Published online Feb 15, 2013. doi: 10.4251/wjgo.v5.i2.29
Revised: December 29, 2012
Accepted: January 17, 2013
Published online: February 15, 2013
Processing time: 154 Days and 12.3 Hours
Primary gallbladder (GB) carcinoma and Crohn’s disease (CD) of the GB are individually rare. We present a case of a pregnant woman with CD found to have GB involvement and primary GB carcinoma. A 34-year-old female at 6 wk gestation with a 21 year history of CD of uncertain extent presented with 3 mo of diarrhea, urgency and abdominal pain. During work-up, she was found to have elevated transaminases and an abnormal alkaline phosphatase. Imaging revealed two gallbladder polyps both greater than 1 cm in size. Resection and histological evaluation was consistent with Crohn’s involvement of the GB, poorly differentiated adenocarcinoma of the GB with invasion through the muscularis propria and matted lymph nodes in the porta hepatis positive for metastatic carcinoma (stage pT2N1). Six cases of CD involving the GB, two cases of primary GB carcinoma in CD, and ten cases of cholangiocarcinoma in pregnancy have been published. This is the only case that describes all three factors. Common features in CD of the GB include acute cholecystitis, ileal involvement, and presence independent of active intestinal disease. Common features in CD patients with GB malignancy include younger age of detection, a long history of CD, extensive colonic and ileal involvement of disease, the absence of cholelithiasis, and pre-existing gallbladder disease (primary sclerosing cholangitis and gallbladder polyps). Pregnancy is specific to this case. The role of CD in the development of GB malignancy is not well understood nor is the contribution of pregnancy to the spread of disease. Chronic inflammation and immunosuppression compounded by hormonal influence is implicated.
- Citation: Attraplsi S, Shobar RM, Lamzabi I, Abraham R. Gallbladder carcinoma in a pregnant patient with Crohn's disease complicated with gallbladder involvement. World J Gastrointest Oncol 2013; 5(2): 29-33
- URL: https://www.wjgnet.com/1948-5204/full/v5/i2/29.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v5.i2.29
Primary gallbladder carcinoma (GC) is the second most common primary biliary malignancy and the fifth most common malignancy of the gastrointestinal tract. The prognosis of GC is dismal, with five-year survival rates of 0%-10% and median survival of less than 6 mo. Patients with inflammatory bowel disease (IBD) are at increased risk for cholangiocarcinoma. Several case reports[1-10], population-based case control and cohort studies[11-18] report IBD as a risk factor for cholangiocarcinoma. The 10-year cumulative risk of cholangiocarcinoma in IBD was found to be 0.07% in a national Danish cohort study[19] with a four-fold increase among IBD patients compared to the general population. However the absolute risk of cholangiocarcinoma and more specifically GC in patients with IBD remains unclear[19]. Furthermore, little is known about the impact of IBD on the development of GC. We herein report a case of a pregnant woman with Crohn’s disease (CD) complicated with involvement of the gallbladder (GB) and primary GC. The purpose of this case is to illustrate the presentation of GB involvement in CD and primary GC and to discuss the putative risk factors that interplay to contribute to the development of these complications in the setting of pregnancy.
A 34-year-old female at 6 wk gestation with a 21-year history of IBD of uncertain classification and extent presented with 3 mo of diarrhea, urgency and abdominal pain. Her disease had been indolent for the majority of her life. Her medications included an oral contraceptive (OC), which she had been on for the past 6 years, and mesalamine and mercaptopurine, which she took as needed at times of flare symptoms. Initial work up included a sigmoidoscopy that was consistent with mild patchy colitis of the sigmoid and descending colon, extending beyond the limit of the exam, with rectal sparing. Pathology showed patchy moderately active chronic colitis with crypt distortion and cryptitis without granuloma. The endoscopic distribution of disease was more consistent with CD, however she was unable to provide information on previous endoscopies. Her pregnancy limited further examination at that time. She was started on oral and topical mesalamine and symptomatically responded.
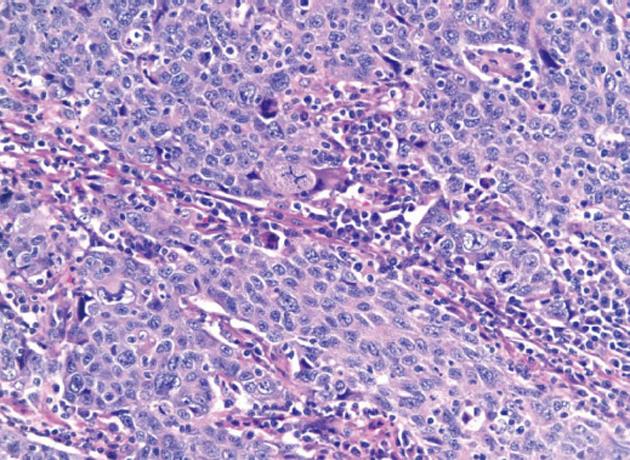
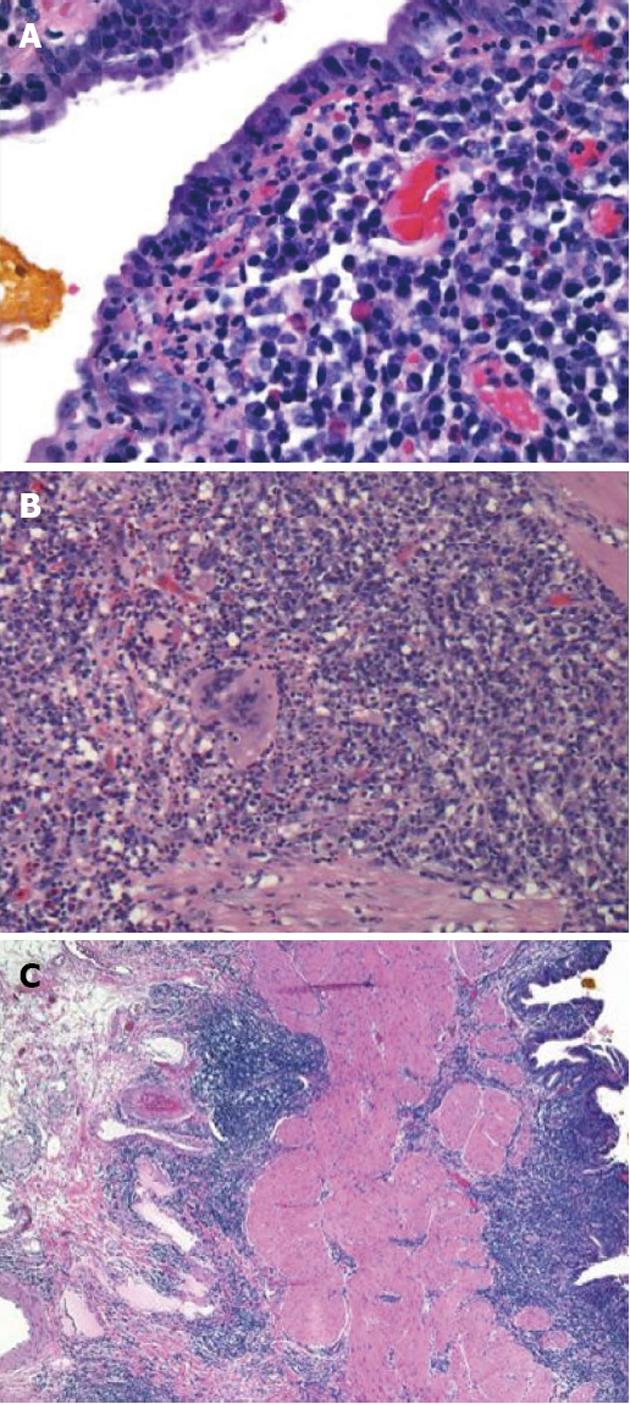
Initial labs were obtained including elevated alkaline phosphatase 515 U/L, aspartate aminotransferase 96 U/L, and alanine aminotransferase 181 U/L. Further labs included normal bilirubin, negative anti-mitochondrial antibody (AB), anti-smooth muscle AB, celiac and viral hepatitis serologies and anti-nuclear AB. An ultrasound revealed a large polypoid mass in the GB prompting further imaging. A non-contrast magnetic resonance cholangiopancreatography was obtained revealing two hypointense smooth margined masses with small stalks in the neck and fundus of the GB, 1.4 cm × 1.8 cm and 1.4 cm × 2.3 cm respectively. No GB wall thickening, cholilithiasis, ductal dilation, strictures, or liver parenchymal abnormalities were present. Laparoscopic cholecystecomy was performed at 18 wk gestation. Histology revealed a poorly differentiated adenocarcinoma of the GB (Figure 1) with invasion through the muscularis propria and an adjacent tubulovillous adenoma with highgrade dysplasia without nodal involvement (stage pT2N0). Additionally, there was widespread epithelial dysplasia in the GB with acute superficial inflammation, transmural chronic inflammation with numerous plasma cells and one granuloma consistent with CD (Figure 2). Muscularis propria invasion prompted partial liver resection and portal lymphadenectomy, revealing matted lymph nodes in the porta hepatis positive for metastatic carcinoma (stage pT2N1). Liver tissue was negative for primary sclerosing cholangitis (PSC).
The immediate post-operative course was uncomplicated and the patient was discharged one week later. The patient was given the option to terminate the pregnancy and proceed with adjuvant radiation and chemotherapy or to carry out her pregnancy and delay further treatment until the post-partum period. She elected to defer radiation and chemotherapy until after she delivered. She has since had an uncomplicated vaginal delivery and is starting radiation and chemotherapy.
Cholangiocarcinoma in ulcerative colitis is well established particularly with the presence of PSC[2,20-23]. In contrast cholangiocarcinoma in CD is less frequent. Primary GC in CD is even more infrequent with only 2 reported cases in the literature[24,25]. With inclusion of our case, common clinical features include detection at a young age (32 years, 34 years and 50 years), absence of gallstone formation (all cases), a long duration of disease (12 years, 13 years and 21 years), absence of biliary symptoms (all cases), and a history of ileal and pan-colonic disease (unknown extent in our case). Clinically active colitis was absent in two cases[24,25] and present in our case.
Primary GC in CD is rare enough that a true association can be questioned, despite consistent commonalities between cases. A study looking at 2645 CD patients and cancer incidence over a 17-year period from the Danish National Registry compared the rate of cancer in the CD population to the expected rate of cancer in the general Danish population[14]. In total, the authors found 143 malignant neoplasms in CD patients compared to 123 in the general population (95%CI: 0.97-1.36) of which only 2 were cholangiocarcinomas in CD (not location specified) compared to 1 in the general population. This agrees with the rare incidence of GB carcinoma in CD and the questionable contribution of IBD as an independent factor to the development of cholangiocarcinoma. Pre-existing GB diseases, include gallstones, chronic cholecystitis, polyps and premalignant lesions, adenomyomatosis, an anomalous junction of the pancreato-biliary duct, chronic infection, PSC and hormonal changes in women[26], are known risk factors for the development of GC in the general population. These same factors may similarly be required for the development of GC in IBD however temporally accelerated in the setting of inflammation and immunosuppression. This is illustrated in the above cited cases with two of the three carcinomas originating from GB polyps[25] and one in the setting of concomitant PSC[24].
Persistent inflammation is thought to promote carcinogenesis by causing DNA damage, activating tissue reparative proliferation, and by creating a local environment that is enriched with cytokines and other growth factors for autonomous proliferation and escape from apoptosis[27]. Population studies previously mentioned hint that IBD (i.e., inflammation) alone does not account for these changes. A large survey investigating inflammatory patterns in post-cholecystectomy patients with IBD noted the absence of any specimens containing granulomatous disease or GC, including the 78 patients with CD. This lack of presence may be due to the low threshold to perform cholecystectomy in IBD patients preventing the progression of chronic inflammation, thus aborting the full development of biliary epithelial dysplasia and its associated malignancy as suggested by the authors[28]. Potentially, the presence of pre-existing immune modulating medications and the relative immunosuppressed state of IBD may act to down-regulate the immune response and in turn have a role in creation and progression of malignancy in the setting of long standing inflammation. Chronic GB inflammation as CD involvement was present in our case.
CD involvement of the GB itself is very rare with only six reported cases in the literature[29-34]. Common pathological features include transmural inflammation, granulomatous change, and lymphoid aggregation[29-34]. Similarly, our case demonstrated chronic transmural inflammation and granuloma formation. Common clinical characteristics include initial presentation with acute cholecystitis, as demonstrated in 4 cases[29-31,33] and ileal involvement occurred in 5 cases[29-32,34]. Of note, patients with ileal disease have a 10 fold increase in the incidence of cholelithiasis due to the disruption in bile salt metabolism[35]. Suggested mechanisms include disturbances in bile acid metabolism due to loss of absorptive function of the terminal ileum resulting in depletion of the bile acid pool and precipitation of cholesterol with subsequent stone formation[36,37]. However 2 of the 7 total cases, including ours, occurred in the absence of gallstone formation[31]. Alternatively, ileal disease may result in disturbance in the microbiome and colonization of the terminal ileum with anaerobic bacteria[38]. This results in deconjugation of bile acids to products that have an irritating effect on the mucosa of the GB resulting in inflammation[39]. The potential role of these mechanisms in its development is not clear.
And finally, the effect of pregnancy on the progression of our patient’s disease is not clear. Ten cases of cholangiocarcinoma in pregnancy exist in the literature[40-48]. Pregnancy is associated with high estrogen levels and theoretically can aggravate a preexisting malignant lesion. The relative immunosuppressed state that exists during pregnancy may also play a role in enhancing aggressiveness of the malignancy. Human intrahepatic cholangiocarcinomas express the receptors for both estrogens and insulin-like growth factor-1 (IGF-1)[49] indicating that estrogens and IGF-1 coordinately regulate cholangiocarcinoma growth and apoptosis[49]. Additionally, GC is more common in women. Secreted mutagenic toxins persist in the GB in women due to stasis which results from impaired contractility associated with progesterone[50]. This protracted exposure allows environmental carcinogens to then cause malignant transformation[43]. Additionally, the use of hormone based contraception remains a controversial issue. In some OC studies, no difference between the incidence of cholangiocarcinoma and healthy controls was found[51,52] but in other studies a positive association between OC use and extrahepatic bile cancer was found[53,54]. Our patient was on an OC agent for about six years.
GC carries the worst prognosis of any gastrointestinal or hepatobiliary neoplasm. The prognosis is equally grave independent of the presence of IBD. It is essential to identify IBD patients at high risks for developing GC. At this time, annual ultrasound examination of the GB is recommended in patients with PSC. Cholecystectomy is recommended in all PSC patients with mass lesions of any size due to high malignant potential[55]. The same recommendations should be extended to patients with chronic immunosuppressive states and chronic inflammation, such as IBD.
P- Reviewers Girelli CM, Diehl LJ S- Editor Song XX L- Editor A E- Editor Xiong L
| 1. | Klingenberg-Noftz RD, Homann N, Bos I, Bruch HP, Ludwig D. Simultaneous detection of synchronous colonic and biliary carcinoma by abdominal ultrasonography in two patients with ulcerative colitis. Dig Dis Sci. 2004;49:1922-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Choi PM, Nugent FW, Zelig MP, Munson JL, Schoetz DJ. Cholangiocarcinoma and Crohn’s disease. Dig Dis Sci. 1994;39:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Chong VF, Heng A. Cholangiocarcinoma complicating ulcerative colitis. Ann Acad Med Singapore. 1993;22:798-801. [PubMed] |
| 4. | Haworth AC, Manley PN, Groll A, Pace R. Bile duct carcinoma and biliary tract dysplasia in chronic ulcerative colitis. Arch Pathol Lab Med. 1989;113:434-436. [PubMed] |
| 5. | Van Steenbergen W, Fevery J, Vandenbrande P, Desmet V, Ponette E, Kerremans R, de Groote J. Ulcerative colitis, primary sclerosing cholangitis, bile duct carcinoma, and generalized sarcoidosis. Report of a unique association. J Clin Gastroenterol. 1987;9:574-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Berman MD, Falchuk KR, Trey C. Carcinoma of the biliary tree complicating Crohn’s disease. Dig Dis Sci. 1980;25:795-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Wee A, Ludwig J, Coffey RJ, LaRusso NF, Wiesner RH. Hepatobiliary carcinoma associated with primary sclerosing cholangitis and chronic ulcerative colitis. Hum Pathol. 1985;16:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 120] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Krause JR, Ayuyang HQ, Ellis LD. Occurrence of three cases of carcinoma in individuals with Crohn’s disease treated with metronidazole. Am J Gastroenterol. 1985;80:978-982. [PubMed] |
| 9. | Williams SM, Harned RK. Bile duct carcinoma associated with chronic ulcerative colitis. Dis Colon Rectum. 1981;24:42-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Akwari OE, Van Heerden JA, Foulk WT, Baggenstoss AH. Cancer of the bile ducts associated with ulcerative colitis. Ann Surg. 1975;181:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Mellemkjaer L, Olsen JH, Frisch M, Johansen C, Gridley G, McLaughlin JK. Cancer in patients with ulcerative colitis. Int J Cancer. 1995;60:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Broomé U, Glaumann H, Hellers G, Nilsson B, Sörstad J, Hultcrantz R. Liver disease in ulcerative colitis: an epidemiological and follow up study in the county of Stockholm. Gut. 1994;35:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 385] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 14. | Mellemkjaer L, Johansen C, Gridley G, Linet MS, Kjaer SK, Olsen JH. Crohn’s disease and cancer risk (Denmark). Cancer Causes Control. 2000;11:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Broomé U, Löfberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404-1408. [PubMed] |
| 16. | Persson PG, Karlén P, Bernell O, Leijonmarck CE, Broström O, Ahlbom A, Hellers G. Crohn’s disease and cancer: a population-based cohort study. Gastroenterology. 1994;107:1675-1679. [PubMed] |
| 17. | Ekbom A, Helmick C, Zack M, Adami HO. Extracolonic malignancies in inflammatory bowel disease. Cancer. 1991;67:2015-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Welzel TM, Mellemkjaer L, Gloria G, Sakoda LC, Hsing AW, El Ghormli L, Olsen JH, McGlynn KA. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer. 2007;120:638-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Erichsen R, Jepsen P, Vilstrup H, Ekbom A, Sørensen HT. Incidence and prognosis of cholangiocarcinoma in Danish patients with and without inflammatory bowel disease: a national cohort study, 1978-2003. Eur J Epidemiol. 2009;24:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Mir-Madjlessi SH, Farmer RG, Sivak MV. Bile duct carcinoma in patients with ulcerative colitis. Relationship to sclerosing cholangitis: report of six cases and review of the literature. Dig Dis Sci. 1987;32:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Ritchie JK, Allan RN, Macartney J, Thompson H, Hawley PR, Cooke WT. Biliary tract carcinoma associated with ulcerative colitis. Q J Med. 1974;43:263-279. [PubMed] |
| 22. | Akwari OE, van Heerden JA, Adson MA, Foulk WT, Baggenstoss AH. Bile duct carcinoma associated with ulcerative colitis. Rev Surg. 1976;33:289-293. [PubMed] |
| 23. | Schrumpf EFO. The frequency of cholangiocarcinoma (CC) in primary sclerosing cholangitis. J Hepatol. 1989;9:83. [DOI] [Full Text] |
| 24. | Joffe N, Antonioli DA. Primary carcinoma of the gallbladder associated with chronic inflammatory bowel disease. Clin Radiol. 1981;32:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Ogawa H, Funayama Y, Naito H, Fukushima K, Shibata C, Matsuno S, Sasaki I. Gallbladder carcinoma complicating Crohn’s disease. Am J Gastroenterol. 2001;96:263-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Sheth S, Bedford A, Chopra S. Primary gallbladder cancer: recognition of risk factors and the role of prophylactic cholecystectomy. Am J Gastroenterol. 2000;95:1402-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Wise C, Pilanthananond M, Perry BF, Alpini G, McNeal M, Glaser SS. Mechanisms of biliary carcinogenesis and growth. World J Gastroenterol. 2008;14:2986-2989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Lin J, Shen B, Lee HJ, Goldblum JR. Histopathological characterization of cholecystectomy specimens in patients with inflammatory bowel disease. J Crohns Colitis. 2012;6:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | McClure J, Banerjee SS, Schofield PS. Crohn’s disease of the gall bladder. J Clin Pathol. 1984;37:516-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Post AB, van Stolk R, Broughan TA, Tuthill RJ. Crohn’s disease of the gallbladder. J Clin Gastroenterol. 1993;16:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Rettally CA, Treviño HH, Molina K, Kane SV. Crohn’s disease involving the gallbladder. case report and review of the literature. Am J Gastroenterol. 2003;98:509-511. [PubMed] |
| 32. | Andoh A, Endo Y, Kushima R, Hata K, Tsujikawa T, Sasaki M, Mekata E, Tani T, Fujiyama Y. A case of Crohn’s disease involving the gallbladder. World J Gastroenterol. 2006;12:977-978. [PubMed] |
| 33. | Sharma AK, Nawroz IM, Evgenikos N, Daniel T. Isolated involvement of the gallbladder by Crohn’s disease manifesting as acute cholecystitis. J Gastrointest Surg. 2005;9:357-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Bouteloup C, Guédon C, François A, Hemet J, Lerebours E. [Gallbladder involvement in Crohn disease]. Gastroenterol Clin Biol. 1997;21:153-155. [PubMed] |
| 35. | Lapidus A, Bångstad M, Aström M, Muhrbeck O. The prevalence of gallstone disease in a defined cohort of patients with Crohn’s disease. Am J Gastroenterol. 1999;94:1261-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Kurchin A, Ray JE, Bluth EI, Merritt CR, Gathright JB, Pehrsson BF, Ferrari BT. Cholelithiasis in ileostomy patients. Dis Colon Rectum. 1984;27:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Vantrappen G, Ghoos Y, Rutgeerts P, Janssens J. Bile acid studies in uncomplicated Crohn’s disease. Gut. 1977;18:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Van de Merwe JP, Schröder AM, Wensinck F, Hazenberg MP. The obligate anaerobic faecal flora of patients with Crohn’s disease and their first-degree relatives. Scand J Gastroenterol. 1988;23:1125-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Heaton KW. Disturbances of bile acid metabolism in intestinal disease. Clin Gastroenterol. 1977;6:69-89. [PubMed] |
| 40. | Sadoon S, Hodgett S. Unusual cause of itching in a pregnancy (cholangiocarcinoma). J Obstet Gynaecol. 2008;28:230-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Purtilo DT, Clark JV, Williams R. Primary hepatic malignancy in pregnant women. Am J Obstet Gynecol. 1975;121:41-44. [PubMed] |
| 42. | Marasinghe JP, Karunananda SA, Angulo P. Cholangiocarcinoma in pregnancy: a case report. J Obstet Gynaecol Res. 2008;34:635-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Liao WS, Wang TE, Lin SC, Chang KM, Yang TL, Yang YC, Chen YJ, Yung YY. Chloangiocarcinoma in pregnancy - A case report. Neikexue Zhi. 2002;13:286-292. |
| 44. | Devoe LD, Moossa AR, Levin B. Pregnancy complicated by extrahepatic biliary tract carcinoma. A case report. J Reprod Med. 1983;28:153-155. [PubMed] |
| 45. | Zelissen PM, van Hattum J. [A young woman with a liver tumor and hypercalcemia]. Ned Tijdschr Geneeskd. 1986;130:1705-1707. [PubMed] |
| 46. | Sophia S, Girling JC. Deranged liver function tests in pregnancy: the importance of postnatal follow-up. Obstet Med. 2009;2:32-33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Ismael G, de Azambuja E, Awada A. Molecular profiling of a tumor of unknown origin. N Engl J Med. 2006;355:1071-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | DePalma F, Henry P, Hahm A, Lee T, Elfant A. Cholangiocarcinoma during pregnancy diagnosed by ERCP with cholangioscopy (Abstract). Available from: http://universe.gi.org/contentlist.asp?page=31&eventtype=51. |
| 49. | Alvaro D, Barbaro B, Franchitto A, Onori P, Glaser SS, Alpini G, Francis H, Marucci L, Sterpetti P, Ginanni-Corradini S. Estrogens and insulin-like growth factor 1 modulate neoplastic cell growth in human cholangiocarcinoma. Am J Pathol. 2006;169:877-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Venniyoor A. Cholesterol gallstones and cancer of gallbladder (CAGB): molecular links. Med Hypotheses. 2008;70:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Combined oral contraceptives and gallbladder cancer. The WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Int J Epidemiol. 1989;18:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Moerman CJ, Berns MP, Bueno de Mesquita HB, Runia S. Reproductive history and cancer of the biliary tract in women. Int J Cancer. 1994;57:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Altaee MY, Johnson PJ, Farrant JM, Williams R. Etiologic and clinical characteristics of peripheral and hilar cholangiocarcinoma. Cancer. 1991;68:2051-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Yen S, Hsieh CC, MacMahon B. Extrahepatic bile duct cancer and smoking, beverage consumption, past medical history, and oral-contraceptive use. Cancer. 1987;59:2112-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |