Published online Dec 15, 2013. doi: 10.4251/wjgo.v5.i12.230
Revised: November 3, 2013
Accepted: November 15, 2013
Published online: December 15, 2013
Processing time: 66 Days and 23.7 Hours
Post transplant lymphoproliferative disorder (PTLD) represents a life threatening disorder occurring after transplantation, ranging from a polyclonal mononucleosis like illness to a monomorphic high grade neoplasm with cytologic and histopathologic evidence indicative of transformation to lymphoma. PTLD of diffuse large B-cell lymphoma (DLBCL) subtype, isolated to the esophagus is a rare diagnosis. We describe the first case of an immunocompromised adult patient diagnosed with DLBCL-PTLD limited to his esophagus without an associated mass or locoregional lymphadenopathy on imaging since the institution of the revised Cheson criteria, which includes positron emission tomography-computed tomography as the standard staging modality. Even more unique to our case was the suggestion of underlying cytomegalovirus (CMV) gastritis leading to a hypothesis about a less well understood relationship between CMV and Epstein Barr virus (EBV). In the post transplant setting, immunocompromised state, or EBV positive state, upper gastrointestinal symptoms should prompt investigation with an upper endoscopy (EGD). Additionally, specific to our case, the fact that the patients’ presentation was suspicious for CMV gastritis raises the possibility that the CMV infection predated his PTLD increasing his risk of acquiring PTLD. This reemphasizes the importance and diagnostic utility of early screening with EGD in patients after transplantation.
Core tip: Post transplant lymphoproliferative disorder (PTLD) are a heterogeneous group of lymphoid proliferations associated with immunosuppression following solid organ transplantation (SOT) or allogeneic hematopoietic stem cell transplantation (HSCT). PTLD associated with B-cell esophageal lymphoma is a rare diagnosis. Our patient was identified to have isolated PTLD at the gastroesophageal junction, which has not been reported in the modern era of positron emission tomography-computer tomography. Previous reported cases describe esophageal lymphoma associated with symptoms of dysphagia, odynophagia or esophogastroduodenoscopy (EGD) findings that revealed an associated mass or locoregional lymphadenopathy. However, given the variation in presentation in post-transplant and immunocompromised patients, gastrointestinal symptoms should prompt further investigation with an EGD.
- Citation: Haverkos BM, Oza VM, Johnson A, Walker J, Shana’ah A. Rare presentation of post-transplant lymphoproliferative disorder isolated to gastroesophageal junction. World J Gastrointest Oncol 2013; 5(12): 230-234
- URL: https://www.wjgnet.com/1948-5204/full/v5/i12/230.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v5.i12.230
Post-transplant lymphoproliferative disorder (PTLD) represents a life threatening disorder occurring after transplantation, ranging from a polyclonal mononucleosis like illness to a monomorphic high grade neoplastic disease with cytologic and histopathologic evidence indicative of non-Hodgkin as well as Hodgkin’s lymphoma. Monomorphic disease includes diffuse large B-cell lymphoma (DLBCL), Burkitts lymphoma, plasma cell myeloma/plasmacytoma, and rarely T-cell neoplasms[1]. The majority of PTLD following both allogeneic HSCT and SOT are of B cell origin, and are usually associated with the Epstein Barr virus (EBV). In the pediatric population, the highest incidence of lymphoma has been observed during the first year after transplantation with 47% of cases occurring within 6 mo, 62% within 1 year, and 90% within 5 years of transplant[2]. In adults slightly more than 50% of patients are diagnosed beyond 12 mo with a predilection for EBV associated tumors to occur sooner than EBV negative tumor types. In children at ten years, the relative risk of lymphoma is 11.8 fold greater higher than in persons in the non-transplant population[3]. The incidence of PTLD is generally higher among pediatric patients as compared to adults. Among renal transplant recipients, the prevalence of PTLD has been reported to be about 5%[4] and recent studies suggest that risk factors for partial or no clinical remission are multiple site disease, performance status, and nondetection of EBV in the tumor[5].
The clinical features of PTLD are multiple and varied and can range from asymptomatic EBV seroconversion or peripheral blood viremia to monoclonal B cell proliferation with nodal, extra-nodal, and disseminated disease[6]. In a retrospective review of 78 PTLD patients at the University of Michigan the majority were identified with extensive symptomatic disease: extranodal disease (79%), poor performance status (68%), elevated lactate dehydrogenase (71%), and advanced stage by Ann Arbor criteria (68%)[1].
The esophagus is one of the more infrequent sites for gastrointestinal lymphomas, usually accounting for < 1% of cases[7]. Furthermore, fewer than 30 cases of lymphoma limited to the esophagus have been reported[8]. Approximately 2/3 of these cases are diffuse large B cell lymphomas. Our case represents the first documented case of PTLD of DLBCL type with isolated gastroesophageal involvement without local or disseminated lymphadenopathy in the modern revised Cheson criteria staging era.
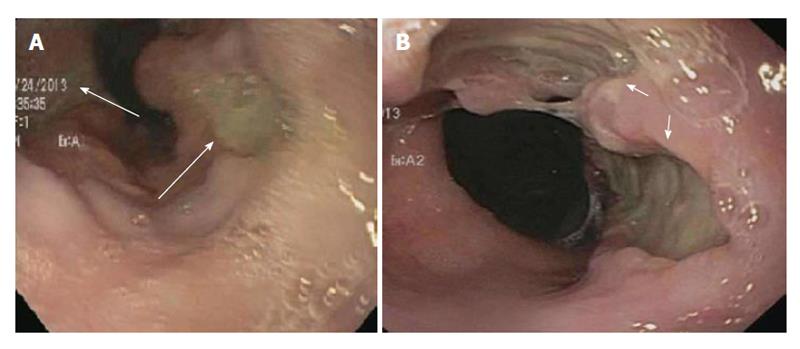
We present the case of a 25-year-old African American male with a history of gastroesophageal reflux disease (GERD) and systemic lupus nephritis status post living related donor kidney transplantation, 3 years prior, on chronic immunosuppression presented to the emergency department for evaluation of four weeks of progressive retrosternal burning, nausea, vomiting and odynophagia. He had previously been diagnosed six years prior with GERD and was placed on daily proton pump inhibitor (PPI) therapy, but had discontinued PPI use after symptoms resolved. He denied solid and/or liquid dysphagia, but reported an approximate ten pound weight loss because of worsening symptoms. There was no history of any fevers, chills or night-sweats. At the time of admission immunosuppression for his renal transplant included Prednisone, Sirolimus and Mycophenolate mofetil. Admission labs were within normal limits with the exception of a lipase level of 130 U/L (normal range of 18-51 U/L). Gastroenterology service was then consulted for further evaluation. An upper endoscopy (EGD) was pursued to evaluate for cytomegalovirus (CMV), Herpes Simplex virus (HSV) and/or candidal esophagitis. EGD revealed Los Angeles grade A esophagitis with two small cratered esophageal non-bleeding ulcers at the gastroesophageal (GE) junction (Figure 1A). The gastric antrum was also mildly erythematous. Given the broad differential diagnosis for esophagitis including non-infectious as well as infectious etiologies biopsies were obtained from the gastroesophageal (GE) junction. Gastric biopsies were also obtained to evaluate for Helicobacter pylori infection.
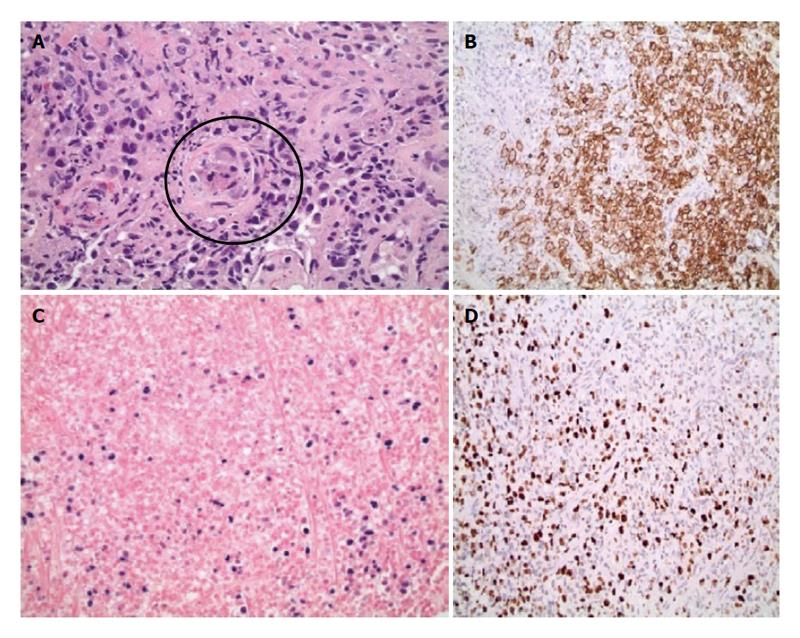
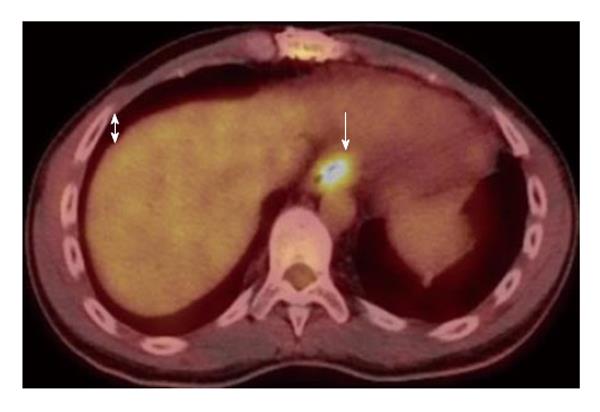
Biopsy results of the stomach only demonstrated mild chronic gastritis with reactive epithelial changes whereas biopsies of the GE junction demonstrated ulceration, a diffuse infiltrate of large atypical cells with irregular nuclei and prominent nucleoli as well as prominent necrosis and rare CMV inclusions within an endothelial cell (Figure 2A). Based on immunohistochemical and in-situ hybridization stains the large lymphoma cells were strongly and uniformly positive for CD20 (Figure 2B), CD30, CD45, PAX5, BCL2, BCL6, MUM1, and in-situ hybridization for Epstein Barr virus encoded RNA (EBER) (Figure 2C). The Ki-67 stain was positive in 100% of large cells consistent with a high proliferation fraction (Figure 2D). The large B cells were negative for CD5, CD10, CD15, BCL1, and GCET1. The pathology was diagnostic of a post-transplant lymphoproliferative discorder, DLBCL type expressing a non-germinal cell immunophenotype. A CMV immunostain did not demonstrate positive results due to loss of the rare endothelial cell on deeper levels. The patient was subsequently started on valganciclovir for treatment of CMV esophagitis (given CMV inclusions and ulcers) and Hematology/Oncology service was consulted for further management and treatment of PTLD-DLBCL type. He subsequently underwent staging workup to evaluate this new diagnosis, which included a bilateral bone marrow biopsy, a positive emission tomography (PET) and a computed tomography (CT) scan of chest, abdomen and pelvis. Bone marrow biopsy was negative for involvement by lymphoma as was the peripheral blood flow cytometric analysis. PET scan showed focal uptake within the region of the GE junction only but no other significant uptake throughout the chest, abdomen, or pelvis, nor was the patient noted to have any paraesophageal or gastric involvement (Figure 3). CT scans showed only subtle prominence at the GE junction. He was discharged with plan to start chemotherapy as an outpatient. Unfortunately, he was re-admitted one week later for severe epigastric pain, nausea and vomiting. Repeat endoscopy was done which showed significantly enlarged GE junction ulcers (Figure 1B). He was then started on treatment with dose adjusted R-EPOCH (Rituximab, Etoposide, Prednisone, Vincristine, Doxorubicin) for his limited stage esophageal PTLD, DLBCL type. After his first cycle his laboratory values were consistent with tumor lysis syndrome, requiring a dose of Rasburicase. Throughout the inpatient stay, our patient continued to have delayed nausea and vomiting. He had a diagnostic lumbar puncture to rule out central nervous system disease (which was negative) and was also administered intrathecal Methotrexate at the time. A repeat PET/CT scan done to reassess disease after chemotherapy showed interval improvement in patient’s lymphoma at the GE junction. Patient was then discharged on prophylactic Dapsone, Valacyclovir and Fluconazole. He has received four cycles of chemotherapy and is currently doing well, and is scheduled to complete six cycles followed by restaging.
In summary, we present an immunosuppressed renal transplant patient with a prior history of GERD and four weeks of progressive retrosternal burning, nausea, vomiting and odynophagia. The differential was broad and included both infectious and non-infectious etiologies. He underwent an EGD with biopsy of GE junction consistent with non-germinal center DLBCL and CMV inclusions. His laboratory findings were remarkable for a normal CBC and liver profile, and elevated LDH. PET/CT imaging was only remarkable for focal uptake at the GE junction. So the ensuing plan was to get 6 cycles of dose adjusted R-EPOCH and intrathecal prophylaxis with methotrexate.
Approximately 85% of PTLDs are B cell in origin and most commonly of the monomorphic DLBCL type (50%) typically occurring after solid organ transplant. The major risk factor in developing PTLD is type, degree, and cumulative length of immunosuppression as well as EBV status[6]. PTLDs have a diverse clinical presentation, commonly with extranodal manifestations (approx. 70%) with gastrointestinal tract involvement occurring in up to 30% of patients who usually present with GI complaints (i.e., weight loss, bowel perforation, bleeding, fever, and pain). PTLD with multiple lesions throughout the gastrointestinal tract has been described in a child[9]. Specifically, in renal transplant recipients who acquire EBV associated PTLD, the allograft is affected in approximately one-third of all cases[10]. In general, data on gastrointestinal lesions in PTLD is limited. In the study by Shitrit et al[12] that included 17 patients with gastrointestinal manifestations, median time of occurrence of PTLD was 36 mo, and in 6 patients, PTLD was diagnosed within 18 mo. In 29%, or 5 out of 17 patients, involvement of organs other than the GI tract were found[11]. However, it must be noted that much of this data is extracted from pediatric populations who tend to develop PTLD earlier than adults.
Our patient was identified to have isolated PTLD of the DLBCL type at the gastroesophageal junction. Primary esophageal B cell lymphoma outside of the post-transplant setting has been reported but is also very rare[13]. In the most extensive case series on GI involvement, O’Conner et al[14] reported the findings of 6/14 children who were found to have isolated gastroesophageal PTLD on EGDs after presenting with GI symptoms. The incidence of PTLD in pediatric patients is higher than in the adult transplant population presumably secondary to the population being more frequently EBV naive. O’Conneret al[14] reviewed the possibility of using EGD as a screening tool for PTLD, which has not been utilized in the adult population.
Our patient was treated with induction R-EPOCH (Rituximab, Etoposide, Vincristine, Cyclophosphamide, and Doxorubicin) chemotherapy, prophylactic intrathecal Methotrexate, and Valganciclovir for his presumptive CMV gastritis. He has responded well to treatment thus far. In general, the treatment for EBV driven PTLD remains a controversial subject, with no SE based treatment or multi-centered trials to support any particular treatment modality. However, a reduction in immunosuppression is the most common management with less efficacy against the monomorphic disease therefore typically necessitating immunochemotherapy with Rituximab.
In our case it is important to comment on his presumptive CMV gastritis. CMV infection has been an identified risk factor in the development of PTLD through the modification of inflammatory cytokines[15]. In a group of patients with primary CMV disease, the post-transplant risk of PTLD was found to be 4 to 6 fold higher[16]. Also in one small study, children with simultaneous CMV and EBV infection had earlier onset, worse symptoms, and a greater rise in serum creatinine than was seen with primary CMV or EBV disease alone[17]. This provides rationale to further study the relationship between CMV and EBV.
From this case we have learned the importance of prompt investigation with EGD in the post-transplant setting, immunocompromised state, or EBV positive state when upper gastrointestinal symptoms occur. Additionally, specific to our case, the fact that the patients’ presentation was suspicious for CMV gastritis raises the possibility that his CMV disease predated his PTLD increasing his risk of acquiring PTLD. This reemphasizes the take home message that early screening with EGD in a patient after transplantation has important diagnostic utility.
It highlights the spectrum of presentation found in an uncommon diagnosis of post transplant lymphoproliferative disorder (PTLD).
It allows for a review of the more common clinical manifestations to PTLD with a focus on the workup of such patients.
It most importantly highlights a unique unreported presentation in an unambiguous case of PTLD.
One grey area was the diagnosis of cytomegalovirus (CMV), although it is certainly not the most salient point to the case.
In the patient there was no peripheral blood CMV viremia, which would verify the CMV infection.
The paper is worth being published.
P- Reviewer: Kim ST S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
| 1. | Knight JS, Tsodikov A, Cibrik DM, Ross CW, Kaminski MS, Blayney DW. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27:3354-3362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Penn I. De novo malignances in pediatric organ transplant recipients. Pediatr Transplant. 1998;2:56-63. [PubMed] |
| 3. | Smets F, Vajro P, Cornu G, Reding R, Otte JB, Sokal E. Indications and results of chemotherapy in children with posttransplant lymphoproliferative disease after liver transplantation. Transplantation. 2000;69:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Walker RC, Paya CV, Marshall WF, Strickler JG, Wiesner RH, Velosa JA, Habermann TM, Daly RC, McGregor CG. Pretransplantation seronegative Epstein-Barr virus status is the primary risk factor for posttransplantation lymphoproliferative disorder in adult heart, lung, and other solid organ transplantations. J Heart Lung Transplant. 1995;14:214-221. [PubMed] |
| 5. | Leblond V, Dhedin N, Mamzer Bruneel MF, Choquet S, Hermine O, Porcher R, Nguyen Quoc S, Davi F, Charlotte F, Dorent R. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19:772-778. [PubMed] |
| 6. | Shroff R, Rees L. The post-transplant lymphoproliferative disorder-a literature review. Pediatr Nephrol. 2004;19:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 8. | Kalogeropoulos IV, Chalazonitis AN, Tsolaki S, Laspas F, Ptohis N, Neofytou I, Rontogianni D. A case of primary isolated non-Hodgkin’s lymphoma of the esophagus in an immunocompetent patient. World J Gastroenterol. 2009;15:1901-1903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Sebire NJ, Malone M, Risdon RA, Ramsay AD. Epstein-Barr virus-associated lymphoproliferative disorder presenting as apparently isolated gastrointestinal lesions in childhood. Pediatr Dev Pathol. 2005;8:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Cohen JI. Epstein-Barr virus lymphoproliferative disease associated with acquired immunodeficiency. Medicine (Baltimore). 1991;70:137-160. [PubMed] |
| 11. | Herida M, Mary-Krause M, Kaphan R, Cadranel J, Poizot-Martin I, Rabaud C, Plaisance N, Tissot-Dupont H, Boue F, Lang JM. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21:3447-3453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Shitrit D, Shitrit AB, Dickman R, Sahar G, Saute M, Kramer MR. Gastrointestinal involvement of posttransplant lymphoproliferative disorder in lung transplant recipients: report of a case. Dis Colon Rectum. 2005;48:2144-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Basseri RJ, Cole J, Jamil LH. Primary esophageal B-cell lymphoma. Clin Gastroenterol Hepatol. 2011;9:e23-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | O'Connor JA, Cogley C, Burton M, Lancaster-Weiss K, Cordle RA. Posttransplantation lymphoproliferative disorder: endoscopic findings. J Pediatr Gastroenterol Nutr. 2000;31:458-461. [PubMed] |
| 15. | Dharnidharka VR, Sullivan EK, Stablein DM, Tejani AH, Harmon WE. Risk factors for posttransplant lymphoproliferative disorder (PTLD) in pediatric kidney transplantation: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Transplantation. 2001;71:1065-1068. [PubMed] |
| 16. | Newell KA, Alonso EM, Kelly SM, Rubin CM, Thistlethwaite JR, Whitington PF. Association between liver transplantation for Langerhans cell histiocytosis, rejection, and development of posttransplant lymphoproliferative disease in children. J Pediatr. 1997;131:98-104. [PubMed] |
| 17. | Shroff R, Trompeter R, Cubitt D, Thaker U, Rees L. Epstein-Barr virus monitoring in paediatric renal transplant recipients. Pediatr Nephrol. 2002;17:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |