Published online Aug 15, 2011. doi: 10.4251/wjgo.v3.i8.123
Revised: July 25, 2011
Accepted: August 1, 2011
Published online: August 15, 2011
Colorectal cancer is a common cancer and the fourth leading cause of death in Korea. The incidence and mortality of colorectal cancer varies according to risk factors, such as age, family history, genetic history, food habits, and physical activities. Some studies have focused on the association between vitamin D and colorectal cancer. Today, there is growing evidence that high vitamin D intake and a plasma level of 25(OH)D3 reduce the incidence of colorectal cancer by modifying cancer angiogenesis, cell apoptosis, differentiation, and proliferation. Taken together, these results suggest that vitamin D supplementation alone, or in combination with anti-cancer agents, might reduce the incidence of colorectal cancer. In this review, we discuss the function and mechanism of vitamin D including the effect of vitamin D on colorectal cancer.
- Citation: Kang W, Lee S, Jeon E, Yun YR, Kim KH, Jang JH. Emerging role of vitamin D in colorectal cancer. World J Gastrointest Oncol 2011; 3(8): 123-127
- URL: https://www.wjgnet.com/1948-5204/full/v3/i8/123.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v3.i8.123
Cancer of the colon or rectum is called colorectal cancer. It is a common cancer and the fourth leading cause of cancer-related death in the world. Colorectal cancer is also the third most common cancer in men and the second in women worldwide. It counted for an estimated, 1.2 million new cases and 0.6 million deaths in 2008[1]. In addition, colorectal cancer is the second leading cause of cancer death in the United States. Colorectal cancer incidence rates are rapidly increasing in several historically low risk areas including Spain, and a number of countries within Eastern Asia and Eastern Europe[2]. The risk factors for colorectal cancer are age, family history, and lifestyle, such as food habits and physical activities.
Vitamin D is associated with bone growth. Vitamin D insufficiency causes abnormal bone growth, while sufficiency prevents rickets and osteomalacia, as well as osteoporosis. Vitamin D enhances the immune system[3]. In addition, vitamin D is known to prevent cancer[4] and cardiovascular diseases[5]. Vitamin D and its analogues have the ability to prevent cancer in vitro and in animal models. However, the anti-cancer effect of dietary vitamin D remains controversial. Recently, a study reported that vitamin D is not effective in preventing cancer and cardiovascular diseases[6]. Of vitamin D forms, 1,25(OH)2D3 (calciferol) induces biological effects by binding to the vitamin D receptor (VDR). The activation of VDR leads to the maintenance of calcium and phosphorus levels in the blood as well as of bone content. VDR is also involved in cell proliferation and differentiation.
For more than 20 years, epidemiological, experimental and clinical studies have shown that vitamin D has significant protective effect against the development of cancer[4]. The mechanism of vitamin D works through several molecular pathways, such as growth-factor signaling, and transforming growth factor-β-SMAD signaling. The anti-cancer activities of vitamin D exerted by 1,25(OH)2D3 are produced by regulating the cell cycle, apoptosis, and adhesion, as well as by cellular differentiation and proliferation. Interestingly, vitamin D reduces the incidence of colorectal cancer, when vitamin D intake, the plasma level of 25(OH)D3 and UV exposure is particularly high. In addition, the combination of vitamin D with other anti-cancer agents efficiently controls the development of colorectal cancer growth[7].
Here, the function and mechanism of vitamin D is briefly introduced, and the beneficial effect of vitamin D on colorectal cancer is discussed.
The molecular pathogenesis of colorectal cancer has been one of the most prominent study areas in recent years. Colorectal cancer exhibits two major forms: sporadic colorectal cancer and inherited colorectal cancer.
First, sporadic cancer occurs in people who have no family history or very little of the disease. Although cancer sometimes has a hereditary or familial component, it is not common. Approximately 70%-75% of colorectal cancer is sporadic cancer. Second, inherited colorectal cancer comprises familial and hereditary cancer. Familial and hereditary cancer cancer occurs in families who have a faulty gene inherited from the father or mother. Generally, 5% of colorectal cancer is familial cancer. Familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer are the two forms of inherited colorectal cancer[8].
Ten to thirty percent of cases are attributed to familial risk and the rest to sporadic cancer. The majority of cancers are considered to be sporadic cancer. As stated above, most colorectal cancers are sporadic cancer, and only 5%-10% are inherited cancers.
Vitamin D exerts its various functions through molecular pathways. Vitamin D pathways are highly complex. The factors, affecting the vitamin D pathway, are P21, P27, CDKs, P53, BRCA-1.-2, ß-catenin and c-myc. Depending on which factor, vitamin D is involved in cell adhesion, apoptosis, differentiation and division[9]. Primarily, vitamin D plays an important role in muscle and bone health. Vitamin D deficiency results in impaired bone mineralization and leads to bone softening diseases including rickets and osteomalacia[10]. Further, vitamin D deficiency is involved in high bone turnover[11]. Vitamin D deficiency can also play a role in the pathogenesis of auto-immune diseases such as multiple sclerosis, diabetes type 1, cancer[12] and cardiovascular disease[13]. Conversely, vitamin D deficiency increases parathyroid hormone levels leading to mobilization of calcium from bone, thereby compromising bone development in the adolescent[14]. In contrast, vitamin D supplementation enhances bone density[15]. Next, vitamin D exerts an anti-cancer activity[16]. These activities of vitamin D functions are regulated by circulating vitamin D forms, the increasing concentration of 25(OH)D3 and increasing activity of 1,25(OH)2D3. Vitamin D induces cellular proliferation, differentiation, and apoptosis of cancer and normal cells through the regulatory mechanism[17-19]. These studies show that low intake levels of vitamin D increase the risk of colorectal cancer. Some studies show vitamin D exerts growth-restraining, anti-carcinogenic effects on colorectal cancer[20,21]. In addition, vitamin D affects growth factors, regulation of cell division, cytokine synthesis, signaling, cell cycle control, and apoptosis pathway[5,22]. In a study in vitro, a similar result was reported. When LOVO cells were treated for 8 d with various concentrations of 1,25(OH)2D3, cell proliferation was inhibited significantly[23].
Table 1 summarizes the anti-cancer effects of vitamin D in vivo in mice and rats.
| Treatment | Inducer | Species | Results | Ref. |
| Supplement | VDR knockout mouse | Inhibition of hyperproliferation and adenoma formation | [24] | |
| Supplement | Apc1638 mice | Inhibition of carcinoma incidence | [25] | |
| Deficiency | Balb/C mice | Enhancement of cancer cell growth | [26] | |
| Supplement | Balb/C mice | Inhibition of tumor growth | [27] | |
| Supplement | C57BL/6J mice | Inhibition of hyperproliferation | [28] | |
| Inhibition of tumor incidence | ||||
| Deficiency | DMH | SD rat | No effect | [29] |
| Deficiency | SD rat | Enhancement of carcinogenesis | [30] | |
| Supplement | DMH | Fisher344 rat | No effect | [31] |
| Supplement | DMH | Fisher344 rat | Inhibition of tumor incidence | [32] |
Vitamin D and its metabolites reduce the incidence of various cancers by inhibiting cancer angiogenesis, stimulating normal cells[33-37] and also by promoting the inhibition of proliferation. Vitamin D metabolites also help to maintain a standard calcium gradient in the various colonic epithelial cells. High levels of blood serum 25(OH)D3 are associated with a noticeable decrease in proliferation of non-cancerous cells[38,39]. The anti-proliferative effect of vitamin D is attained by inducing G1 cell-cycle arrest, which is probably mediated by up-regulation of cell cycle inhibitors. Vitamin D modulates the activation of these cell cycle related genes by various mechanisms. Vitamin D also exerts anti-carcinogenic effects by interfering with the synthesis of growth factors and cytokines and by modulating their signaling pathways. In addition to the growth inhibitory effects, vitamin D induces the differentiation of colon cancer cells. The 1,25(OH)2D3 and its analogs exert anti-carcinogenic activities in human colon cancer cells by inhibition of proliferation and induction of differentiation and apoptosis[22]. The 1,25(OH)2D3 significantly increases the expression and activity of alkaline phosphatase, a marker of colonic differentiation. VDR activation by 1,25(OH)2D3 produces changes in stick junction integrity, increases differentiation and reduces oncogenic cell signaling. Induction of these genes affects cell oncogenesis, and tissue development. Thus, treatment with 1,25(OH)2D3 supresses oncogenic genes in colon cancer cells. Finally, VDR genotypes are associated with anti-cancer activity in colorectal cancer. There are several VDR genotypes. For example, the most important VDR genotype is Bsm I, which has 3 variants: BB, Bb, and bb in America. The bb genotype is associated with lower concentrations of circulating 1,25(OH)2D3, leading to an increased incidence of colorectal cancer[40,41]. Taken together, these observations demonstrate that vitamin D exerts anti-cancer activity in colon cancer.
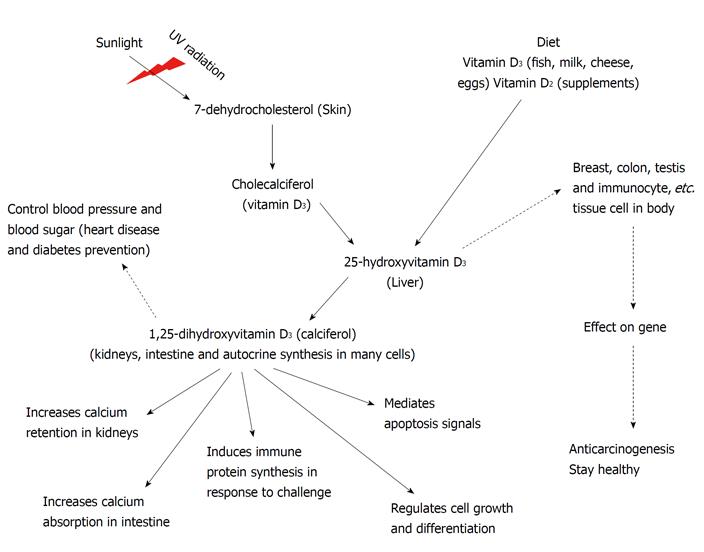
Figure 1 describes the mechanisms of vitamin D in various tissues. In the figure, the dotted arrow shows a newly discovered function of vitamin D.
Previous research has shown the efficacy of taking vitamin D for reducing cancer risk[42]. There is strong evidence that vitamin D can change and inhibit the development of colon cancers[22]. These protective effects are likely due to the regulatory effects of 1,25-dihydroxyvitamin D3 (calciferol) on cellular mechanisms involved in cancer development, including apoptosis, cell adhesion, cell cycle control, regulation of cellular differentiation and proliferation. A clinical study group will set up guidelines for vitamin D intake and develop models to define levels of serum 25(OH)D3 that prevent the growth of cancer. Elevation of vitamin D levels may protect against diverse cancers. Many studies show that vitamin D assists in prevention and therapy of cancer[9]. The new guidelines will lead to more effective physical condition policies, resulting in substantially fewer cases of cancer of the colon in the future[5].
Peer reviewers: Runjan Chetty, Professor, Department of Pathology and Gene Regulation, University of Glasgow, Western Infirmary (Pathology), Dumbarton Road, Glasgow, G11 6NT, Scotland, United Kingdom; Ioannis A Voutsadakis, MD, PhD, Department of Medical Oncology, University Hospital of Larissa, Larissa 41110, Greece
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7953] [Cited by in RCA: 8101] [Article Influence: 506.3] [Reference Citation Analysis (2)] |
| 2. | Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 865] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 3. | Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832-2838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1249] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 4. | Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin. 2008;24:139-149. [PubMed] |
| 5. | Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:1179-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer--ready for prime time? N Engl J Med. 2011;364:1385-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Cross HS, Nittke T, Peterlik M. Modulation of vitamin D synthesis and catabolism in colorectal mucosa: a new target for cancer prevention. Anticancer Res. 2009;29:3705-3712. [PubMed] |
| 8. | Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1426] [Cited by in RCA: 1370] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 9. | Bulathsinghala P, Syrigos KN, Saif MW. Role of vitamin d in the prevention of pancreatic cancer. J Nutr Metab. 2010;2010:721365. [PubMed] |
| 10. | Ebeling PR. Megadose therapy for vitamin D deficiency. Med J Aust. 2005;183:4-5. [PubMed] |
| 11. | Diamond TH, Levy S, Smith A, Day P. High bone turnover in Muslim women with vitamin D deficiency. Med J Aust. 2002;177:139-141. [PubMed] |
| 12. | Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 782] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 13. | Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 278] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Harkness L, Cromer B. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int. 2005;16:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Vitamin D and adult bone health in Australia and New Zealand: a position statement. Med J Aust. 2005;182:281-285. [PubMed] |
| 16. | Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326:469. [PubMed] |
| 17. | Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci. 2001;952:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Studzinski GP, McLane JA, Uskoković MR. Signaling pathways for vitamin D-induced differentiation: implications for therapy of proliferative and neoplastic diseases. Crit Rev Eukaryot Gene Expr. 1993;3:279-312. [PubMed] |
| 19. | Ylikomi T, Laaksi I, Lou YR, Martikainen P, Miettinen S, Pennanen P, Purmonen S, Syvälä H, Vienonen A, Tuohimaa P. Antiproliferative action of vitamin D. Vitam Horm. 2002;64:357-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women's Health Study. Am J Epidemiol. 1993;137:1302-1317. [PubMed] |
| 21. | Zheng W, Anderson KE, Kushi LH, Sellers TA, Greenstein J, Hong CP, Cerhan JR, Bostick RM, Folsom AR. A prospective cohort study of intake of calcium, vitamin D, and other micronutrients in relation to incidence of rectal cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7:221-225. [PubMed] |
| 22. | Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3:601-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 382] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 23. | Lointier P, Wargovich MJ, Saez S, Levin B, Wildrick DM, Boman BM. The role of vitamin D3 in the proliferation of a human colon cancer cell line in vitro. Anticancer Res. 1987;7:817-821. [PubMed] |
| 24. | Kallay E, Pietschmann P, Toyokuni S, Bajna E, Hahn P, Mazzucco K, Bieglmayer C, Kato S, Cross HS. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Lipkin M, Yang K, Edelmann W, Newmark H, Fan KH, Risio M, Kucherlapati R. Inherited and acquired risk factors in colonic neoplasia and modulation by chemopreventive interventions. J Cell Biochem Suppl. 1996;25:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Tangpricha V, Spina C, Yao M, Chen TC, Wolfe MM, Holick MF. Vitamin D deficiency enhances the growth of MC-26 colon cancer xenografts in Balb/c mice. J Nutr. 2005;135:2350-2354. [PubMed] |
| 27. | Spina C, Tangpricha V, Yao M, Zhou W, Wolfe MM, Maehr H, Uskokovic M, Adorini L, Holick MF. Colon cancer and solar ultraviolet B radiation and prevention and treatment of colon cancer in mice with vitamin D and its Gemini analogs. J Steroid Biochem Mol Biol. 2005;97:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 29. | Sitrin MD, Halline AG, Abrahams C, Brasitus TA. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazine-induced colonic carcinogenesis in the rat. Cancer Res. 1991;51:5608-5613. [PubMed] |
| 30. | Millan MJ, Bervoets K, Colpaert FC. 5-hydroxytryptamine (5-HT)1A receptors and the tail-flick response. I. 8-hydroxy-2-(di-n-propylamino) tetralin HBr-induced spontaneous tail-flicks in the rat as an in vivo model of 5-HT1A receptor-mediated activity. J Pharmacol Exp Ther. 1991;256:973-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 132] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Comer PF, Clark TD, Glauert HP. Effect of dietary vitamin D3 (cholecalciferol) on colon carcinogenesis induced by 1,2-dimethylhydrazine in male Fischer 344 rats. Nutr Cancer. 1993;19:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Beaty MM, Lee EY, Glauert HP. Influence of dietary calcium and vitamin D on colon epithelial cell proliferation and 1,2-dimethylhydrazine-induced colon carcinogenesis in rats fed high fat diets. J Nutr. 1993;123:144-152. [PubMed] |
| 33. | Iseki K, Tatsuta M, Uehara H, Iishi H, Yano H, Sakai N, Ishiguro S. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer. 1999;81:730-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Majewski S, Skopinska M, Marczak M, Szmurlo A, Bollag W, Jablonska S. Vitamin D3 is a potent inhibitor of tumor cell-induced angiogenesis. J Investig Dermatol Symp Proc. 1996;1:97-101. [PubMed] |
| 35. | Shokravi MT, Marcus DM, Alroy J, Egan K, Saornil MA, Albert DM. Vitamin D inhibits angiogenesis in transgenic murine retinoblastoma. Invest Ophthalmol Vis Sci. 1995;36:83-87. [PubMed] |
| 36. | Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214-220. [PubMed] |
| 37. | Pálmer HG, González-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 616] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 38. | Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med. 1985;313:1381-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 316] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Holt PR, Arber N, Halmos B, Forde K, Kissileff H, McGlynn KA, Moss SF, Kurihara N, Fan K, Yang K. Colonic epithelial cell proliferation decreases with increasing levels of serum 25-hydroxy vitamin D. Cancer Epidemiol Biomarkers Prev. 2002;11:113-119. [PubMed] |
| 40. | Ma J, Stampfer MJ, Gann PH, Hough HL, Giovannucci E, Kelsey KT, Hennekens CH, Hunter DJ. Vitamin D receptor polymorphisms, circulating vitamin D metabolites, and risk of prostate cancer in United States physicians. Cancer Epidemiol Biomarkers Prev. 1998;7:385-390. [PubMed] |
| 41. | Slatter ML, Yakumo K, Hoffman M, Neuhausen S. Variants of the VDR gene and risk of colon cancer (United States). Cancer Causes Control. 2001;12:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 42. | Lilliu H, Pamphile R, Chapuy MC, Schulten J, Arlot M, Meunier PJ. Calcium-vitamin D3 supplementation is cost-effective in hip fractures prevention. Maturitas. 2003;44:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |