsy for diagnosing and staging superficial esophageal tumors and can substantially modify the diagnostic grade of a lesion. Therefore EMR may facilitate optimal therapeutic decisions by avoiding undertreatment and overtreatment based on inaccurate grading and staging[76].
Early use of PET in the staging of patients with esophageal cancer could facilitate treatment planning and identifying unsuspected distant metastases in up to 20% of patients with a negative metastatic survey by conventional staging[77]. Positron emission tomography detects more distant lymph node and organ metastases compared with conventional diagnostics, allowing a more accurate selection of the most appropriate treatment. CT/PET has inadequate assessment in the superficial esophageal adenocarcinoma. Moreover, the addition of PET to a complete EUS examination did not alter regional-node or celiac-node staging in patients with esophageal cancer[78]. SUVmax ratio was only associated with tumor invasion depth on CT/PET. A recent study evaluated the use of CT/PET in early esophageal adenocarcinoma using a cut-off of 1.48, the sensitivity and specificity of SUVmax ratio for identification of T1a lesions were 43.3% and 80.9%, respectively[79]. Thus more data is needed on the role of CT/PET in early EAC.
The management of patients with early esophageal cancer considered for treatment should take place in a specialty multidisciplinary team including GI pathologist, esophageal surgeon, therapeutic endoscopist, radiologist and oncologist. The endoscopic treatment should commence in high volume tertiary referral centers with availability and expertise in the multiple modalities of endoscopic therapy of BE. Moreover, the center must possess expertise in the management of complications of each modality. The British Society of Gastroenterology recommended a minimum of 30 supervised cases of endoscopic resection and 30 cases of endoscopic ablation should be performed to acquire competence in technical skills, management pathways and complications. Patients with EAC should be informed about the benefits, risks and alternatives of endoscopic and surgical approach. Initially, endoscopic mapping of the Barrett’s segment with intestinal metaplasia should be undertaken prior to any endoscopic therapy. The American Gastroenterological Association (AGA) recommends endoscopic eradication therapy for patients with high-grade dysplasia. Risk stratification based on histopathologic assessment should be performed and any nodularity seen on white-light forward viewing upper endoscopy should undergo resection prior to any local ablative therapy (Figure 1). Lymph node metastasis should be excluded. Endoscopic therapy appears to be a good alternative to esophagectomy for patients with low risk pT1b sm1 EAC, on the basis of macroscopic and histologic analyses[55,80]. Data obtained from the Surveillance Epidemiology and End Results database of the NCI to compare cancer-free survival in patients with early esophageal cancer who were either treated with endoscopic therapy (n = 99) or surgical resection (n = 643) did not reveal a difference in esophageal cancer-specific mortality between the two groups[81]. In a population-based analysis, the use of endoscopic therapy for superficial EAC tended to increase from 1998-2009 and the long-term survival of patients with EAC did not appear to differ between those who received endoscopic therapy and those treated with surgery[82]. Several curative modalities are available for local treatment of BE with HGD. Among these modalities are radiofrequency ablation, argon plasma coagulation, thermal laser therapy, cryotherapy and photodynamic treatment. Here we review the efficacy and risks of each modality. Long term outcome of patients with BE and HGD who underwent endoluminal therapy revealed recurrence of intestinal metaplasia occurs in one-third of cases and supports continued endoscopic surveillance even after complete eradication[83].
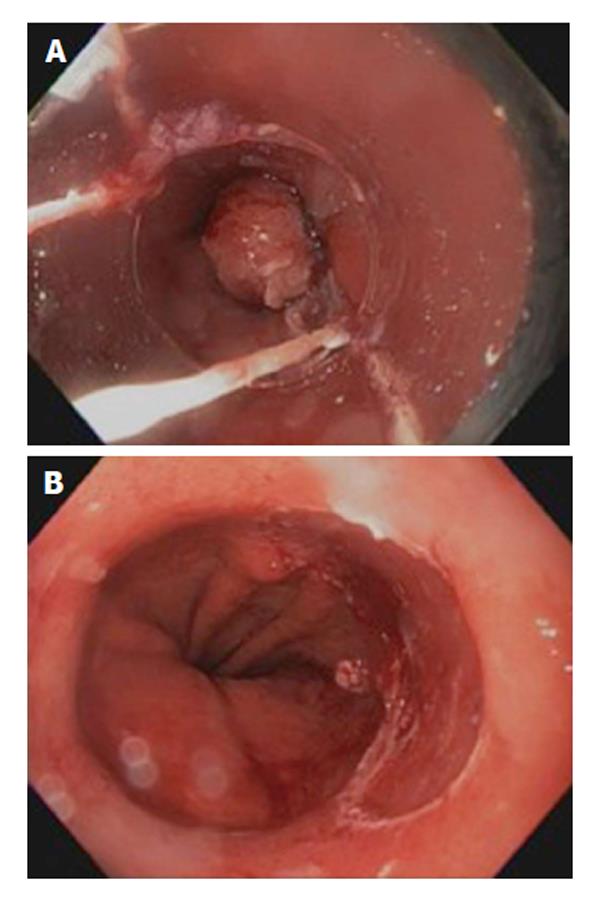
Endoscopic mucosal resection provided a primary role in the endoscopic therapy of patients with early EAC (HGD, T1a). EMR should not be attempted if lymph node invasion is suspected. EMR should be performed by an expert therapeutic endoscopist. The principle of EMR is to capture the entire mucosa and submucosa using a suction cup fitted on the tip of the endoscope (Cap-assisted suck and cut or band and cut technique) or lifting the submucosa from the muscularis propria through submucosal injection of saline or indigo carmine (freehand technique). The entire specimen is then excised en bloc using a diathermy snare resection or performing multiband mucosectomy[84] (Figures 2 and 3). Total en bloc resection is preferred to reduce risk of recurrence and provide accurate histologic assessment. The distinct advantage of EMR over ablative therapy is providing large specimen of resected tissue for histopathologic assessment. One must understand the limitations of EMR include the assessment of base and lateral margin of the tumor resected specimen. The depth of infiltration is better assessed using quantitative micrometric measure in microns of the depth of submucosal invasion from the bottom of muscularis mucosae. This is deemed to be more accurate than classifying tumor invasion based on depth of submucosal involvement (sm1, sm2, and sm3) as the entire submucosa may not be available in the specimen of all cases[85]. EMR can also be performed in patients with early esophageal adenocarcinoma with previous antireflux surgery[86]. Risk of recurrence after EMR appears low. In one study evaluating 22 patients (16 with HGD), 82% had no evidence of HGD or cancer after a median follow-up of two years[87]. Another long-term follow up study carried in 7 patients for more than 10 years, in 43 for 5-10 years, in 31 for 3-5 years and in 66 for less than 3 years after endoscopic resection. Of the 11 patients who died during the follow up, 10 died of other diseases, only 1 of recurrence of tumor. The 5-year survival rate was 96.2% for early-stage esophageal cancer[88]. Risks of EMR include bleeding, perforation and stricture formation which can occur in up to 37% of cases[61].
Endoscopic submucosal dissection is an advanced endoscopic procedure to resect early gastrointestinal neoplasms. It is technically more difficult, carries a high risk when used to treat early esophageal tumors and currently is not widely available in the United States. Studies have been published and reported its efficacy and safety in patients with early EAC[86,89]. In a phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms to assess the efficacy and safety of endoscopic submucosal dissection (ESD) in 56 lesions, the en bloc resection rate and R0 resection rate were 100% and 94.6%, respectively. The median treatment time for completing the procedure was 69 min (24-168 min)[90]. The rates of adverse events during and after ESD were 22.2% and 53.8%, respectively, but most events were mild. Another study evaluated ESD in combination with radiofrequency ablation in 30 patients with biopsy-proven mucosal adenocarcinoma. Endoscopic follow-up (median 17 mo) showed complete remission of neoplasia in 27/28 (96.4%) patients who underwent successful ESD using waterjet-assisted system[90]. A Meta-analysis by Cao et al[91] of endoscopic submucosal dissection vs endoscopic mucosal resection for tumors of the gastrointestinal tract showed higher en bloc and curative resection rates (OR = 13.87, 95%CI: 10.12-18.99; OR = 3.53, 95%CI: 2.57-4.84) irrespective of lesion size. Subgroup analysis showed higher en bloc and curative resection rates with ESD for esophageal, gastric, and colorectal neoplasms, and for lesions of size < 10 mm, 10 mm < 20 mm, and > 20 mm and lower local recurrence. However, ESD was more time-consuming than EMR and showed high procedure-related bleeding and perforation rates (OR = 2.20, 95%CI: 1.58-3.07; OR = 4.09, 95%CI: 2.47-6.80). Similarly, in a previous study evaluating the role of ESD in comparison to EMR in 171 lesions ≤ 20 mm of esophageal cancer (168 were squamous-cell carcinoma and 3 were adenocarcinoma), the curative resection rate of ESD was 97% significantly higher than endoscopic mucosal resection cap-assisted (87%)[92]. However, EMR would be an alternative to lesions < 15 mm in diameter. One must note that ESD in the esophagus has been associated with perforation rates of 2% to 5% and stricture rates between 5% and 17.2%[90,93]. More data is needed to evaluate the utility of ESD for early esophageal adenocarcinoma in the United Stated.
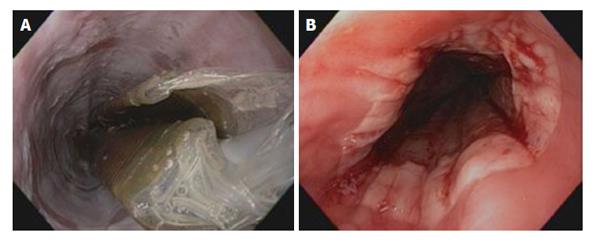
Radiofrequency ablation of BE with HGD is the most commonly used therapy, which has been shown to produce reproducible superficial injury in the esophagus (Figure 4). Its ease of use and better safety profile makes it a favorable therapy for flat lesions with HGD. The system generator is capable of delivering 10 to 12 J at a setting of 40 W/cm² with a depth of ablation between 500 and 1000 mm. Two delivery systems are currently available in use. A 3-cm-long balloon ablation catheter (HALO 360) intended to treat long-segment circumferential BE, and an endoscope-mounted targeted device (HALO 90) to treat short segments and tongues of BE. In a recent large series of 335 patients with BE and neoplasia (72% with HGD, 24% with IMC, 4% with low-grade dysplasia) in the United Kingdom who underwent RFA for BE-related neoplasia. The authors found that by 12 mo after treatment, dysplasia was cleared from 81% of patients. Shorter segments of BE respond better to radiofrequency ablation (RFA)[94]. In another study of 70 patients who were treated. Seventy-four per cent had dysplasia (44 LGD, 8 HGD). Complete response was accomplished in 81% of patients[95]. A United Kingdom registry that follows the outcomes of 335 patients with BE who have undergone RFA for neoplasia and received endoscopic mucosal resection if nodules are found revealed HGD was cleared from 86% of patients, all dysplasia from 81%, and BE from 62% at the 12-mo time point, after a mean of 2.5 (range, 2-6) RFA procedures[94]. Of interest, endoscopic mucosal resection before RFA did not provide any benefit. Moreover, RFA appears to have a higher rate of complete histologic resolution response in comparison to photodynamic therapy (PDT) without any serious adverse events and was less costly than PDT for endoscopic treatment of Barrett’s dysplasia[96]. Complications of RFA include chest and cervical pain, abdominal pain, dysphagia and stricture formation. Subsquamous neoplasia have been reported to develop after RFA for BE[97]. Currently, RFA is reserved for patients with BE with high-grade dysplasia with no visualized nodules. Its application for patients without dysplasia is debatable giving risks of complications and cost[98].
Photodynamic therapy has been used to photochemically eliminate abnormal mucosa. Porfimer sodium (POR) PDT use has been limited by serious side effects including prolonged cutaneous photosensitivity and stricture formation. In a randomized phase III trial using POR and photodynamic therapy for ablating HGD in conjunction with omeprazole, POR PDT appears to be an effective therapy for ablating HGD in patients with BE and in reducing the incidence of esophageal adenocarcinoma[99]. PDT is associated with increased risks of stricture formation and of buried intestinal metaplasia or malignancy underneath neosquamous epithelium. In a study by Weiss et al[100] on 17 patients treated with PDT. High-grade dysplasia or early adenocarcinoma was completely eliminated in nine of 60% patients. Complications included stricture, sunburn, urticaria, small pleural effusions, esophageal spasm and transient atrial fibrillation. A recent randomized controlled trial of 5-Aminolaevulinic acid (ALA) vs Photofrin photodynamic therapy for high-grade dysplasia arising in BE showed no difference in complete reversal of HGD between the two groups. On sub-group analysis for BE ≤ 6 cm, complete reversal of HGD was significantly higher with ALA-PDT than Photofrin-PDT. Strictures and skin photosensitivity were significantly more common after treatment with Photofrin-PDT than ALA-PDT (33% vs 9% and 43% vs 6%, respectively, P < 0.05)[101].
Argon plasma coagulation is a noncontact thermal tissue coagulation in which argon gas provides the medium for the delivery of an electric current[102]. This is accomplished with passing a probe through the working channel of the endoscope. The general setting for ablation of Barrett’s mucosa is a high power setting 60-90 W at 1-2 L/min. Earlier study showed complete eradication of HGD and in situ adenocarcinoma was achieved after a mean number of 3.3+/-1.5 V. Argon plasma coagulation (APC) sessions in (80%)[103]. In a randomized controlled trial of 35 patients who received ablation of BE with multipolar electrocoagulation (16) vs argon plasma coagulation (19), the authors concluded complete reversal of BE can be maintained in approximately 70% of patients, irrespective of the technique[104]. Similarly, previous studies showed similar outcome with eradication of BE and restoration of squamous epithelium[105]. However, progression to HGD can still occur despite APC ablation[106]. Thus APC is effective ablative therapy for BE but the long term benefits are unknown. More data is needed on its use in early EAC.
Cryoablation is a relatively new technique with studies focusing on high-grade dysplasia and early-stage cancer in high-risk patients. It has an acceptable safety profile, and early results show response in a significant number of patients in whom other modalities have failed[107]. Its ease of use and lower chance of complication make it an attractive procedure. Although cryoablation is a non-tissue acquiring procedure that requires liquid nitrogen spray application it is not devoid of potential risk of gastric perforation due to gas insufflation. Data on its use in early EAC is limited. In a multicenter, retrospective cohort study of 79 patients with esophageal carcinoma in whom conventional therapy failed, refused and/or were ineligible for conventional therapy[108]. The study included all T staging and showed complete response of intraluminal disease in 31 of 49 subjects (61.2%), including 18 of 24 (75%) with mucosal cancer with an overall follow up of 10.6 months. No serious adverse events were reported. A recent study by Gosain et al[109] evaluated 32 patients with BE-HGD of any length who were treated with liquid nitrogen spray cryotherapy every 8 wk until complete eradication of HGD and intestinal metaplasia. Complete eradication of HGD achieved in 100% (32/32), and IM in 84% at 2-year follow-up. Recurrent HGD occurred in 18% with HGD. BE segment length ≥ 3 cm was associated with a higher recurrence of IM but not HGD. No serious adverse events occurred although stricture was seen in 9% of cases. Thus, cryoablation therapy appears comparable to other treating modality in BE and in early EAC, spray cryotherapy appears to have a unique role, eliminating mucosal cancer in 75% of patients[110].
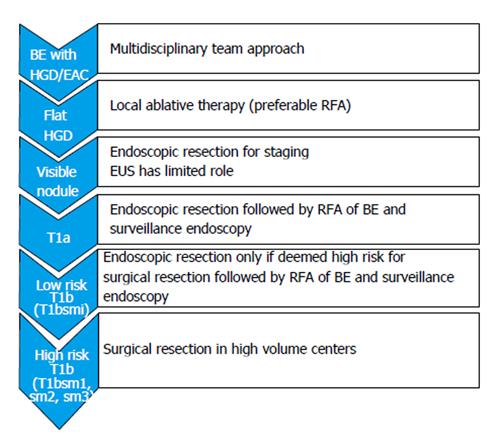
A recent meta-analysis of seven studies involving 870 patients who underwent endotherapy (n = 510) or surgery (n = 360) concluded that endotherapy has similar efficacy to surgery but with lower adverse event rates. However, endotherapy was associated with a higher neoplasia recurrence rate[111]. Limitation to this study included small number of retrospective studies and different types of endoscopic treatments used. Figure 5 shows the current practical approach to the management of patients with early EAC.
Esophageal adenocarcinoma is characterized by increasing incidence, male predominance and lack of preventive measures. Future preventive therapy might include the treatment of gastroesophageal acid reflux, obesity and/or chemoprevention with nonsteroidal antiinflammatory (NSAIDs) drugs or statins. Today, there is no evidence-based preventive measures are currently available for patients with EAC. Proton pump inhibitors are effective in reducing esophageal acid exposure and improve reflux symptoms however, they are not recommended for use as chemopreventive agents in EAC. Weight loss, exercise and bariatric surgery may potentially improve obesity. Studies have shown up-regulation of cyclooxygenase (COX)-2 in BE-metaplastic and dysplastic tissue and in Barrett’s adenocarcinoma[112-114]. Others showed conflicting results[115]. NSAIDs and COX inhibitors have been proposed and shown to reduce risk of metaplasia in BE and EAC[116]. Statins have been suggested to induce anticancer effects against a variety of cancers in several studies[117]. Agents targeting the vascular endothelial growth factor and epidermal growth factor receptor pathways are currently in progress. The AGA recommendation for the chemoprevention of cancer in patients with BE is screening patients to identify cardiovascular risk factors for which aspirin therapy is indicated and against the use of aspirin solely to prevent esophageal adenocarcinoma in the absence of other indications[22].
Esophageal cancer is one of the most serious gastrointestinal cancers worldwide, owing to its rapid development and fatal prognoses in most cases. Major risk factors for EAC include BE, GERD, smoking, and obesity. Improved survival is achievable when the disease is confined to the more superficial mucosal layers and treated. Endoscopic luminal therapy is feasible and proven useful in BE with HGD and early esophageal adenocarcinoma.
P- Reviewer: Bustamante-Balen M, Lisotti A S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Edgren G, Adami HO, Weiderpass E, Nyrén O. A global assessment of the oesophageal adenocarcinoma epidemic. Gut. 2013;62:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, Feuer EJ. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 3. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11834] [Article Influence: 845.3] [Reference Citation Analysis (4)] |
| 4. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 5. | Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br J Cancer. 2009;101:855-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 6. | Orloff M, Peterson C, He X, Ganapathi S, Heald B, Yang YR, Bebek G, Romigh T, Song JH, Wu W. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA. 2011;306:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther. 2010;32:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Hoyo C, Cook MB, Kamangar F, Freedman ND, Whiteman DC, Bernstein L, Brown LM, Risch HA, Ye W, Sharp L. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41:1706-1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 9. | Alemán JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146:357-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Nyrén O, Blot WJ. Helicobacter pylori infection: mainly foe but also friend? J Natl Cancer Inst. 2006;98:1432-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Xie FJ, Zhang YP, Zheng QQ, Jin HC, Wang FL, Chen M, Shao L, Zou DH, Yu XM, Mao WM. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol. 2013;19:6098-6107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Sharma P. Clinical practice. Barrett’s esophagus. N Engl J Med. 2009;361:2548-2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Prade E, Tobiasch M, Hitkova I, Schäffer I, Lian F, Xing X, Tänzer M, Rauser S, Walch A, Feith M. Bile acids down-regulate caveolin-1 in esophageal epithelial cells through sterol responsive element-binding protein. Mol Endocrinol. 2012;26:819-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Hong J, Behar J, Wands J, Resnick M, Wang LJ, Delellis RA, Lambeth D, Cao W. Bile acid reflux contributes to development of esophageal adenocarcinoma via activation of phosphatidylinositol-specific phospholipase Cgamma2 and NADPH oxidase NOX5-S. Cancer Res. 2010;70:1247-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Wang A, Mattek NC, Holub JL, Lieberman DA, Eisen GM. Prevalence of complicated gastroesophageal reflux disease and Barrett‘s esophagus among racial groups in a multi-center consortium. Dig Dis Sci. 2009;54:964-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 979] [Article Influence: 69.9] [Reference Citation Analysis (1)] |
| 17. | Rastogi A, Puli S, El-Serag HB, Bansal A, Wani S, Sharma P. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc. 2008;67:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 291] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 18. | Bird-Lieberman EL, Dunn JM, Coleman HG, Lao-Sirieix P, Oukrif D, Moore CE, Varghese S, Johnston BT, Arthur K, McManus DT. Population-based study reveals new risk-stratification biomarker panel for Barrett’s esophagus. Gastroenterology. 2012;143:927-35.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol. 2012;107:850-862; quiz 863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 20. | Leers JM, DeMeester SR, Oezcelik A, Klipfel N, Ayazi S, Abate E, Zehetner J, Lipham JC, Chan L, Hagen JA. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 21. | Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 875] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 22. | Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 382] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 23. | Gupta N, Gaddam S, Wani SB, Bansal A, Rastogi A, Sharma P. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012;76:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 646] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 25. | Abrams JA, Kapel RC, Lindberg GM, Saboorian MH, Genta RM, Neugut AI, Lightdale CJ. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol. 2009;7:736-742; quiz 710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 26. | Kariv R, Plesec TP, Goldblum JR, Bronner M, Oldenburgh M, Rice TW, Falk GW. The Seattle protocol does not more reliably predict the detection of cancer at the time of esophagectomy than a less intensive surveillance protocol. Clin Gastroenterol Hepatol. 2009;7:653-668; quiz 606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Gordon LG, Mayne GC, Hirst NG, Bright T, Whiteman DC, Watson DI. Cost-effectiveness of endoscopic surveillance of non-dysplastic Barrett’s esophagus. Gastrointest Endosc. 2014;79:242-256.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Pech O, Gossner L, Manner H, May A, Rabenstein T, Behrens A, Berres M, Huijsmans J, Vieth M, Stolte M. Prospective evaluation of the macroscopic types and location of early Barrett’s neoplasia in 380 lesions. Endoscopy. 2007;39:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Ragunath K, Krasner N, Raman VS, Haqqani MT, Cheung WY. A randomized, prospective cross-over trial comparing methylene blue-directed biopsy and conventional random biopsy for detecting intestinal metaplasia and dysplasia in Barrett’s esophagus. Endoscopy. 2003;35:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Sharma P, Topalovski M, Mayo MS, Weston AP. Methylene blue chromoendoscopy for detection of short-segment Barrett’s esophagus. Gastrointest Endosc. 2001;54:289-293. [PubMed] |
| 31. | Gossner L, Pech O, May A, Vieth M, Stolte M, Ell C. Comparison of methylene blue-directed biopsies and four-quadrant biopsies in the detection of high-grade intraepithelial neoplasia and early cancer in Barrett’s oesophagus. Dig Liver Dis. 2006;38:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Olliver JR, Wild CP, Sahay P, Dexter S, Hardie LJ. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet. 2003;362:373-374. [PubMed] |
| 33. | Lim CH, Rotimi O, Dexter SP, Axon AT. Randomized crossover study that used methylene blue or random 4-quadrant biopsy for the diagnosis of dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2006;64:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Canto MI, Kalloo A. Chromoendoscopy for Barrett’s esophagus in the twenty-first century: to stain or not to stain? Gastrointest Endosc. 2006;64:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Sharma P, Weston AP, Topalovski M, Cherian R, Bhattacharyya A, Sampliner RE. Magnification chromoendoscopy for the detection of intestinal metaplasia and dysplasia in Barrett’s oesophagus. Gut. 2003;52:24-27. [PubMed] |
| 36. | Guelrud M, Herrera I, Essenfeld H, Castro J. Enhanced magnification endoscopy: a new technique to identify specialized intestinal metaplasia in Barrett’s esophagus. Gastrointest Endosc. 2001;53:559-565. [PubMed] |
| 37. | Curvers W, Baak L, Kiesslich R, Van Oijen A, Rabenstein T, Ragunath K, Rey JF, Scholten P, Seitz U, Ten Kate F. Chromoendoscopy and narrow-band imaging compared with high-resolution magnification endoscopy in Barrett’s esophagus. Gastroenterology. 2008;134:670-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Kara MA, Ennahachi M, Fockens P, ten Kate FJ, Bergman JJ. Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett’s esophagus by using narrow band imaging. Gastrointest Endosc. 2006;64:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 217] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Curvers WL, van den Broek FJ, Reitsma JB, Dekker E, Bergman JJ. Systematic review of narrow-band imaging for the detection and differentiation of abnormalities in the esophagus and stomach (with video). Gastrointest Endosc. 2009;69:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Alvarez Herrero L, Curvers WL, Bansal A, Wani S, Kara M, Schenk E, Schoon EJ, Lynch CR, Rastogi A, Pondugula K. Zooming in on Barrett oesophagus using narrow-band imaging: an international observer agreement study. Eur J Gastroenterol Hepatol. 2009;21:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Curvers WL, Singh R, Song LM, Wolfsen HC, Ragunath K, Wang K, Wallace MB, Fockens P, Bergman JJ. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 42. | Curvers WL, van Vilsteren FG, Baak LC, Böhmer C, Mallant-Hent RC, Naber AH, van Oijen A, Ponsioen CY, Scholten P, Schenk E. Endoscopic trimodal imaging versus standard video endoscopy for detection of early Barrett’s neoplasia: a multicenter, randomized, crossover study in general practice. Gastrointest Endosc. 2011;73:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Evans JA, Poneros JM, Bouma BE, Bressner J, Halpern EF, Shishkov M, Lauwers GY, Mino-Kenudson M, Nishioka NS, Tearney GJ. Optical coherence tomography to identify intramucosal carcinoma and high-grade dysplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:38-43. [PubMed] |
| 44. | Suter MJ, Vakoc BJ, Yachimski PS, Shishkov M, Lauwers GY, Mino-Kenudson M, Bouma BE, Nishioka NS, Tearney GJ. Comprehensive microscopy of the esophagus in human patients with optical frequency domain imaging. Gastrointest Endosc. 2008;68:745-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 45. | Pouw RE, Heldoorn N, Alvarez Herrero L, ten Kate FJ, Visser M, Busch OR, van Berge Henegouwen MI, Krishnadath KK, Weusten BL, Fockens P. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest Endosc. 2011;73:662-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Savoy AD, Wolfsen HC, Raimondo M, Woodward TA, Noh K, Pungpapong S, Hemminger LL, Wallace MB. The role of surveillance endoscopy and endosonography after endoscopic ablation of high-grade dysplasia and carcinoma of the esophagus. Dis Esophagus. 2008;21:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Young PE, Gentry AB, Acosta RD, Greenwald BD, Riddle M. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol. 2010;8:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Thosani N, Singh H, Kapadia A, Ochi N, Lee JH, Ajani J, Swisher SG, Hofstetter WL, Guha S, Bhutani MS. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 49. | Larghi A, Lightdale CJ, Memeo L, Bhagat G, Okpara N, Rotterdam H. EUS followed by EMR for staging of high-grade dysplasia and early cancer in Barrett’s esophagus. Gastrointest Endosc. 2005;62:16-23. [PubMed] |
| 50. | Sharma P, Meining AR, Coron E, Lightdale CJ, Wolfsen HC, Bansal A, Bajbouj M, Galmiche JP, Abrams JA, Rastogi A. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011;74:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 51. | Bergholt MS, Zheng W, Lin K, Ho KY, Teh M, Yeoh KG, So JB, Huang Z. In vivo diagnosis of esophageal cancer using image-guided Raman endoscopy and biomolecular modeling. Technol Cancer Res Treat. 2011;10:103-112. [PubMed] |
| 52. | Almond LM, Hutchings J, Lloyd G, Barr H, Shepherd N, Day J, Stevens O, Sanders S, Wadley M, Stone N. Endoscopic Raman spectroscopy enables objective diagnosis of dysplasia in Barrett’s esophagus. Gastrointest Endosc. 2014;79:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 53. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [PubMed] |
| 54. | Endo M, Yoshino K, Kawano T, Nagai K, Inoue H. Clinicopathologic analysis of lymph node metastasis in surgically resected superficial cancer of the thoracic esophagus. Dis Esophagus. 2000;13:125-129. [PubMed] |
| 55. | Sgourakis G, Gockel I, Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol. 2013;19:1424-1437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 56. | Odze RD. Diagnosis and grading of dysplasia in Barrett’s oesophagus. J Clin Pathol. 2006;59:1029-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 57. | Keswani RN, Noffsinger A, Waxman I, Bissonnette M. Clinical use of p53 in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2006;15:1243-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Scheil-Bertram S, Lorenz D, Ell C, Sheremet E, Fisseler-Eckhoff A. Expression of alpha-methylacyl coenzyme A racemase in the dysplasia carcinoma sequence associated with Barrett’s esophagus. Mod Pathol. 2008;21:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 786] [Article Influence: 46.2] [Reference Citation Analysis (1)] |
| 60. | Takubo K, Vieth M, Aida J, Matsutani T, Hagiwara N, Iwakiri K, Kumagai Y, Hongo M, Hoshihara Y, Arai T. Histopathological diagnosis of adenocarcinoma in Barrett’s esophagus. Dig Endosc. 2014;26:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Chennat J, Konda VJ, Ross AS, de Tejada AH, Noffsinger A, Hart J, Lin S, Ferguson MK, Posner MC, Waxman I. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol. 2009;104:2684-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 62. | Estrella JS, Hofstetter WL, Correa AM, Swisher SG, Ajani JA, Lee JH, Bhutani MS, Abraham SC, Rashid A, Maru DM. Duplicated muscularis mucosae invasion has similar risk of lymph node metastasis and recurrence-free survival as intramucosal esophageal adenocarcinoma. Am J Surg Pathol. 2011;35:1045-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Bennett C, Vakil N, Bergman J, Harrison R, Odze R, Vieth M, Sanders S, Gay L, Pech O, Longcroft-Wheaton G. Consensus statements for management of Barrett’s dysplasia and early-stage esophageal adenocarcinoma, based on a Delphi process. Gastroenterology. 2012;143:336-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 64. | Griffin SM, Burt AD, Jennings NA. Lymph node metastasis in early esophageal adenocarcinoma. Ann Surg. 2011;254:731-736; discussion 736-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Gockel I, Sgourakis G, Lyros O, Polotzek U, Schimanski CC, Lang H, Hoppo T, Jobe BA. Risk of lymph node metastasis in submucosal esophageal cancer: a review of surgically resected patients. Expert Rev Gastroenterol Hepatol. 2011;5:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 66. | Puli SR, Batapati Krishna Reddy J, Bechtold ML, Antillon MR, Ibdah JA. How good is endoscopic ultrasound for TNM staging of gastric cancers? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:4011-4019. [PubMed] |
| 67. | Thomas T, Gilbert D, Kaye PV, Penman I, Aithal GP, Ragunath K. High-resolution endoscopy and endoscopic ultrasound for evaluation of early neoplasia in Barrett’s esophagus. Surg Endosc. 2010;24:1110-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Manner H, May A, Pech O, Gossner L, Rabenstein T, Günter E, Vieth M, Stolte M, Ell C. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol. 2008;103:2589-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 69. | Buskens CJ, Westerterp M, Lagarde SM, Bergman JJ, ten Kate FJ, van Lanschot JJ. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc. 2004;60:703-710. [PubMed] |
| 70. | Lee L, Ronellenfitsch U, Hofstetter WL, Darling G, Gaiser T, Lippert C, Gilbert S, Seely AJ, Mulder DS, Ferri LE. Predicting lymph node metastases in early esophageal adenocarcinoma using a simple scoring system. J Am Coll Surg. 2013;217:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Bulsiewicz WJ, Dellon ES, Rogers AJ, Pasricha S, Madanick RD, Grimm IS, Shaheen NJ. The impact of endoscopic ultrasound findings on clinical decision making in Barrett’s esophagus with high-grade dysplasia or early esophageal adenocarcinoma. Dis Esophagus. 2014;27:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | May A, Günter E, Roth F, Gossner L, Stolte M, Vieth M, Ell C. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut. 2004;53:634-640. [PubMed] |
| 73. | Chemaly M, Scalone O, Durivage G, Napoleon B, Pujol B, Lefort C, Hervieux V, Scoazec JY, Souquet JC, Ponchon T. Miniprobe EUS in the pretherapeutic assessment of early esophageal neoplasia. Endoscopy. 2008;40:2-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Crabtree TD, Yacoub WN, Puri V, Azar R, Zoole JB, Patterson GA, Krupnick AS, Kreisel D, Meyers BF. Endoscopic ultrasound for early stage esophageal adenocarcinoma: implications for staging and survival. Ann Thorac Surg. 2011;91:1509-1515; discussion 1515-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Wani S, Abrams J, Edmundowicz SA, Gaddam S, Hovis CE, Green D, Gupta N, Higbee A, Bansal A, Rastogi A. Endoscopic mucosal resection results in change of histologic diagnosis in Barrett’s esophagus patients with visible and flat neoplasia: a multicenter cohort study. Dig Dis Sci. 2013;58:1703-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 76. | Hull MJ, Mino-Kenudson M, Nishioka NS, Ban S, Sepehr A, Puricelli W, Nakatsuka L, Ota S, Shimizu M, Brugge WR. Endoscopic mucosal resection: an improved diagnostic procedure for early gastroesophageal epithelial neoplasms. Am J Surg Pathol. 2006;30:114-118. [PubMed] |
| 77. | Luketich JD, Schauer PR, Meltzer CC, Landreneau RJ, Urso GK, Townsend DW, Ferson PF, Keenan RJ, Belani CP. Role of positron emission tomography in staging esophageal cancer. Ann Thorac Surg. 1997;64:765-769. [PubMed] |
| 78. | Keswani RN, Early DS, Edmundowicz SA, Meyers BF, Sharma A, Govindan R, Chen J, Kohlmeier C, Azar RR. Routine positron emission tomography does not alter nodal staging in patients undergoing EUS-guided FNA for esophageal cancer. Gastrointest Endosc. 2009;69:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 79. | Sun G, Tian J, Gorospe EC, Johnson GB, Hunt CH, Lutzke LS, Leggett CL, Iyer PG, Wang KK. Utility of baseline positron emission tomography with computed tomography for predicting endoscopic resectability and survival outcomes in patients with early esophageal adenocarcinoma. J Gastroenterol Hepatol. 2013;28:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Manner H, Pech O, Heldmann Y, May A, Pohl J, Behrens A, Gossner L, Stolte M, Vieth M, Ell C. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11:630-635; quiz e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 81. | Singh S, Sharma P. How effective is endoscopic therapy in the treatment of patients with early esophageal cancer? Nat Clin Pract Gastroenterol Hepatol. 2009;6:70-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol. 2013;11:1424-1429.e2; quiz e81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 83. | Guarner-Argente C, Buoncristiano T, Furth EE, Falk GW, Ginsberg GG. Long-term outcomes of patients with Barrett’s esophagus and high-grade dysplasia or early cancer treated with endoluminal therapies with intention to complete eradication. Gastrointest Endosc. 2013;77:190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Moss A, Bourke MJ, Hourigan LF, Gupta S, Williams SJ, Tran K, Swan MP, Hopper AD, Kwan V, Bailey AA. Endoscopic resection for Barrett’s high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 85. | Seerden TC, Larghi A. Staging of early adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc Clin N Am. 2011;21:53-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Van Den Eynde M, Jouret-Mourin A, Sempoux C, Piessevaux H, Deprez PH. Endoscopic mucosal or submucosal resection of early neoplasia in Barrett’s esophagus after antireflux surgery. Gastrointest Endosc. 2010;72:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Brahmania M, Lam E, Telford J, Enns R. Endoscopic mucosal resection: early experience in British Columbia. Can J Gastroenterol. 2010;24:239-244. [PubMed] |
| 88. | Wang SJ, Wu ML, Zhang LW, Guo XQ, Xu ZB, Er LM, Wang SP, Gao Y, Cong QW. [The value of endoscopic mucosal resection for dysplasia and early-stage cancer of the esophagus and gastric cardia]. Zhonghua Zhongliu Zazhi. 2008;30:853-857. [PubMed] |
| 89. | Neuhaus H, Terheggen G, Rutz EM, Vieth M, Schumacher B. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett’s esophagus. Endoscopy. 2012;44:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 90. | Higuchi K, Tanabe S, Azuma M, Katada C, Sasaki T, Ishido K, Naruke A, Katada N, Koizumi W. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc. 2013;78:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 91. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 92. | Ishihara R, Iishi H, Uedo N, Takeuchi Y, Yamamoto S, Yamada T, Masuda E, Higashino K, Kato M, Narahara H. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 93. | Evans JA, Early DS, Chandraskhara V, Chathadi KV, Fanelli RD, Fisher DA, Foley KQ, Hwang JH, Jue TL, Pasha SF. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc. 2013;77:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 94. | Haidry RJ, Dunn JM, Butt MA, Burnell MG, Gupta A, Green S, Miah H, Smart HL, Bhandari P, Smith LA. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013;145:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 95. | Zemlyak AY, Pacicco T, Mahmud EM, Tsirline VB, Belyansky I, Walters A, Heniford BT. Radiofrequency ablation offers a reliable surgical modality for the treatment of Barrett’s esophagus with a minimal learning curve. Am Surg. 2012;78:774-778. [PubMed] |
| 96. | Ertan A, Zaheer I, Correa AM, Thosani N, Blackmon SH. Photodynamic therapy vs radiofrequency ablation for Barrett’s dysplasia: efficacy, safety and cost-comparison. World J Gastroenterol. 2013;19:7106-7113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Titi M, Overhiser A, Ulusarac O, Falk GW, Chak A, Wang K, Sharma P. Development of subsquamous high-grade dysplasia and adenocarcinoma after successful radiofrequency ablation of Barrett’s esophagus. Gastroenterology. 2012;143:564-6.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 98. | Hur C, Choi SE, Rubenstein JH, Kong CY, Nishioka NS, Provenzale DT, Inadomi JM. The cost effectiveness of radiofrequency ablation for Barrett’s esophagus. Gastroenterology. 2012;143:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 99. | Overholt BF, Lightdale CJ, Wang KK, Canto MI, Burdick S, Haggitt RC, Bronner MP, Taylor SL, Grace MG, Depot M. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 348] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 100. | Weiss AA, Wiesinger HA, Owen D. Photodynamic therapy in Barrett’s esophagus: results of treatment of 17 patients. Can J Gastroenterol. 2006;20:261-264. [PubMed] |
| 101. | Dunn JM, Mackenzie GD, Banks MR, Mosse CA, Haidry R, Green S, Thorpe S, Rodriguez-Justo M, Winstanley A, Novelli MR. A randomised controlled trial of ALA vs. Photofrin photodynamic therapy for high-grade dysplasia arising in Barrett’s oesophagus. Lasers Med Sci. 2013;28:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 102. | American Society for Gastrointestinal Endoscopy Technology Committee. Mucosal ablation devices. Gastrointest Endosc. 2008;68:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Van Laethem JL, Jagodzinski R, Peny MO, Cremer M, Devière J. Argon plasma coagulation in the treatment of Barrett’s high-grade dysplasia and in situ adenocarcinoma. Endoscopy. 2001;33:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 104. | Sharma P, Wani S, Weston AP, Bansal A, Hall M, Mathur S, Prasad A, Sampliner RE. A randomised controlled trial of ablation of Barrett’s oesophagus with multipolar electrocoagulation versus argon plasma coagulation in combination with acid suppression: long term results. Gut. 2006;55:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Familiari L, Scaffidi M, Bonica M, Consolo P, Giacobbe G, Fichera D, Familiari P. Endoscopic treatment of Barrett’s epithelium with Argon Plasma Coagulation. Long-term follow-up. Minerva Gastroenterol Dietol. 2003;49:63-70. [PubMed] |
| 106. | Sie C, Bright T, Schoeman M, Game P, Tam W, Devitt P, Watson D. Argon plasma coagulation ablation versus endoscopic surveillance of Barrett’s esophagus: late outcomes from two randomized trials. Endoscopy. 2013;45:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 107. | Dumot JA, Vargo JJ, Falk GW, Frey L, Lopez R, Rice TW. An open-label, prospective trial of cryospray ablation for Barrett’s esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc. 2009;70:635-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 108. | Greenwald BD, Dumot JA, Abrams JA, Lightdale CJ, David DS, Nishioka NS, Yachimski P, Johnston MH, Shaheen NJ, Zfass AM. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 109. | Gosain S, Mercer K, Twaddell WS, Uradomo L, Greenwald BD. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc. 2013;78:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 110. | Greenwald BD, Dumot JA. Cryotherapy for Barrett’s esophagus and esophageal cancer. Curr Opin Gastroenterol. 2011;27:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Wu J, Pan YM, Wang TT, Gao DJ, Hu B. Endotherapy versus surgery for early neoplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc. 2014;79:233-241.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 112. | Lagorce C, Paraf F, Vidaud D, Couvelard A, Wendum D, Martin A, Fléjou JF. Cyclooxygenase-2 is expressed frequently and early in Barrett’s oesophagus and associated adenocarcinoma. Histopathology. 2003;42:457-465. [PubMed] |
| 113. | Lurje G, Vallbohmer D, Collet PH, Xi H, Baldus SE, Brabender J, Metzger R, Heitmann M, Neiss S, Drebber U. COX-2 mRNA expression is significantly increased in acid-exposed compared to nonexposed squamous epithelium in gastroesophageal reflux disease. J Gastrointest Surg. 2007;11:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 114. | Kandil HM, Tanner G, Smalley W, Halter S, Radhika A, Dubois RN. Cyclooxygenase-2 expression in Barrett’s esophagus. Dig Dis Sci. 2001;46:785-789. [PubMed] |
| 115. | Mehta S, Boddy A, Johnson IT, Rhodes M. Systematic review: Cyclo-oxygenase-2 in human oesophageal adenocarcinogenesis. Aliment Pharmacol Ther. 2006;24:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Anderson LA, Johnston BT, Watson RG, Murphy SJ, Ferguson HR, Comber H, McGuigan J, Reynolds JV, Murray LJ. Nonsteroidal anti-inflammatory drugs and the esophageal inflammation-metaplasia-adenocarcinoma sequence. Cancer Res. 2006;66:4975-4982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 117. | Hawk ET, Viner JL. Statins in esophageal cancer cell lines: promising lead? Am J Gastroenterol. 2008;103:838-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 118. | Wang KK, Okoro N, Prasad G, WongKeeSong M, Buttar NS, Tian J. Endoscopic evaluation and advanced imaging of Barrett’s esophagus. Gastrointest Endosc Clin N Am. 2011;21:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |