Published online Nov 15, 2012. doi: 10.4251/wjgo.v4.i11.216
Revised: September 4, 2012
Accepted: October 20, 2012
Published online: November 15, 2012
AIM: To review a single institutional experience in clinical management of gastrointestinal stromal tumors (GIST) and analyze for factors determining treatment outcome.
METHODS: Clinicopathological data of patients with a diagnosis of GIST who were treated at our institute during November 2004 to September 2009 were retrospectively reviewed.
RESULTS: Ninety-nine cases were included in the analysis. Primary tumor sites were at the stomach in and small bowel in 44% and 33%, respectively. Thirty-one cases already had metastasis at presentation and the most common metastatic site was the liver. Sixty-four cases (65%) were in the high-risk category. Surgical treatment was performed in 77 cases (78%), 3 of whom received upfront targeted therapy. Complete resection was achieved in 56 cases (73% of operative cases) and of whom 27 developed local recurrence or distant metastasis at a median duration of 2 years. Imatinib was given as a primary therapy in unresectable cases (25 cases) and as an adjuvant in cases with residual tumor (21 cases). Targeted therapy gave partial response in 7 cases (15%), stable disease in 27 cases (57%) and progressive disease in 13 cases (28%). Four-year overall survival was 74% (95% CI: 61%-83%). Univariate survival analysis found that low-risk tumor, gastric site, complete resection and response to imatinib were associated with better survival.
CONCLUSION: The overall outcomes of GIST can be predicted by risk-categorization. Surgery alone may not be a curative treatment for GIST. Response to targeted therapy is a crucial survival determinant in these patients.
- Citation: Pornsuksiri K, Chewatanakornkul S, Kanngurn S, Maneechay W, Chaiyapan W, Sangkhathat S. Clinical outcomes of gastrointestinal stromal tumor in southern Thailand. World J Gastrointest Oncol 2012; 4(11): 216-222
- URL: https://www.wjgnet.com/1948-5204/full/v4/i11/216.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v4.i11.216
Gastrointestinal stromal tumors (GISTs) are relatively uncommon mesenchymal neoplasms arising primarily in the wall of the stomach, small intestine, and colon, and other sites within the abdomen[1]. Although GIST comprises only 0.2% of all gastrointestinal tumors, it is the most common mesenchymal tumor, accounting for 80% of gastrointestinal tract sarcomas. Recent studies have found incidence rates of GISTs of 10-20, 7-15, and 14 cases/million per year in the United States[2], Europe[3], and Taiwan[4], respectively. The primary tumor is most commonly located in the stomach (50%-65%) or small intestine (25%-30%), however it has also been reported in the colon, esophagus and a number of extra-gastrointestinal sites[5-7].
Surgery is the mainstay treatment with a curative aim for localized GISTs without metastasis. Previous studies have found five-year disease free survival in primary GISTs in whom complete surgical resection could be achieved to be 65%[8,9], although another study found recurrent disease in a number of cases after complete surgical resection at a median time of 20 mo[9]. In primarily unresectable or metastatic disease, the current first line treatment is a tyrosine kinase inhibitor, imatinib mesylate. Such molecular targeted therapy gives a varying response rate depending on the tumor location, histological risk stratification and mutation status of the receptor tyrosine kinase KIT. In general, symptomatic GIST cases who were in the high risk group have shown poorer disease free survival rates even when complete surgical resection could be achieved[9-12]. Other than histopathological criteria, risk determinants for disease specific survival include non-gastric primary location, macroscopic residual tumor and tumor rupture. In unresectable cases, various studies have found that factors determining response were primarily biological characteristics, including a high mitotic index and KIT mutation status[13,14].
This study aimed to review the clinical presentations, pathological characteristics and treatment outcomes of GIST cases in a university hospital setting in Southern Thailand, analyzed for factors effecting treatment outcomes.
The study was approved by the Institutional Ethic Committee of the Faculty of Medicine, Prince of Songkla University. A list of patients with the pathological diagnosis of GIST during November 2004 to September 2009 was obtained from the Department of Pathology and the Tumor Registry Unit of our institution, Songklanagarind Hospital. Details on sociodemographic and clinical data, pathological and laboratory findings, and treatment were retrieved from the hospital information system. A diagnosis of GIST was based on a histopathological appearance that was compatible with GIST (spindle or epithelioid cell type) and was confirmed by positive immunohistochemical staining for CD117. Patients who were referred to our institute after a diagnosis was made were included only if the pathological slides were available for review. Patients without adequate follow-up were excluded from this review.
The morphological characteristics of the tumors were evaluated according to the risk stratification criteria of the National Institutes of Health (NIH) consensus (Fletcher’s criteria 2002)[10], which classifies GISTs into very low, low, intermediate, and high risk categories. Our treatment usually began with surgical removal of the tumor if possible. In cases with unresectable tumor or distant metastasis, treatment began with a daily dose of 400 milligrams of imatinib, a tyrosine kinase inhibitor. Response to the treatment was evaluated and assessed by a radiologist, beginning at 12 mo after treatment initiation, based on the Response Evaluation Criteria In Solid Tumors (RECIST) method[15]. Long-term treatment outcomes included overall survival (OS) and progress free survival (PFS) with recurrence, progressive disease and death set as sensors for the PFS analysis.
The mutation status of the tumors was analyzed in cases in which a specimen was available. For analysis, tumor DNA was extracted from formalin-fixed paraffin embedded tissue using a DNeasy Blood and Tissue Kit (Qiagen). The mutation study covered exons 9 and 11 of KIT. The studies used polymerase chain reaction and direct nucleotide sequencing method.
Descriptive statistics were used to describe the baseline characteristics and clinical information of each patient. Univariate survival analysis used the Log-rank test and a stepwise Cox proportional hazard analysis was used for multivariate survival analysis. The statistical significance of each variable was tested by a log-likelihood ratio of successive models at a P value < 0.05. All analysis was done using the Stata version 6.0 program (Stata Corporation, TX).
From November 2004 to September 2009, 100 patients were diagnosed with GIST. One patient was excluded due to being lost to follow up before receiving any treatment, leaving 99 cases in the analysis. Patients who were referred after initial diagnosis accounted for 51% of the total. Gender distribution was 55 male: 44 female or 1.25:1. The median age at diagnosis was 58 years (range 10-82 years). The only case of pediatric GIST was a girl who presented at the age of 10 years. Almost all patients (87%) were symptomatic and about half (57%) presented with an abdominal mass. Twenty-six patients (26%) came with gastrointestinal bleeding, 2 had gut obstruction and 2 had intestinal perforation.
The most common primary tumor sites were the stomach (43 cases, 44%) and small bowel (33 cases, 33%). The other sites were the rectum (5 cases), omentum (2 cases), retroperitoneal (3 cases) and unknown primary (13 cases). Thirty-one cases already had metastasis at presentation and the most common metastatic site was the liver. When the NIH risk criteria was used to categorize the cases, 65% of the patients were in the high risk group, with 17%, 12% and 6% in the intermediate, low and very low risk groups, respectively. On histopathology, 98% of cases were positive for CD117 immunohistochemistry, positive staining for CD34 was 79%, smooth muscle actin 30%, S100 24% and desmin 9%. Mutations of KIT were studied in 35 cases whose specimens were available. The study detected KIT mutations in 19 cases; 17 in exon11 and 2 in exon9.
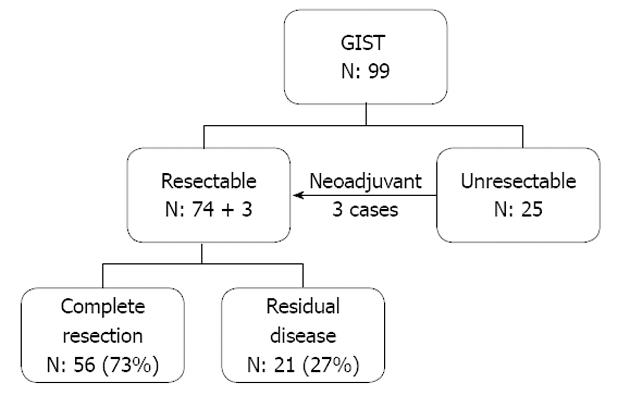
The seventy-seven cases who underwent surgical treatment included 74 cases who had primary surgery and 3 cases who received upfront tyrosine kinase inhibitor therapy prior to their operation (Figure 1). Fifty-six cases in this group (73% of operative cases) achieved complete resection. About half of these 56 (29 cases) were in the high risk group according to the NIH risk classification. Seven of the patients who had a complete resection later developed local recurrence, and 14 distant metastases. Twenty of these 21 cases were in the high risk category and the median time to recurrence was 23.3 mo. In the 25 unresectable cases, 16 cases (64%) originally presented with metastasis, all of which were categorized as high risk according to the NIH risk classification. In the 9 of these cases without metastasis, the main reason for unresectability was structure involvement.
We achieved complete resection in the majority of gastric GIST cases (70%), the complete resection rate was 46% in extra-gastric tumors (Table 1).
| Primary sites | No. of cases | Complete resection | Residual disease | Unresectable |
| Stomach | 43 | 30 (70) | 9 (20) | 4 (9) |
| Small bowel | 33 | 20 (61) | 6 (18) | 7 (21) |
| Rectum | 5 | 2 (40) | 1 (20) | 2 (40) |
| Extra-gastrointestinal | 18 | 4 (22) | 5 (28) | 9 (50) |
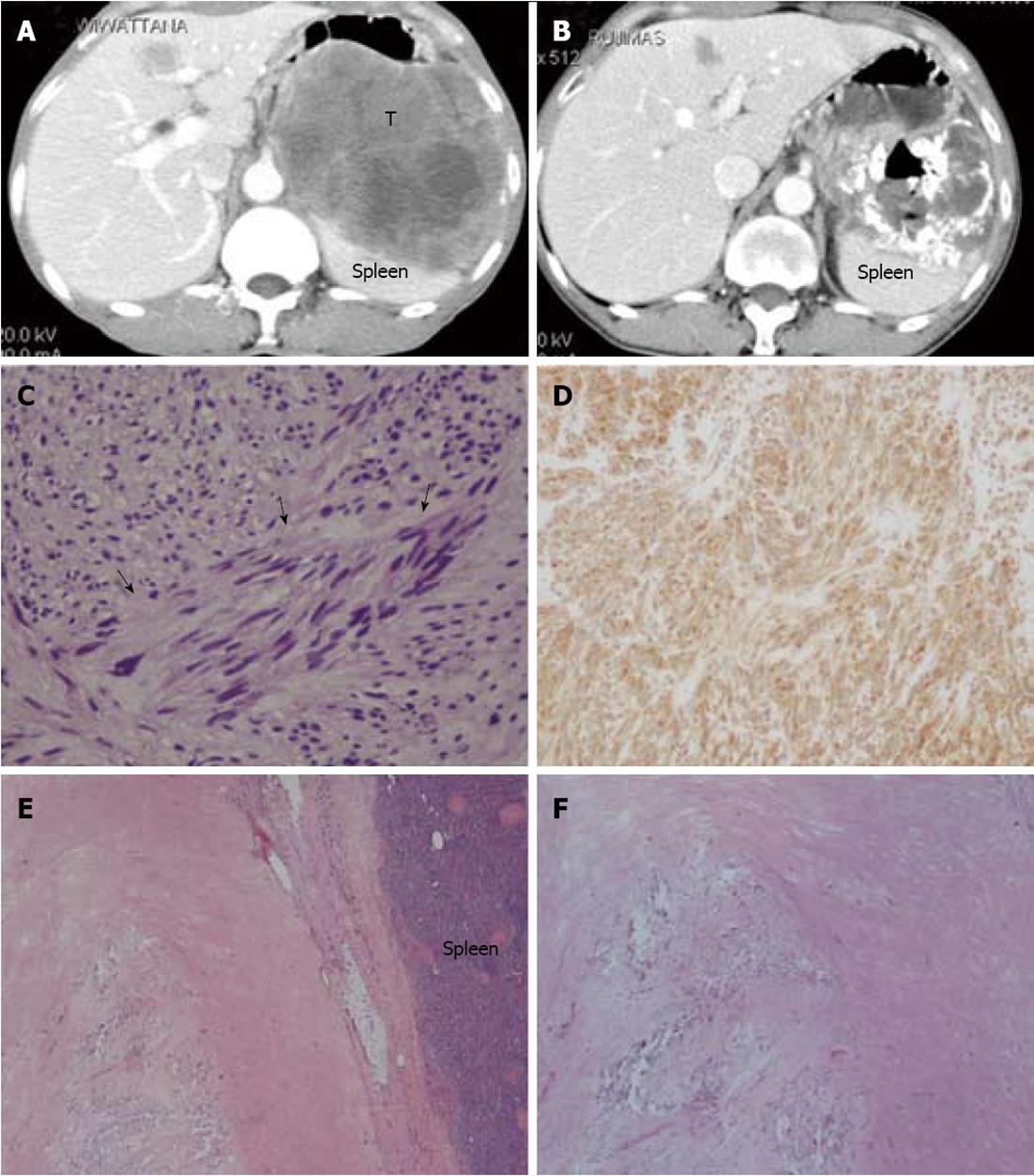
Tyrosine kinase inhibitor therapy was given to our patients when they had an unresectable tumor, residual disease, or recurrence after primary surgical resection. According to the RECIST, of the 47 patients who received tyrosine kinase inhibitor therapy, 7 cases (15%) had partial response, 27 cases (57%) had stable disease, and 13 cases (28%) had progressive disease. Of the 3 cases in which surgical exploration was performed after targeted therapy and the radiologic diagnosis scored stable disease or partial response, one achieved a complete pathological response (Figure 2). Adverse reactions were recorded in 18 cases (38%). The three most common adverse reactions were edema (6 cases, 13%), anemia (5 cases, 11%) and skin rash (3 cases, 6%)
When the primary tumor site was considered, 4 cases (31%) of gastric GIST achieved a partial response, which was significantly higher than in the other sites (P = 0.047) (Table 2).
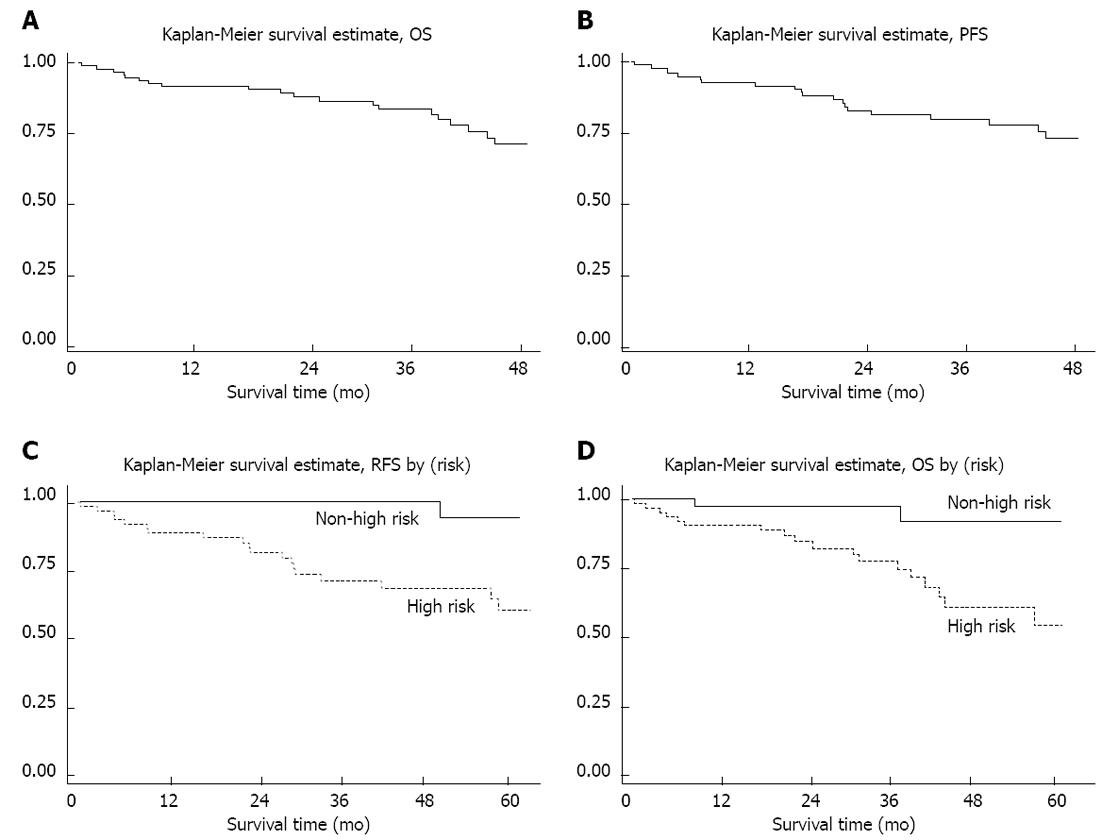
Until the preparation of this manuscript in September 2011, the mean follow-up period was 49 mo. The four-year overall OS and PFS rates (Figure 3) were 74 % (95% CI: 61%-83%) and 72 % (95% CI: 59%-82%), respectively.
On univariate analysis, presence of liver metastasis, presence of residual disease or unresectability, high risk disease, non-gastric primary site, presence of liver metastasis and unresponsiveness to targeted therapy were factors that were significantly associated with poorer OS (Table 3). High risk disease, unresponsiveness to targeted therapy and gastric GIST had significantly poorer PFS. High risk categorization reduced 4-year PFS from 95% in other risk groups to 61 (P < 0.01). Gastric GIST had a 4-year PFS of 89%, compared to 63% in other primary sites (P = 0.04). On multivariate analysis, the NIH risk category was the only factor that most fit the Cox regression model at the hazard ratio of 6.12 (95% CI 1.4-26.4)
| Four-year OS (%) | P value | |
| OS (99 cases) | 72.4 | |
| Primary tumor site | 0.02 | |
| Stomach | 86.5 | |
| Non-stomach | 64.5 | |
| Risk category1 | < 0.01 | |
| Non-high risk (35 cases) | 92 | |
| High risk (64 cases) | 60.9 | |
| Liver metastasis at diagnosis | < 0.01 | |
| Absent (72 cases) | 86.3 | |
| Present (27 cases) | 44.6 | |
| Residual disease after surgery | 0.04 | |
| Absent (56 cases) | 84.1 | |
| Present (21 cases) | 61.5 | |
| Response to targeted therapy | 0.03 | |
| CR + PR (8 cases) | 88.9 | |
| SD + PD (40 cases) | 64.2 |
Considering cases who were primarily resectable, the 4-year recurrent free survival (RFS) was 76.5%. 4-year RFS in cases with high NIH risk (94.1%) was also significantly better than those in other risk categories (62.9%) (P < 0.01) (Figure 3C).
It has only been since around the year 2000 that the term GIST began to appear in pathological reports in our institute. The diagnosis became more common in the following years, possibly due to increasing awareness of this diagnosis of both the pathologists and the clinicians. In general, the tumor is defined as a mesenchymal neoplasm arising in the gastrointestinal tract and expressing KIT (CD117)[16]. The mainstay treatment for a GIST is surgical removal. The five-year survival rate after complete surgical resection was reported at 48%-79%[17]. In situations where complete resection is not possible, tyrosine kinase targeted therapy is the current treatment of choice.
In our study, a majority of our patients were symptomatic cases that belonged to the high risk category. At presentation, 25% of the cases were considered unresectable, either due to anatomical difficulty or presence of distant metastasis. Surgery was performed in 78% and complete resection was achieved in 73% in cases who underwent surgical exploration. However, we found that 48% of the patients who achieved complete tumor removal developed local recurrence or distant metastasis at a median duration of 2 years. This figure was consistent with previous studies that also experienced a medium-term recurrence after the surgical treatment alone[18-20]. Our analysis showed that almost all these failure cases were in the original high risk category, according to the NIH criteria, which raises a question concerning the role of postoperative adjuvant treatment in the high risk patient. Two recent studies have suggested that adjuvant imatinib therapy may improve RFS after the resection of a primary gastrointestinal stromal tumor[21-23], although these studies had only a limited follow-up duration, thus the findings are still not confirmed.
The GIST is not a chemosensitive tumor. Nevertheless, small molecule targeting the specific tyrosine kinase is an effective adjuvant treatment and is a prototype of targeted therapy in human neoplasms. Imatinib mesylate is a compound known to be active against BCR-ABL, KIT receptors and platelet-derived growth factor receptor-α[23]. Imatinib clearly has a role in unresectable GISTs and also resectable GISTs with residual disease after surgery. A number of studies examining the efficacy of imatinib in advanced GISTs found that it gave 5% complete response, 45%-65% partial response and 18%-32% stable disease[24-26]. The 15% partial response and 56% stable disease rates in our patients were relatively low. However, the 4-year OS of 74% in our patients was compatible with other major studies in the post-imatinib era[19,27,28]. We had 3 patients in whom upfront imatinib converted the tumor from unresectable to removable. As mentioned earlier, one of these cases had pathologically complete remission and 2 cases had tumor shrinkage, to an extent that then allowed their complete removal, suggesting a positive role for this drug in pre-operative down-staging of GISTs. In addition, our study found that gastric GISTs responded to the treatment better than other sites, which may explain the better prognosis of GISTs in this location[29]. A recent multi-institutional trial suggested that extension of imatinib treatment duration to 36 mo significantly improved RFS for operable GISTs[30].
On survival analysis, the study found associations between certain clinical parameters and survival, including gastric site, risk categorization and treatment factors. An excellent outcome could be expected if complete resection could be achieved. Up to 95% 3-year OS was observed in cases with complete tumor removal. In cases that could not have their tumor removed in the first place, disease control depended solely on the response to targeted therapy. Unresectable cases which imatinib failed to control the tumor growth had an average 3-year OS of less than 60%, compared to 80% in those who achieved at least stable disease status. Although the resectability and targeted therapy response were crucial outcome determinants, analysis of the whole series showed that the NIH risk categorization was an independent factor that predicted survival probability in our patients. On average, patients who were not in the high-risk group had more than 90% survival probability. This could be partly at least explained by noting that high risk patients were less likely to have a complete tumor removal, as tumor size is one parameter that determines risk in the NIH risk consensus.
In conclusion, our study examined the treatment outcomes of GISTs over a 5-year period in a teaching hospital in southern Thailand. The study found that the outcomes were mainly determined by tumor resectability and response to targeted therapy and that NIH risk categorization could predict the overall prognosis.
The authors thank the Cancer Registry Unit, Songklanagarind Hospital for the survival status data. Dave Patterson edited English language in the manuscripts.
Gastrointestinal stromal tumors (GISTs) are relatively uncommon mesenchymal neoplasms arising primarily in the wall of the stomach, small intestine, and colon, and other sites within the abdomen. Surgery is the mainstay treatment with a curative aim for localized GISTs without metastasis. In primarily unresectable or metastatic disease, the current first line treatment is a tyrosine kinase inhibitor, imatinib mesylate. Such molecular targeted therapy gives a varying response rate depending on the tumor location, histological risk stratification and mutation status of the receptor tyrosine kinase KIT.
This study aimed to review the clinical presentations, pathological characteristics and treatment outcomes of GIST cases in a university hospital setting in Southern Thailand, analyzed for factors effecting treatment outcomes.
On survival analysis, the study found associations between certain clinical parameters and survival, including gastric site, risk categorization and treatment factors. An excellent outcome could be expected if complete resection could be achieved. Up to 95% 3-year overall survival (OS) was observed in cases with complete tumor removal. In cases that could not have their tumor removed in the first place, disease control depended solely on the response to targeted therapy. Although the resectability and targeted therapy response were crucial outcome determinants, analysis of the whole series showed that the NIH risk categorization was an independent factor that predicted survival probability in our patients.
GIST is one of the most common mesenchymal tumors of the gastrointestinal tract (1%-3% of all gastrointestinal malignancies). They are defined as tumors whose behavior is driven by mutations in the Kit gene or PDGFRA gene, and may or may not stain positively for Kit.
This is a retrospective study of GISTs in a single institution in Thailand. Despite the lack of novelty, the manuscript was scientifically well written. It is important to share clinical data on GISTs worldwide and to clarify nation-specific trends of this rare disease. Thus, the reviewer thinks this case series study from Thailand is valuable and potentially worth publishing.
Peer reviewers: Sung-Soo Park, MD, PhD, Department of Surgery, Korea University Anam Hospital, Anam-dong 5-ga Seongbuk-gu, Seoul 136-705, South Korea; Tatsuo Kanda, MD, PhD, Division of Digestive and General Surgery, Graduate School of Medical and Dental Sciences, Niigata University, Niigata City 951-8510, Japan
S- Editor Wang JL L- Editor A E- Editor Xiong L
| 1. | Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002;160:1567-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 323] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1177] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 3. | Mucciarini C, Rossi G, Bertolini F, Valli R, Cirilli C, Rashid I, Marcheselli L, Luppi G, Federico M. Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. BMC Cancer. 2007;7:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Tzen CY, Wang JH, Huang YJ, Wang MN, Lin PC, Lai GL, Wu CY, Tzen CY. Incidence of gastrointestinal stromal tumor: a retrospective study based on immunohistochemical and mutational analyses. Dig Dis Sci. 2007;52:792-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Liegl B, Hornick JL, Lazar AJ. Contemporary pathology of gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009;23:49-68, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, Corless CL, Debiec-Rychter M, DeMatteo RP, Ettinger DS. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5 Suppl 2:S1-29; quiz S30. [PubMed] |
| 7. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. [PubMed] |
| 8. | Roberts PJ, Eisenberg B. Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. Eur J Cancer. 2002;38 Suppl 5:S37-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Hassan I, You YN, Shyyan R, Dozois EJ, Smyrk TC, Okuno SH, Schleck CD, Hodge DO, Donohue JH. Surgically managed gastrointestinal stromal tumors: a comparative and prognostic analysis. Ann Surg Oncol. 2008;15:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 11. | Rutkowski P, Debiec-Rychter M, Nowecki ZI, Wozniak A, Michej W, Limon J, Siedlecki JA, Jerzak Vel Dobosz A, Grzesiakowska U, Nasierowska-Guttmejer A. Different factors are responsible for predicting relapses after primary tumors resection and for imatinib treatment outcomes in gastrointestinal stromal tumors. Med Sci Monit. 2007;13:CR515-CR522. [PubMed] |
| 12. | Rutkowski P, Nowecki ZI, Michej W, Debiec-Rychter M, Woźniak A, Limon J, Siedlecki J, Grzesiakowska U, Kakol M, Osuch C. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007;14:2018-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Högenauer C, Langner C, Lipp RW, Höfler G, Krejs GJ, Hinterleitner TA. Complete remission of a metastatic gastrointestinal stromal tumour with the tyrosine kinase inhibitor imatinib (STI 571): effect of low dosage in an advanced tumour with exon 11 mutation. Eur J Gastroenterol Hepatol. 2003;15:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Rutkowski P, Nowecki ZI, Debiec-Rychter M, Grzesiakowska U, Michej W, Woźniak A, Siedlecki JA, Limon J, vel Dobosz AJ, Kakol M. Predictive factors for long-term effects of imatinib therapy in patients with inoperable/metastatic CD117(+) gastrointestinal stromal tumors (GISTs). J Cancer Res Clin Oncol. 2007;133:589-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13076] [Article Influence: 523.0] [Reference Citation Analysis (0)] |
| 16. | Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Kosmadakis N, Visvardis EE, Kartsaklis P, Tsimara M, Chatziantoniou A, Panopoulos I, Erato P, Capsambelis P. The role of surgery in the management of gastrointestinal stromal tumors (GISTs) in the era of imatinib mesylate effectiveness. Surg Oncol. 2005;14:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 875] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 19. | Wu TJ, Lee LY, Yeh CN, Wu PY, Chao TC, Hwang TL, Jan YY, Chen MF. Surgical treatment and prognostic analysis for gastrointestinal stromal tumors (GISTs) of the small intestine: before the era of imatinib mesylate. BMC Gastroenterol. 2006;6:29. [PubMed] |
| 20. | Nikfarjam M, Kimchi E, Shereef S, Gusani NJ, Jiang Y, Liang J, Sehmbey M, Staveley-O'Carroll KF. Surgical outcomes of patients with gastrointestinal stromal tumors in the era of targeted drug therapy. J Gastrointest Surg. 2008;12:2023-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K, American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 970] [Article Influence: 60.6] [Reference Citation Analysis (1)] |
| 22. | Kanda T, Nishida T, Wada N, Kobayashi O, Yamamoto M, Sawaki A, Boku N, Koseki M, Doi T, Toh Y. Adjuvant therapy with imatinib mesylate after resection of primary high-risk gastrointestinal stromal tumors in Japanese patients. Int J Clin Oncol. 2011;Nov 23 [Epub ahead of print]. [PubMed] |
| 23. | Benjamin RS, Blanke CD, Blay JY, Bonvalot S, Eisenberg B. Management of gastrointestinal stromal tumors in the imatinib era: selected case studies. Oncologist. 2006;11:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3203] [Cited by in RCA: 3110] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 25. | Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1270] [Cited by in RCA: 1205] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 26. | Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki RG. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 768] [Article Influence: 45.2] [Reference Citation Analysis (1)] |
| 27. | Takahashi T, Nakajima K, Nishitani A, Souma Y, Hirota S, Sawa Y, Nishida T. An enhanced risk-group stratification system for more practical prognostication of clinically malignant gastrointestinal stromal tumors. Int J Clin Oncol. 2007;12:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Cao H, Zhang Y, Wang M, Shen DP, Sheng ZY, Ni XZ, Wu ZY, Liu Q, Shen YY, Song YY. Prognostic analysis of patients with gastrointestinal stromal tumors: a single unit experience with surgical treatment of primary disease. Chin Med J (Engl). 2010;123:131-136. [PubMed] |
| 29. | Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. 2008;112:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 30. | Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 682] [Article Influence: 52.5] [Reference Citation Analysis (0)] |