INTRODUCTION
Over the past two decades, many researchers have reported that ion channels and transporters play important roles in fundamental cellular functions. Particularly, their physiological roles in cell proliferation have been considered since cell volume changes, which require the participation of ion movement across the cell membrane, are indispensable in cell cycle progression. Recently, the roles of ion transporters have been studied in cancer cells[1-3] and various types of ion transporters have been found in cancers of digestive organs. Some types of K+ channels, such as voltage-gated Kv1.3 channel, voltage-gated HERG channel and KCNK9 channel, have been reported to be expressed at extremely higher levels in colonic carcinoma specimens than in normal colon tissues[4-6]. In gastric cancer, the voltage-gated HERG channel has revealed cancer-limited expression and its blocker diminishes the G1 to S phase transition[7]. Furthermore, increased mRNA levels of voltage-gated Cav1.2 Ca2+ channels and Ca2+-conducting channels (TRPM8) have been reported in colorectal adenocarcinoma[8,9].
Several reports indicate that Cl- channels/transporters play an important role in gastrointestinal cancer cells. For instance, transcriptional downregulation of Ca2+-activated 2Cl- channels (CLCA1 and CLCA2) genes is detected in colorectal tumor samples[10]. Sarosi et al[11] have reported that the Cl-/HCO3- exchanger influences the proliferation of Barrett’s esophageal adenocarcinoma cells through changes of intracellular pH. In addition, we previously found that mRNA and the functional expression levels of Na+/K+/2Cl- cotransporter (NKCC) were higher in poorly-differentiated type gastric adenocarcinoma cells than in differentiated cells[12]. These reports indicate that the transepithelial Cl- transport plays an important role in cell proliferation of gastrointestinal cancer cells. From this viewpoint, we investigated whether the intracellular chloride concentration ([Cl-]i) regulates cell proliferation and cell cycle progression in human gastric cancer cells.
INTRACELLULAR CHLORIDE AND SIGNAL TRANSDUCTION
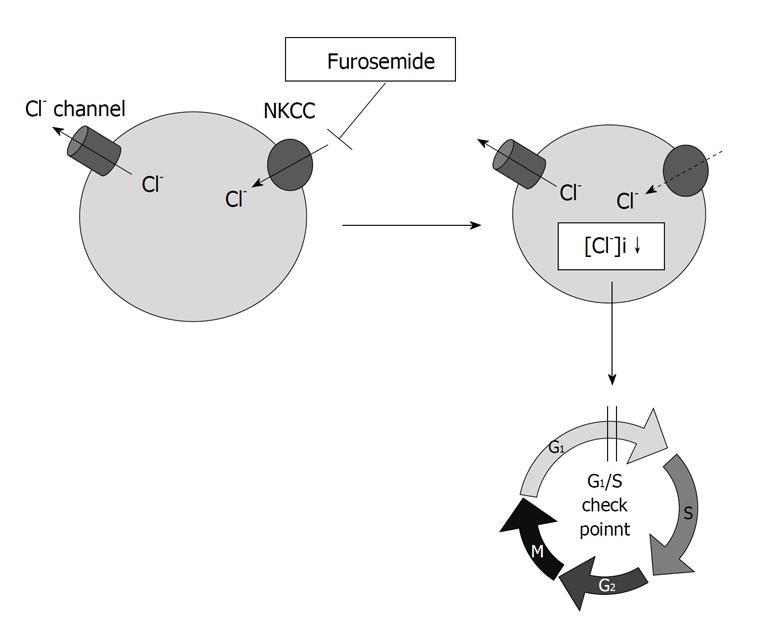
Our study[13] indicates that the intracellular chloride could act as a signal to regulate mRNA expression of ion channel in renal epithelial A6 cells, suggesting that many physiological functions are associated with the change of the [Cl-]i. Similarly, Heimlich et al[14] have shown that an alteration of the [Cl-]i plays an important role in the activation of signaling molecules upstream of the mitochondria, specifically impairing the intrinsic apoptotic pathway. In a mouse macula densa cell line, low chloride stimulates prostaglandin E2 release and COX-2 expression through activation of mitogen activated protein kinases (MAPKs)[15]. These reports suggest that the changes in the [Cl-]i might act as a regulator of various types of intracellular enzymes. We previously showed that furosemide, a blocker of NKCC, diminished cell growth by delaying the G1-S phase progression in gastric cancer cells with high expression and activity of NKCC[12] (Figure 1). NKCC is one of the important transporters controlling the [Cl-]ivia uptake of Cl- into the intracellular space and, therefore, furosemide decreases the [Cl-]i[16] (Figure 1). Based on these findings, we hypothesized that the [Cl-]i would be one of critical messengers regulating cell proliferation and investigated whether the [Cl-]i regulates cell cycle progression in human gastric cancer cells.
Figure 1 Na+/K+/2Cl- cotransporter controls the intracellular chloride concentration via uptake of Cl- into the intracellular space.
Furosemide, a blocker of Na+/K+/2Cl- cotransporter, delays the G1-S phase progression by decreasing the intracellular chloride concentration ([Cl-]i) in gastric cancer cells.
CELL CYCLE PROGRESSION AND [Cl-]i IN GASTRIC CANCER CELLS
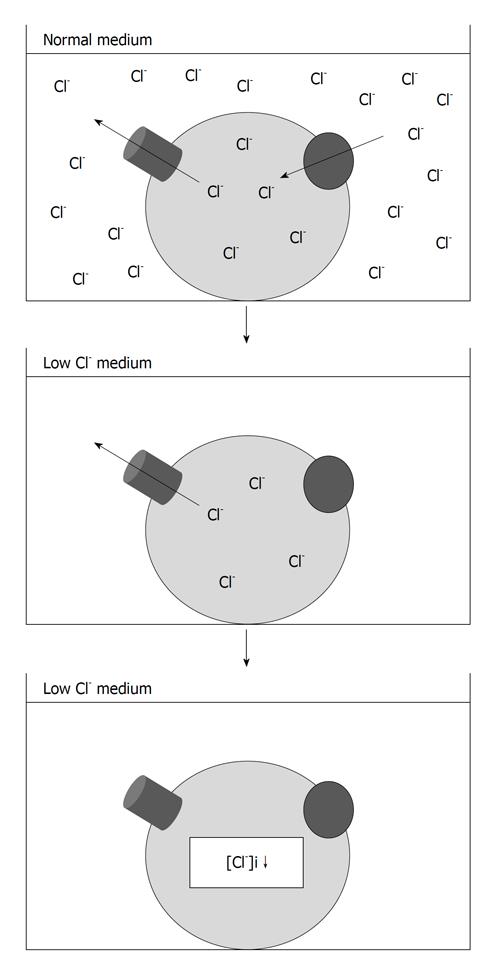
We directed our interest to the roles of the [Cl-]i in cell proliferation and cell cycle progression of gastric cancer cells. We applied media containing various chloride concentrations to human gastric cancer MKN28 cells and measured the [Cl-]i at 48 h after the application. The [Cl-]i of gastric cancer cells incubated in the normal medium was around 30 mmol/L. When cells were incubated in the low Cl- medium (replacement of Cl- by NO3-) for 48 h, the [Cl-]i decreased to around
0 mmol/L. Furthermore, the [Cl-]i of cells cultured in the media containing various chloride concentrations was proportionally dependent on the chloride concentration of the cultured medium[17,18]. These findings indicated that our experimental system using the low Cl- medium can be used as a model of the [Cl-]i regulation (Figure 2). The proliferation rate in MKN28 cells was significantly diminished by the culture in the low Cl- medium compared with that in a normal one. In addition, analysis of cell proliferation of MKN28 cells cultured in the media containing various chloride concentrations indicated that the rate of cell proliferation depends on the extracellular chloride concentration[18]. These results revealed that the [Cl-]i plays a key role in proliferation of gastric cancer cells. Cell cycle analysis revealed that the population of MKN28 cells staying in the G0/G1 phase was significantly increased and that cells staying in the S or G2/M phase were reduced by the culture in the low Cl- medium, suggesting that the decrease of the [Cl-]i shows an inhibitory effect on the proliferation of gastric cancer cells by mainly diminishing the transition from the G1 phase to the S phase[18] (Figure 3).
Figure 2 Experimental method for regulation of the intracellular chloride concentration of cultured cells.
The intracellular chloride concentration ([Cl-]i) of gastric cancer cells is decreased by the culture in the low Cl- medium, which were prepared by substituting, respectively, NaCl and KCl with NaNO3 and KNO3.
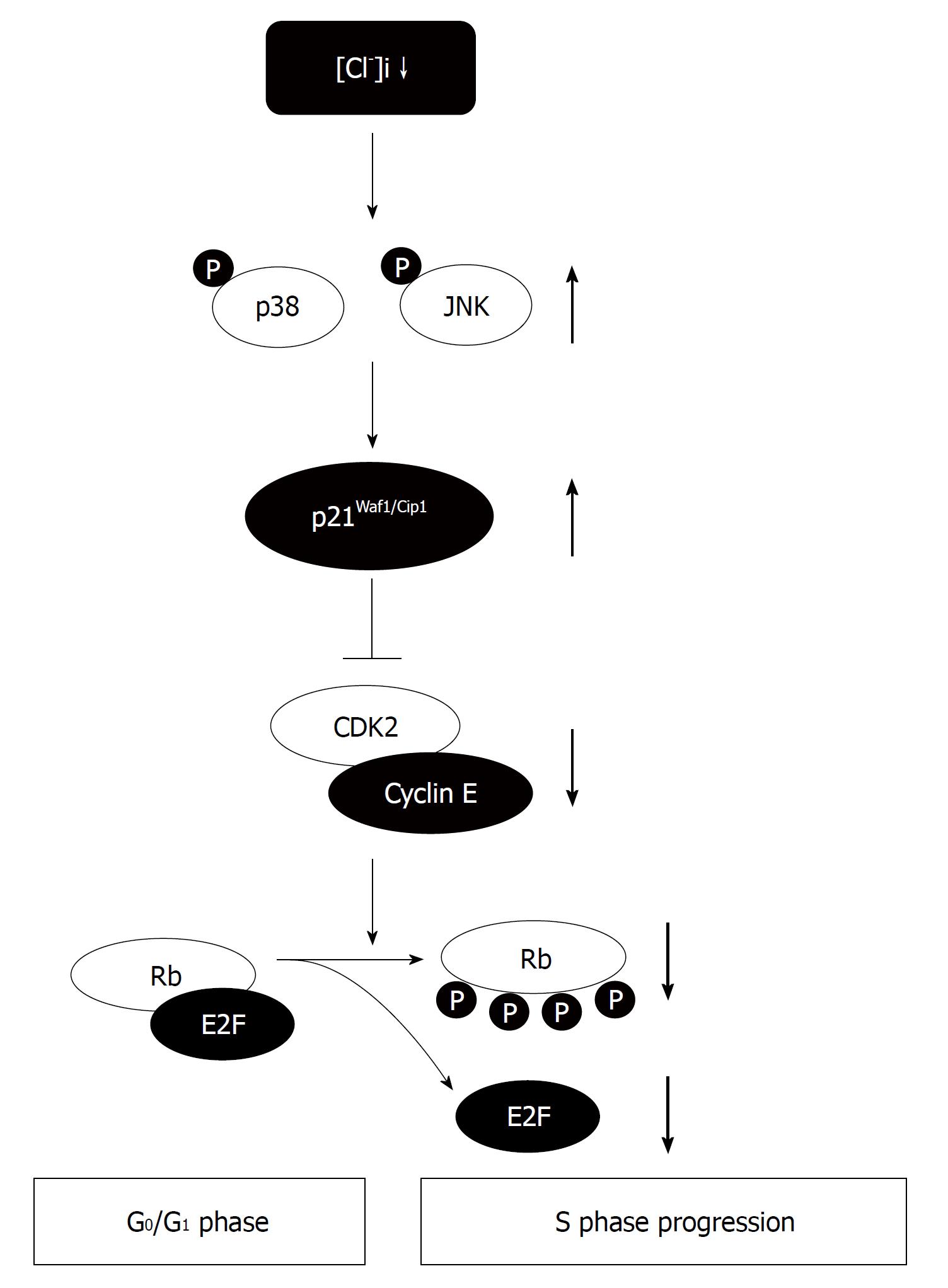
Figure 3 Roles of the intracellular chloride concentration in cell cycle progression of gastric cancer cells.
The intracellular chloride concentration ([Cl-]i) affects the cell proliferation via activation of p38 and/or JNK cascades through upregulation of the p21. The decrease of the [Cl-]i causes the G0/G1 phase arrest by diminishing expression of CDK2 and phosphorylated Rb due to an increase in expression of p21.
[Cl-]i CONTROLS THE G1/S CELL CYCLE CHECK POINT BY REGULATING THE EXPRESSION OF p21 IN GASTRIC CANCER CELLS
We analyzed the expression of cell cycle-associated proteins involved in G1-S phase transition to determine the mechanisms by which the decrease of the [Cl-]i inhibited the proliferation of MKN28 cells. The culture in the low Cl- medium significantly decreased phosphorylation of Rb. The expression of CDK2 protein, which is located at upstream of Rb in a signal pathway, was significantly downregulated by the culture in the low Cl- medium. Furthermore, the low Cl- medium elevated expression of p21[18]. These results suggest that the [Cl-]i of gastric cancer cells acts on the transition from the G1 phase to the S phase by regulating the expression of p21 and its downstream proteins in signal pathways (Figure 3). We found similar effects of the [Cl-]i on cell cycle associated proteins in human prostatic cancer cells and mouse osteoblast cells[16,19].
[Cl-]i REGULATES p21 THROUGH THE ACTIVATION OF MAPKs IN GASTRIC CANCER CELLS
Generally, the induction of p21 is known to be dependent on tumor suppressor protein, p53, leading us to an idea that the upregulation of p21 induced by the decrease of the [Cl-]i is due to activation of p53. However, the total expression and phosphorylation of p53 protein were not affected by application of the low Cl- medium[18], indicating that the upregulation of p21 induced by the decrease of the [Cl-]i was not dependent on activation of p53.
Therefore, we determined that MAPKs are involved in the p21 upregulation and cell cycle arrest induced by reduction of the [Cl-]i. Culture of MKN28 cells in the low Cl- medium significantly induced phosphorylation of MAPKs (ERK, p38, and JNK)[20]. Treatment with an inhibitor of p38 or JNK significantly suppressed p21 upregulation caused by culture in the low Cl- medium and rescued MKN28 cells from the low Cl--induced G1 cell cycle arrest, whereas treatment with an ERK inhibitor had no significant effect on p21 expression or the growth of MKN28 cells in the low Cl- medium[20]. These results suggest that the [Cl-]i affects the cell proliferation via activation of p38 and/or JNK cascades through upregulation of the p21 in gastric cancer cells (Figure 3).
CONCLUSION
We showed that: (1) chloride has significant effects on cell cycle progress in human gastric cancer cells; (2) the decrease of the [Cl-]i causes the G0/G1 phase arrest by diminishing expression of CDK2 and phosphorylated Rb due to an increase in expression of p21; (3) the [Cl-]i has significant effects on the activity of MAPKs cascades; and (4) the activation of p38 and JNK by a low Cl- condition leads to growth inhibition via an increase of p21 expression. Our results suggest that the [Cl-]i regulates intracellular signaling cascades participating in the control of proliferation in gastric cancer cells. A deeper understanding of these mechanisms may lead to the discovery of the [Cl-]i as an important mediator in tumor development and as a novel therapeutic target for gastric cancer.
Peer reviewer: Barbara W Chwirot, Professor, Department of Medical Biology, Institute of General and Molecular Biology, Nicolaus Copernicus University, Gagarina 9, Torun 87-100, Poland
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM