INTRODUCTION
Colorectal cancer is the third leading cause of cancer death in the United States[1]. A special need for non-toxic agents which are easy and effective to use for such cancer prevention and treatment is in demand. Epidemiologic findings may suggest a therapeutic possibility. Turmeric (and its active component curcumin) may be such an agent.
In India, traditional medicine uses turmeric for biliary disorders, anorexia, cough, diabetic wounds, hepatic disorders, rheumatism, and sinusitis[2]. It is pharmacologically safe even when consumed up to 100 mg per day in the Indian diet[3]. The incidence of colon cancer worldwide may vary 20-fold, with a higher prevalence in areas such as North America, Europe, Australia and New Zealand. A lower incidence is seen in countries such as India and less developed areas such as South America and Africa. Epidemiology suggests factors related to socioeconomic and dietary conditions may be important to colorectal cancer development. Significant risk factors include lower fiber intake, high fat diet and low calcium micronutrient intake.
Genetic predisposition to polyposis and cancer is also well established in literature[4]. Colorectal cancer is patterned into sporadic, inherited (10%) and familial (25%) categories. Germline mutations are seen in the two most common forms of inherited colon cancer: familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer.
Curative therapy for colon cancer is largely the province of surgery. Adjunctive chemotherapy and radiotherapy may be used depending on the course of the disease[5]. Exploitation of the over-expression of cyclooxygenase-2 (COX-2) in sporadic colon cancers (90%) and 40% of colon adenomas has shown promise for it as an avenue for chemoprevention of colon cancer. Non-steroidal anti-inflammatory drugs (NSAIDS), such as sulindac, and COX-2 specific inhibitors, such as Celebrex (celecoxib), have shown great utility as a chemopreventive treatment for patients with the FAP genotype. Increased risk of myocardial infarction with COX-2 inhibitors, which led to the removal of valdecoxib and rofecoxib, and the increased risk of gastrointestinal bleeding and renal failure with NSAIDS (sulindac) make their recommendation problematic[6].
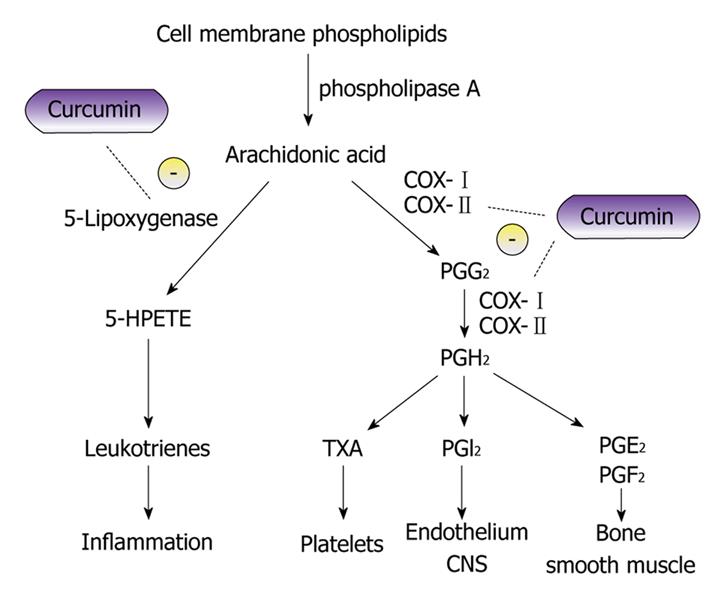
The response of suppressors of COX-2 for prevention of polyp development and cancer production in FAP does show the utility of control of deregulated pathways and, if not for the side effects, COX-2 inhibitors would be strongly recommended as a cancer/polyp chemopreventive agent. Thus agents that inhibit cellular pathways which create or promote carcinogenesis without toxicity are needed. Curcumin is a strong chemopreventive candidate with these properties (Figure 1)[6].
Figure 1 Diagram showing curcumin and its potential inhibitory effects on the metabolic pathway of arachidonic acid.
The anti-inflammatory properties of curcumin can be attributed to its effects on many molecular targets, 5-lipoxygenase and cyclooxygenase to name a few. Curcumin has been found to inhibit 5-lipoxygenase in-vitro in a concentration dependent manner in mouse epidermal cells[6]. The proposed mechanism of cyclooxygenase (COX) inhibition is believed to be due to the inhibition of Nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) activation[53].
Turmeric, a spice common to India and its surrounding regions, is derived from the rhizome of Curcuma Longa. The use of turmeric as a medicinal compound dates back to around 2000 B.C. when it was used as an anti-inflammatory agent. Fractions of turmeric known as curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxy-curcumin) are considered active compounds and possess a yellowish orange color[7]. Curcumin finds potential usefulness as an anti-inflammatory, anti-mutagenic, and anti-cancer molecule[8]. It also functions as an anti-oxidant and is capable of inducing apoptosis[9,10]. A wide variety of effects of curcumin are mediated by its capability to act as a free radical scavenger, to alter gene expression of various stress protein and genes involved in angiogenesis, and to inhibit activity of many important transcription factors such as nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and activator protein 1 (AP-1)[11-15]. Its abilities are often seen to be concentration dependent. At 10 μm, it has an antioxidant effect and at 50 μm it induces apoptosis, possibly in conjunction with generation of superoxide radicals[16]. Oral intake of turmeric at 4-8 g per day in humans can generate plasma levels of as little as 0.41-1.75 μmol/L. When considering its elicited biological effects by regular oral consumption, the concentration of curcumin is very important. In light of low systemic bioavailability, the role of biotransformed moieties, tetrahexahydro-curcumin, has received interest as to their biologic importance.
The anti-oxidant activity of curcumin can arise either from the OH group or from the CH2 group of the β-diketone (heptadiene-dione) moiety and it has been shown that the phenolic OH groups play a major role in the biological activity of curcumin[7,17]. Most of curcumin’s cellular effects are an outcome of its redox characteristics; the phenolic OH groups seem to be the most important moiety in curcumin. Replacement of this group inhibits or eliminates the lipid peroxidation inhibitory and free radical scavenging properties of curcumin[18,19].
Curcumin, in addition to demonstrating anti-tumor action, has been also shown to be an effective chemopreventive agent. Its action in tumors of colon, stomach and skin involve inhibition of cyclooxygenase, phospholipase A2 and phospholipase-Cr1[20,21].
POTENTIAL ROLE OF CURCUMIN IN CARCINOGENESIS
Carcinogenesis is a complex process but may be largely considered to be comprised of three phases: initiation, promotion, and progression[22]. These closely related steps: going from a normal cell to a transformed initiated cell (initiation); from initiated to pre-neoplastic cell (promotion); and from pre-neoplastic to neoplastic (progression); may lend themselves to curcumin intervention.
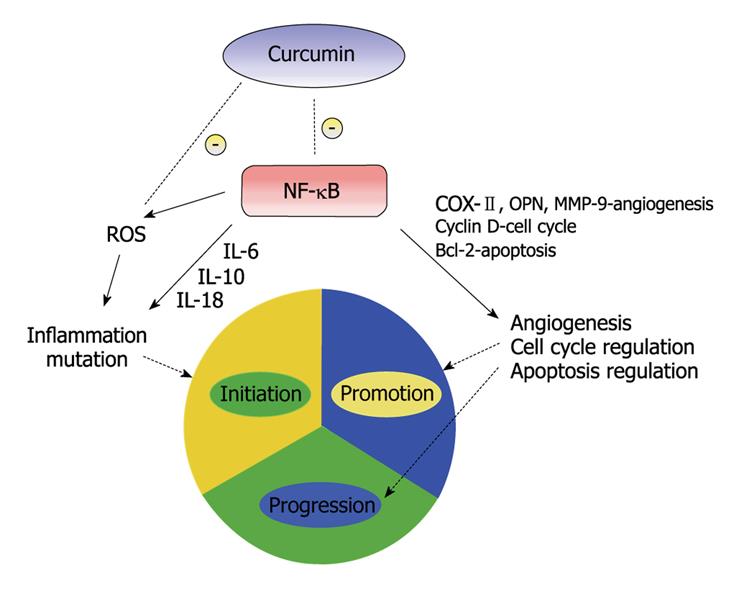
There is suggestive evidence that inflammation may have a role in the three phases of carcinogenesis[23]. Cancer initiation has been produced by oxidative stress and chronic inflammation[24]. Inflammation acts a key regulator in promotion of these initiated cells, possibly by providing them with proliferating signals and by preventing apoptosis[25]. The role of inflammation in tumor induction and subsequent malignant progression has been investigated[26]. Inflammatory response produces cytokines which act as growth and/or angiogenic factors leading transformed cells to proliferate and undergo promotion. Leukocytes produce cytokines, angiogenic factors as well as matrix-degrading proteases that allow the tumor cells to proliferate, invade, and metastasize. Tumor-infiltrating lymphocytes secrete matrix-degrading proteinases like matrix metallopeptidase 9 (MMP-9), thus promoting neoplastic proliferation, angiogenesis, and invasion[26]. These details demonstrate the role of inflammation in all three stages of carcinogenesis. Substantial evidence for the role of inflammation in cancer may be seen by the frequent up regulation of inflammatory mediators like NF-κB. The pathways activated by NF-κB up regulators are implicated not only in tumor growth and progression but also in cancer cell development of resistance to anti-cancer drugs, radiation and death cytokines. NF-κB is an excellent target for anti-cancer therapy[27]. The effect of curcumin on carcinogenesis is felt to be through inhibition of NF-κB as well as other molecular targets (Figure 2).
Figure 2 A simplified illustration of curcumin and its effects on the three stages of carcinogenesis.
NF-κB has been the subject of research for the development of anti-cancer therapeutic agents due to its effects on multiple stages of carcinogenesis. Curcumin has been shown to prevent phosphorylation and degradation of inhibitor κ B α, thereby blocking NF-κB activation[30]. NF-κB, through multiple pathways, can promote inflammation, angiogenesis and disrupt cell cycle and apoptosis regulation, thus promoting carcinogenesis.
Tumor initiation is modified by curcumin in several ways. Many of these seem to involve the blockade or inhibition of NF-κB.
EFFECTS ON TUMOR INITIATION BY CURCUMIN
Inflammation may initiate carcinogenesis through the production of reactive oxygen species (ROS) and reactive nitrogen species by activated neutrophils and macrophages that leads to cancer causing mutations[28]. Curcumin has demonstrated significant reduction of levels of inducible nitric oxide synthesis (iNOS). Curcumin inhibits the induction of nitric oxide synthase and is a potent scavenger of free radicals like nitric oxide[29]. NF-κB has been implicated in the induction of iNOS which produces oxidative stress, one of the causes of tumor initiation. Curcumin prevents phosphorylation and degradation of inhibitor κ B α, thereby blocking NF-κB activation which down regulates iNOS gene transcription[30].
Deregulatory imbalances between adaptive and innate immunity results in chronic inflammation which is associated with epithelial tumorigenesis, the prominent mechanism being NF-κB activation[31]. Curcumin was found to inhibit cell proliferation and cytokine production by inhibiting NF-κB target genes involved in this mitogen induction of T-cell proliferation, interleukin IL-2 production and nitric oxide generation[30]. Reduction induced over expression of cytokines, such as IL-10, IL-6, and IL-18, is accompanied by NF-κB induction which is controlled by and inhibited by curcumin[32].
Curcumin has been demonstrated to increase expression of conjugation enzymes (phase II). These have been shown to suppress ROS-mediated NF-κB, AP-1 and mitogen-activated protein kinases (MAPK) activation[33]. These enzymes, such as sulfotransferase and glutathione-s-transferase, conjugate toxic metabolites (through phase I enzymatic action) and then excrete them[33]. Curcumin modulates cytochrome p450 function and has been demonstrated to reduce aflatoxin B1-DNA adduct formation, an inhibitory step important in chemical carcinogenesis[34]. In various cancer models, curcumin was seen to further counteract ROS by increasing ornithine decarboxylase, glutathione, antioxidant enzymes and phase II metabolizing enzymes[35]. Heme oxygenase-1 (HO-1) has been seen to counteract oxidative stress, modulate apoptosis and inhibit cancer cell proliferation. Curcumin induces HO-1 expression by signaling through nuclear factor (erythroid-derived 2)-related factor 2 (NRF-2) and NF-κB and thereby has the potential to reduce oxidative stress[36-40]. NRF-2 is a transcription factor that regulates the expression of conjugatory enzymes like glutathione-s-transferase via an anti-oxidant response element (ARG)[41]. Curcumin prevents initiation of tumors either by curtailing the pro-inflammatory pathway or by inducing phase II enzymes[42].
TUMOR PROLIFERATION AND PROGRESSION SUPPRESSION BY CURCUMIN
Evidence suggests NF-κB has an important role in cancer initiation, promotion and progression. NF-κB binds to DNA and results in transcription of genes contributory to tumorigenesis: inflammation, anti-apoptosis and positive regulators of cell proliferation and angiogenesis[42]. NF-κB activation occurs primary via inhibitor κ B kinase (IKK)-mediated phosphorylation of inhibitory molecules[43]. Curcumin blocks NF-κB signaling and inhibits IKK activation[44]. Suppression is also noted on cell survival and cell proliferation genes, including Bcl-2, cyclin D1, IL-6, COX-2 and MMP[44,45]. Curcumin also induces apoptosis by caspase activation of a poly (ADP-ribose) polymerase (PARP) cleavage[41,44]. Regulation of NF-κB by curcumin is associated with activation of caspase 3 and 9, decreasing Bcl-X (L) messenger RNA (mRNA) and increasing Bcl-X (S) and c-IAP-2 mRNA[45]. COX-2 is the inducible form of cyclooxygenase that catalyzes the role limiting step in prostaglandin synthesis from arachidonic acid and plays an important role in cancer and tumor promotion[46,47]. Over-expression of COX-2 leads to malignant cell proliferation and invasion and the effect is reversed by non-steroidal anti-inflammatory agents, elucidating the importance of COX-2 inhibitors in cancer chemotherapy[48]. It has been suggested that COX-2 induction is mediated by NF-κB intracellular signaling pathway[49]. Curcumin has also been noted to decrease proliferation of various cancer cells, especially in the colon by down-regulating COX-2[45,50,51]. Curcumin inhibits COX-2 but not COX-1 in colon cancer cells, demonstrating its selectivity[52]. It has been shown to inhibit COX-2 expression by repressing degradation of the inhibitory unit inhibitor κ B α and hindering the nuclear translocation of the functionally active subunit of NF-κB, thereby blocking improper NF-κB activation[53].
Curcumin has been found to reduce the invasion and subsequent metastasis of cancer cells. Curcumin suppresses MMP expression which is believed to play a major role in mediating neovascularization and is increased during tumor progression. MMPs play an important role in endothelial cell migration and tube formation. Two determinants of neovascularization that help in forming new capillaries from preexisting blood vessels are MMP-2 and MMP-9. These two MMPs are known to be involved in tumor angiogenesis mainly through their matrix-degrading capacity[54]. Curcumin down regulates MMP-9 expression by inhibiting NF-κB and AP-1 binding to the DNA promoter region[55]. Adhesion molecules, such as vascular cell adhesion molecules (VCAM), are implicated in cancer progression and they are elevated in patients with advance disease[56].Curcumin has been noted to cause significant inhibition of tumor necrosis factor α induced VCAM-1 expression, related to the activation of the MAPK NF-κB pathway[57]. Curcumin has been shown to reduce cell migration and invasion induced by osteopontin, an extracellular matrix protein, through the NF-κB pathway[58]. Curcumin may inhibit cancer cell growth through down regulation of IL-1 and IL-8 induced receptor internalization[59]. Curcumin controls cancer progression by either blocking tumor growth or inhibiting its invasive and aggressive potential. Most of the effects in either case are exerted by curcumin-induced NF-κB inhibition.
Certain molecular targets of curcumin’s chemoprotective action are β-catenin, β-catenin/T cell factor (TCF), and lymphoid enhance factor (LEF) which are often disrupted in many cancer cells, especially colorectal carcinoma[60-62]. Dysregulated β-catenin (TCF) is implicated in cancer progression and poor prognosis. β-catenin in the cytoplasmic pool is phosphorylated by the axin adenomatous polyposis coli-glycogen synthase kinase 3β complex and subjected to degradation by the ubiquitin proteasome pathway[63]. Non-degraded β-catenin either enters the nucleus to transactivate the TCF/LEF transcription factors, leading to the up regulation of many genes responsible for cell proliferation, or binds to the E-cadherin adhesion complex. Reduction or loss of E-cadherin and/or increased localization of β-catenin in the nucleus is associated with invasive metastatic cancer progression and poor prognosis[64,65]. Curcumin has been found to decrease nuclear β-catenin and TCF4 and hence inhibit β-catenin /TCF signaling in various cell cancer lines[66]. Curcumin induced G2/M phase arrest in the cell cycle and apoptosis in colon cancer cells by impairing Wnt signaling and decreasing transactivation of β-catenin /TCF/LEF, subsequently alternating tumor progression[67]. The anti-tumor effect of curcumin was evidenced by its ability to decrease intestinal tumors in an animal model of FAP by reducing the expression of the oncoprotein β-catenin[68]. Some human β-catenin /TCF target genes, including cyclin D, MMP7, OPN, IL-8 and matrilysin, play a role in tumor promotion and progression[69]. NF-κB repression and decreased β-catenin signaling are some of the mechanisms by which curcumin suppresses the promotion and progression of cancer.
CURCUMIN CLINICAL TRIALS
Every clinical trial with curcumin has shown it to be safe with minimal adverse effect. Doses of up to 8000 mg per day were well tolerated.
Sharman and colleagues assessed the pharmacodynamic and pharmacokinetic properties of curcumin in 15 Caucasian patients with a history of colorectal cancer[70]. One patient had visible disease at the time of the study and the rest had complete surgical resection. Side effects were minimal, transient and not always determined to be due to curcumin. The one patient with local colonic disease saw a decline in a cancer biomarker, carcinoembryonic antigen, from 310 ± 15 to 175 ± 9 after 2 mo of treatment (440 mg/d). Computed tomography scan revealed that disease of the colon stabilized but metastasis was noted in the liver. This was felt due to probable low systemic bioavailability of curcumin though serum levels were not measured. Safety and tolerability of curcumin doses up to 2.2 g for 4 mo were documented.
A phase I clinical trial assessed tolerability of curcumin in 25 subjects from Taiwan with high risk or premalignant lesions[71]. In this study, curcumin was provided as 500 mg capsules for 3 mo. Twenty four of the twenty five subjects finished the study. Higher doses produced higher systemic levels. Subjects who consumed 2000 mg or less had curcumin levels barely detectable in serum and no detectable levels in the urine. Histological improvements independent of dosage were observed in precancerous lesion in 7 of the 25 subjects. Frank malignancies were observed in 2 of the 25 subjects during the 3 mo treatment regimen. This study showed the possible activity of chemoprevention, safety and tolerability in doses up to 8000 mg per day, warranting further studies.
A second phase I clinical study by Sharma et al[72] assessed curcumin biomarkers for systemic activity. This was investigated in 15 patients with histologically proven adenocarcinoma of the colon and rectum. Two of the patients had disease seemingly limited to the colon and thirteen beyond the colon. Patients received between 450-3600 mg curcumin per day with water after a 2 h fast in the morning as a single dose. Side effects were mild and some elevation of alkaline phosphatase and lactate dehydrogenase were noted. Patients consuming 3.6 g of curcumin saw a 46% decrease in Prostaglandin E2 (PGE2) levels (P = 0.028). Mean plasma levels of 11.1 ± 0.6 mmol/L were shown at the 1 h point in 3 patients consuming 3.6 g of curcumin. The levels were 1/40 of that noted in the previous study. The previous study used a synthetic version of curcumin while this study used a natural curcumin with the presence of other curcumanoid properties[71,72]. A question of ethnically related nucleotide polymorphism in the metabolizing enzyme UGT1A1 gene which might produce altered metabolism should be considered[73]. No partial responses were seen and no reduction in tumor markers was observed. Safety and tolerability of curcumin was seen up to daily dosage of 8000 mg.
A phase I study was based on evaluating the presence of curcumin metabolites in hepatic tissue and portal blood on 12 patients. Dosages ranged from 450-3600 mg of curcumin capsules which were taken for 7 d before surgery. Only 3 of 12 patients receiving 3600 mg of curcumin had detectable curcumin metabolites. Curcumin, curcumin sulfate and curcumin glucuronide were not present in bile or liver tissues in any patient. Low oral availability was noted in this study but the possibility of an oral agent to treat distant metastases of the gastrointestinal tract was advanced.
Garcea et al[74] studied curcumin levels in the colorectum and the pharmacodynamics of curcumin in 12 patients with confirmed colorectal cancer. The staging of patients was noted; 2 patients with Duke A, 3 patients were Duke B, and 7 patients were Duke C. Patients were assigned to 450 , 1800 or 3600 mg of curcumin per day for 7 d prior to surgery. Detectable curcumin levels were seen in the serum of only one patient (who was taking 3600 mg per day). Every patient had detectable curcumin levels in normal and malignant colorectal tissue ranging from 7 nmol/g to 20 nmol/g of tissue. Curcumin levels were highest in the normal tissue of the cecum and the ascending colon as opposed to the transverse, splenic flexure and the descending colon, which suggests a local effect. COX-2 levels were undetectable in normal tissue but detectable in malignant colorectal tissue. Curcumin was not found to modulate the expression of Cox-2 in malignant tissues. It appears from this study that doses of 3600 mg of curcumin are safe and sufficient to see pharmacodynamic changes in the gastrointestinal tract.
Colonic polyps are considered to be a precursor to cancer. The effect of curcumin has been studied on humans and animals (mice) with FAP coli.
The CS7B1/6J Min/+ mouse is an established model for the study of FAP coli[75]. A study where 0.2% and 0.5% of curcumin in the diet reduced adenoma multiplicity by 39% and 40% compared to control. Concentration in the small intestine mucosa was noted to be between 39 nmol/g and 240 nmol/g of tissue. Curcumin disappeared from the tissues and plasma within 2-8 h after dosing. A suggested dosage for humans was estimated by extrapolation to be 1.6 g per day. Tumorigenesis was noted in the small bowels of the animal model.
A human study of 5 patients with familial adenomatous polyps was performed using 480 mg of curcumin and 20 mg quercetin three times a day[76]. Four patients had a retained rectum and one had an ileoanal anastomosis. This study spanned 6 mo. All five patients had a decrease in number and size of polyps from their baseline. A mean decrease in polyp number by 60.4% (P < 0.05) and size by 50.9% (P < 0.05) was noted. No adverse effects were noted to any patient and no related laboratory abnormalities were seen. This is the first human demonstration of the reduction in size and number of ileal and rectal polyps in patients with FAP by a curcumin containing agent. The lack of toxicity coupled with the benefits demonstrated makes larger studies compelling.
CONCLUSION
The preponderance of colon cancer is a subject of paramount importance. A need has been demonstrated for compounds that target multiple molecular and cellular pathways which may be important to chemoprevention and/or chemotherapy. Curcumin has demonstrated these chemopreventive properties in cell cultures, animal models and human investigations. Human trials have concluded that curcumin is safe and poses minimal adverse effects. Doses up to 8000 mg per day were well tolerated. Effectiveness in altering pathologic changes was demonstrated. Further studies and possible developments are necessary to fully confirm cardiovascular safety due to suppression of COX-2, albeit the mode of suppression is more difficult than COX-2 inhibitors such as celecoxib, valdecoxib, and rofecoxib. Further studies related to the relevance of bioavailability and curcumin effect on carcinogenesis are important. The development of standardized criteria for preparations of curcumin is critical for further in depth studies. There needs to be further investigation of the role of nucleotide polymorphism and altered metabolism of curcumin (i.e. VGT enzymes) and its impact on carcinogenesis.
Curcumin chemotherapy and chemoprevention of colon cancer presents many exciting possibilities. Many things need to be evaluated, investigated, and developed but the prospects for curcumin as a therapeutic agent are indeed promising.
Peer reviewer: Joseph J Cullen, MD, Professor of Surgery, University of Iowa Carver College of Medicine, Department of Surgery, 4605 JCP, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52242, United States
S- Editor Li LF L- Editor Roemmele A E- Editor Yang C