Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.107412
Revised: May 5, 2025
Accepted: June 19, 2025
Published online: August 15, 2025
Processing time: 141 Days and 15 Hours
Circulating tumor DNA (ctDNA) is the free DNA released by tumor or circulating tumor cells, which is associated with many tumor characteristics and can be used as a biomarker for early screening, monitoring, prognosis, and prediction of therapeutic response in patients with cancer. The field of gastric cancer is very attractive because there are no high-quality screening, monitoring, or prediction methods. Gastric cancer is characterized by great tumor heterogeneity, great differences in genetic and epigenetic characteristics among different subgroups of gastric cancer, and high sensitivity and specificity of methylated ctDNA, which is conducive to the identification of tumor genotypes and the formulation of accu
Core Tip: Circulating tumor DNA (ctDNA) has emerged as a promising noninvasive biomarker for early cancer detection and monitoring. Aberrant DNA methylation is one of the earliest and most frequent epigenetic alterations in gastric cancer. This review summarizes recent advances in understanding the role of ctDNA methylation in gastric cancer initiation and progression, and its potential clinical applications. We highlight the advantages of ctDNA methylation analysis in disease diagnosis, prognosis prediction, and therapeutic monitoring. A better understanding of ctDNA methylation dynamics may provide new insights into personalized medicine and improve outcomes in patients with gastric cancer.
- Citation: Huang HY, Lan J, Zhuang W. Role of circulating tumor DNA methylation in gastric cancer initiation and progression: A comprehensive review. World J Gastrointest Oncol 2025; 17(8): 107412
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/107412.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.107412
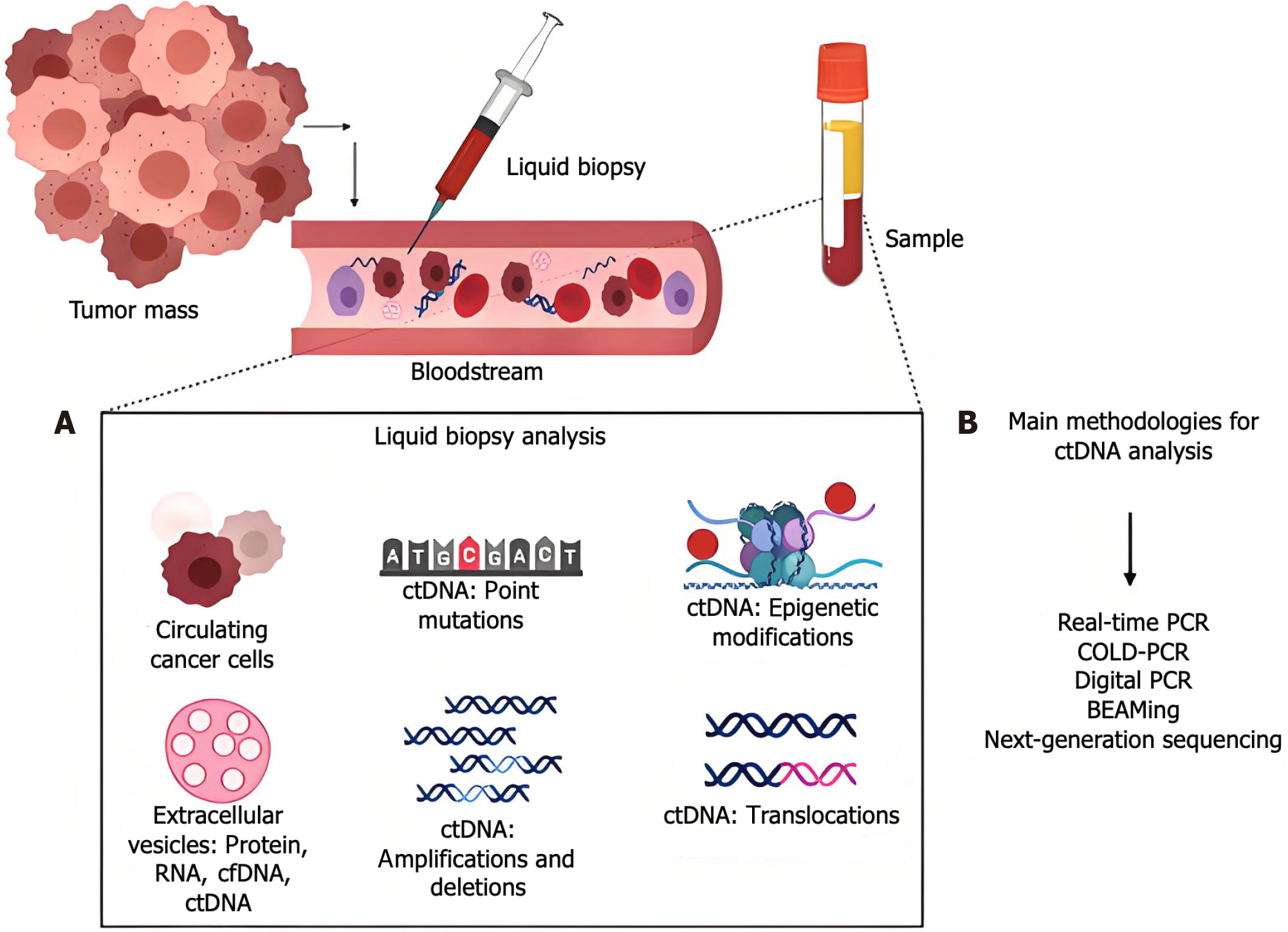
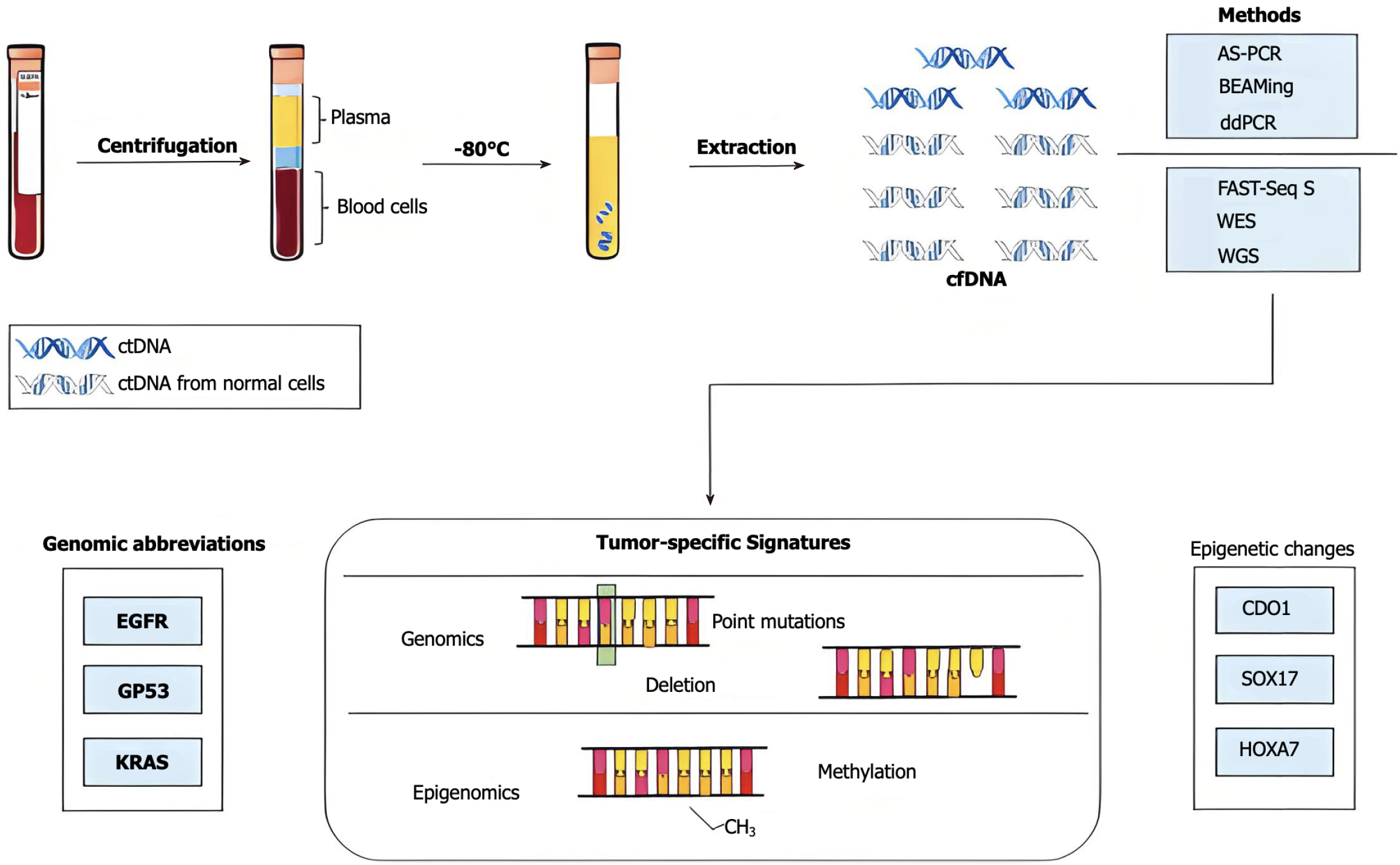
The emergence and progression of gastric cancer involve many genetic pathways and epigenetic alterations[1-4]. A significant alteration in gene regulation that contributes to gastric cancer is aberrant methylation, which manifests as excessive methylation in specific gene regions or a global loss of methylation throughout the genome[5-7]. During apoptosis or necrosis, nucleic acid fragments termed circulating free DNA (cfDNA) are released into the bloodstream. A portion of cfDNA originates from tumors and has tumor-specific molecular markers; thus, it is referred to as circulating tumor DNA (ctDNA)[8-12]. Numerous intrinsic characteristics of ctDNA, such as its DNA methylation state and histone methylation patterns, can serve as indicators for early cancer detection, monitoring recurrence, and assessing therapy efficacy. Recent research[13-15] has demonstrated that alterations in DNA methylation status occur in precancerous lesions, which can facilitate noninvasive early screening and detection of stomach cancer. Furthermore, alterations in methylation status offer substantial benefits in tracking postoperative recurrence and predicting patient prognosis[16-20]. Moreover, numerous studies have indicated that aberrant gene methylation is correlated with recurrence, metastasis, and treatment efficacy[21-24]. The methylation status of ctDNA can facilitate early tumor identification, monitor tumor growth and recurrence during treatment, and assist in guiding specific tumor therapies. For patients requiring adjuvant therapy, medication resistance is an unavoidable and significant issue[25-28]. Research indicates that DNA methylation is correlated with treatment resistance in gastric cancer. Nonetheless, it is reassuring because DNA methylation is a reversible phenomenon[29-33]. Consequently, reversing aberrant epigenetic alterations may provide a viable approach to overcome drug resistance. The clinical manifestations of early stomach cancer are subtle, and imaging studies have low sensitivity for early detection, complicating the diagnostic process. Research on the methylation state of ctDNA[34-36], both nationally and internationally, is progressing swiftly owing to advancements in the comprehension of epigenetic mechanisms[37-40]. Numerous studies[41-46] have established that aberrant DNA methylation significantly contributes to the development of gastric cancer and has substantial potential for early screening, monitoring of recurrence and metastasis, evaluating therapy response, and managing drug-resistant patients. In summary, recent advancements in research regarding ctDNA methylation status as a biomarker for gastric cancer have been reported, and its clinical applicability in early diagnosis, recurrence monitoring, and treatment response prediction has been assessed.
The type of tissue, degree of tumor differentiation, extent of dissemination, location of the tumor, and anticipated prognosis may all be associated with changes in the DNA methylation of critical genes in gastric cancer[47-50]. Furthermore, the methylation status of genes related to gastric cancer is influenced by environmental factors, including infection, chronic inflammation, diet, physical activity, age, and smoking[51-54]. Furthermore, the specific methylation status of gastric carcinoma exhibits a substantial age-related methylation pattern. This may be related to the vulnerability of elderly individuals to cancer[55-60].
The chronic inflammation caused by Helicobacter pylori (H. pylori) is one of the primary external factors that can influence gastric cancer because it may alter the methylation patterns of genes[61-65]. The methylation status of the cancer-related genes CDH1 and MGMT is entirely or partially reversed following H. pylori eradication; nevertheless, their DNA methylation levels do not recover to noninfected levels, which could lead to permanent gene imprinting. In contrast to H. pylori, which results in persistent inflammation and increased DNA methylation[66-70], Epstein-Barr virus (EBV) does not seem to cause an inflammatory phase, and its elevated DNA methylation is thought to be a direct consequence of infection. Blood or plasma sample-based liquid biopsies have demonstrated great promise in cancer research, particularly in the early identification and tracking of stomach cancer[71-76]. A noninvasive technique called liquid biopsy uses blood ctDNA analysis to provide vital information about cancers[77-80]. The occurrence and progression of malignancies are tightly linked to changes in the methylation state of ctDNA, and a small amount of DNA is released into the bloodstream by tumor cells (Figure 1).
The occurrence and progression of stomach cancer are facilitated by the epigenetic mechanism of cells, which frequently involves hundreds of active proteins, somatic mutations, and changes in the expression of epigenetic genome regulators[81-84]. Nonetheless, the relevant mechanism remains unclear, and the current study on the epigenetic mechanism of somatic mutations and epigenome regulators is insufficiently thorough[85-88]. One of the most prevalent mutations in ARID1A, a driver gene of gastric cancer, is frequently enriched in the EBV and microsatellite instability subtypes; nevertheless, how this mutation affects the overall chromatin or DNA methylation status of gastric cancer is unknown[89-93]. Gastric cancer has also been linked to changes in the transcription of genes encoding epigenetic enzymes, primarily as a result of decreased expression of the DNA demethylases TET1, DNMT1, and EZH2 and the histone methyltransferase SETDB2[94-98].
To increase the survival rate and quality of life of patients with stomach cancer, early detection and treatment are crucial[99-103]. To increase survival or lower incidence, the best early detection strategy is to find precancerous lesions or malignancies that may later emerge at the earliest stage of the disease as soon as possible[104-107]. The early detection of stomach cancer is aided by DNA methylation, which takes place during the early stages of tumor growth and can offer potential information for early diagnosis and treatment[108-110]. An aberrant DNA methylation status is not only a characteristic of advanced malignant tumors but also an early event and driving force in the pathogenesis of gastric cancer, according to recent studies. The methylation status of several genes varies in gastric cancer[111-115]. The DNA methylation status of genes linked to cancer was altered in the gastric mucosa of precancerous tissues, intestinal metaplasia tissues, and surrounding tissues in addition to actual gastric tumors. Inhibiting DNA methylation in animal models has also been shown by some researchers to lower the risk of stomach cancer[116-120]. Chronic inflammatory response-induced epigenetic alterations are believed to compound over time in H. pylori-positive gastric cancer, increasing the likelihood of cancer development[121-126].
There is either very little of these gene mutations in plasma or less mutant DNA released into the blood than what can be detected[127-130]. The high frequency of epigenetic aberrations in polyps and early gastric cancer, as well as the release of more methylated DNA into the blood, may be the reason why other studies[131-136] have demonstrated that changes in ctDNA methylation status are more common in these conditions than DNA mutations[137-140]. Therefore, we believe that finding methylated DNA could be more helpful for early screening of gastric cancer, even though the new detection technologies and methods being developed focus on improving the ability to detect DNA mutations (Figure 2).
The pathological results of tissue biopsies are currently the gold standard for diagnosing stomach cancer; nevertheless, endoscopic biopsy has numerous drawbacks, and operating on large numbers of asymptomatic individuals can be challenging[141]. Additionally, various factors in laboratory tests and live studies affect common blood tests (such as CA72-4, CA19-9, and CEA) and stool tests for hidden blood, which also present problems in terms of accuracy and reliability. These markers have limited relevance in early-stage cancer screening, and even studies that combine numerous serological markers have demonstrated poor sensitivity[142]. However, these genes are strongly correlated with tumor stage and patient survival. It is therefore imperative to develop a simple, sensitive, and efficient detection method.
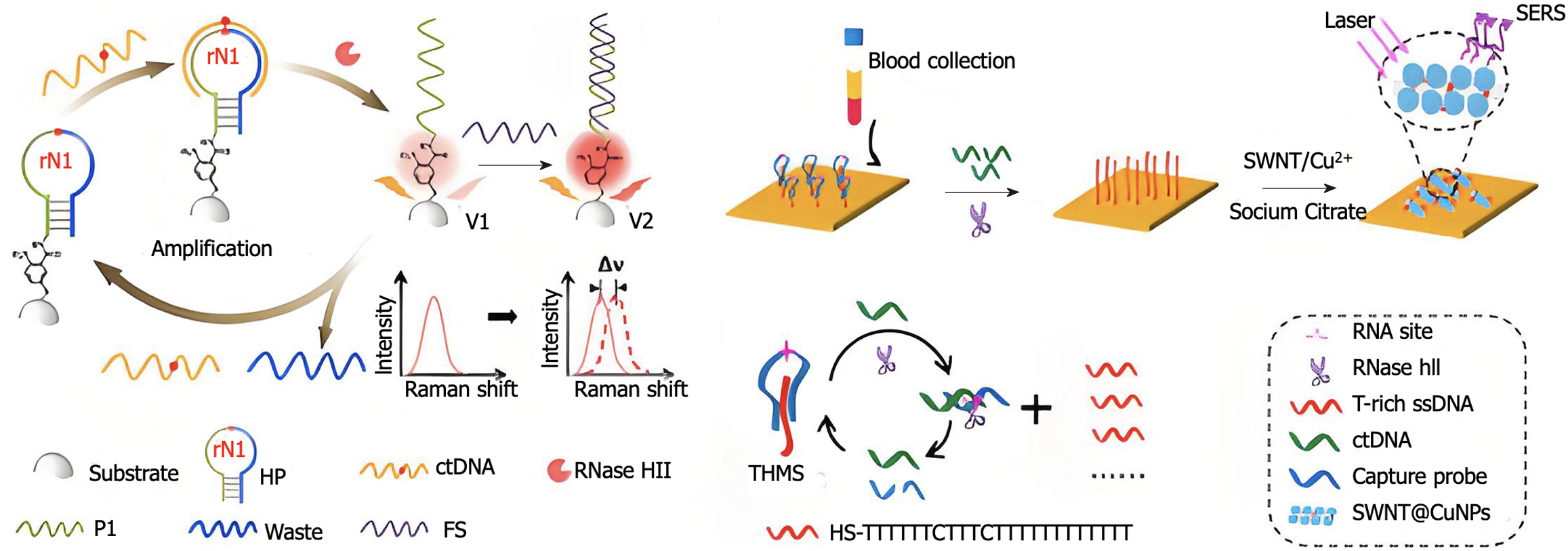
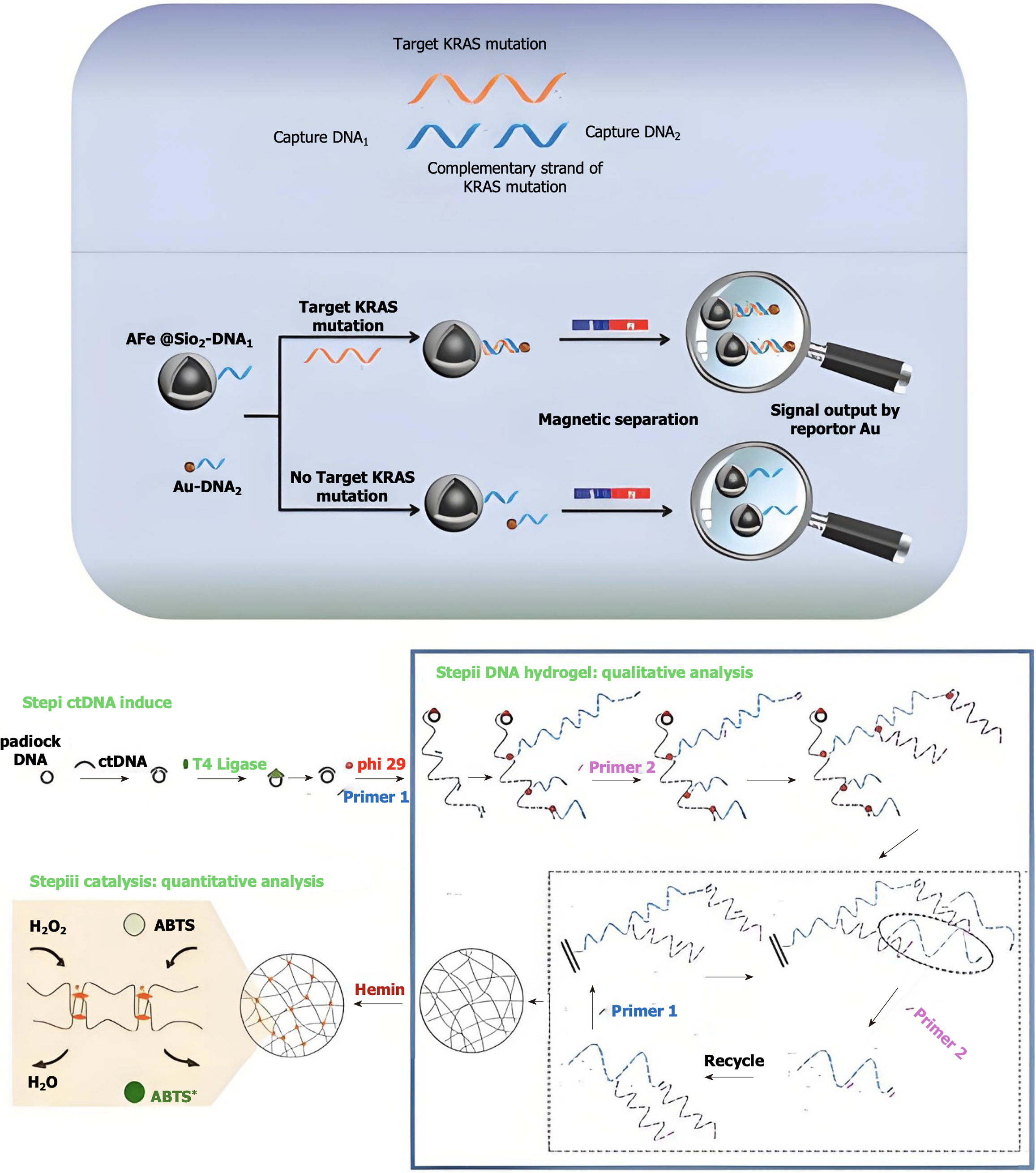
Identifying driver gene mutations in plasma cfDNA was the original strategy, and liquid biopsies are appealing for early cancer screening. However, in people without cancer, mutations related to clonal hematopoiesis of indeterminate potential that are not clearly important might affect this approach[143]. As technology has improved, we can now create products for clinical use by studying the differences in methylation gene patterns between the cfDNA of healthy individuals and that of patients with stomach cancer. SEPT9 has been used in clinical settings for more than a decade and is the first tumor biomarker authorized for the detection of colorectal cancer[144]. The amount of SEPT9 gene methylation was significantly greater in the blood of gastric cancer patients than in that of healthy controls, suggesting that this methylation in the serum could help in the early diagnosis of gastric cancer. The tumor suppressor gene RNF180 is highly methylated in gastric cancer, and its expression is markedly downregulated in primary gastric cancer[145]. It might be possible to use methylation of the promoter region, which is connected to a decrease in RNF180 gene expression, to screen for gastric cancer. It was discovered that SEPT9 methylation in blood had a sensitivity of 17.7% and specificity of 90.6% for detecting gastric cancer, indicating that this method can be helpful for early gastric cancer screening. However, the sensitivity was insufficient for clinical use. In a recent study on gastric cancer screening, the SEPT9 methylation marker alone had a sensitivity of 42.6%, whereas the use of CEA along with a protein marker increased the sensitivity to 86.4%, greatly improving the ability to diagnose the disease[146]. By combining detection with the protein marker CA72-4, their diagnostic capabilities could be further enhanced[147,148]. In summary, the combination of several markers and multitarget detection may be an effective early screening method for stomach cancer. The development of extremely sensitive detection techniques has made it possible to identify trace methylation alterations and low-frequency mutations, which are essential for early cancer detection and disease progression tracking. Currently, the two most popular techniques for detecting ctDNA are digital PCR (dPCR) and second-generation sequencing. dPCR can accurately identify small amounts of ctDNA molecules by using detailed measurements and very sensitive methods to separate them. Additionally, the use of bioinformatics along with machine learning and artificial intelligence (AI) can greatly improve the accuracy and reliability of detecting ctDNA (Figure 3).
Stool is one of the most common analytes used in gastrointestinal screening tests. However, many challenges remain to be overcome in the early detection of gastric cancer via the DNA of shed cells[149]. The stomach is an upper digestive tract organ, and a large amount of gastric acid and a long gastrointestinal tract can damage the DNA of shed cells in the stomach. Some studies[150-152] have indicated that the detection of gastric cancer via DNA in feces may be ineffective.
Recent studies[153-155] have shown that the methylation status of the DNA of shed cells from gastric cancer patients can be detected from feces, and a diagnostic tool named "Colocaller" has been developed according to the methylation status of four targets; this tool has the potential to be used as an auxiliary diagnostic tool for gastric cancer. In addition, some scholars have reported that the promoter regions of TERT, RASSF2, and SFRP2 in the stool of patients with gastric cancer are hypermethylated. This also provides us with a reference method for exploring the diagnosis of gastric cancer using shedding cell DNA, that is, whether one or several "pancancer" markers can have a high screening effect on both gastric cancer and colorectal cancer. In the future, if fecal-based methylation detection has high sensitivity and specificity and retains simple and noninvasive characteristics similar to those of fecal occult blood tests, it will help popularize and improve gastric cancer screening and further improve the detection rate of early gastric cancer.
For patients with lymph node-positive gastric cancer, the recurrence rate can still be as high as 88%, and the 5-year overall survival rate is only approximately 20%, even if they receive radical surgery and standard postoperative adjuvant chemotherapy. At present, there is a lack of means for monitoring gastric cancer recurrence, and the commonly used CEA and CA19-9 can monitor only approximately 40% of cases of tumor recurrence[156]. Key studies in gastric cancer have shown that ctDNA testing can identify patients with minimal residual disease (MRD) after surgery and assess patients' risk of postoperative recurrence. ctDNA has a short half-life, and if ctDNA is continuously detected in the blood after surgery, it may indicate the presence of tumor cells or micro-metastases in the blood circulation[157]. Therefore, the presence of postoperative ctDNA theoretically indicates the possible presence of MRD. Some studies have shown that the methylation status of ctDNA after surgery is an independent predictor of MRD, and patients with positive methylation are associated with an increased risk of MRD and recurrence. Another study revealed that CHFR, RUNX3, MGMT, and hMLH1 are highly methylated in primary gastric cancer and that these methylation genes help predict excessive tumor cells in regional lymph nodes. Machine learning also showed high accuracy in predicting the risk of gastric cancer lymph node metastasis via multitarget methylation analysis. This provides us with a new method and approach to predict early lymph node metastasis in the future, but high-quality studies on the relationship between methylated genes and early regional lymph node metastasis are lacking. Interestingly, MINT2 methylation levels in tumor tissues are very close to those in preoperative peritoneal lavage fluid (PPLF) and blood samples[158]. These findings suggest that abnormal MINT2 methylation in PPLF/blood may indicate the presence of peritoneal micro-metastases in patients with gastric cancer. If MINT2 methylation is positive before surgery, a variety of treatments, such as HIPEC therapy, may be used to reduce the possibility of postoperative recurrence and metastasis (Figure 4).
A 2020 study by Japanese researchers revealed that high levels of long-fragment LINE-1s after surgery might indicate MRD and a greater chance of cancer returning, whereas the amount of methylation in LINE-1s after surgery might not indicate MRD. Since a large amount of cfDNA comes from normal cells, the low methylation level of LINE-1 cfDNA indicates that many cancer cells with high methylation in the bloodstream might grow or spread easily. Additionally, the small number of gastric cancer patients suggests that there is uncertainty regarding the relationship between the degree of methylation and MRD[159].
The association between MRD in stomach cancer and ctDNA methylation status is still debatable, despite the paucity of research on this subject. However, existing research indicates that the ctDNA methylation status is a reliable indicator of MRD[160]. It can predict potential early lymph node metastases and peritoneal micro-metastases, determine the patient's risk of recurrence, and direct adjuvant therapy in the future. It can also reveal whether there are small residual lesions that are not visible on imaging examinations following surgery. While MRD patients can be found after standard follow-up treatment, which suggests that more tailored treatments, such as extra immunotherapy, targeted therapy, and personalized antigen vaccines, might be needed, the ctDNA methylation status can help identify high-risk patients for more aggressive treatment if it is found before follow-up therapy. The final stage of treatment ultimately erases the ctDNA methylation condition.
According to research on animals, stopping the aberrant methylation of DNA can prevent stomach cancer from developing. DNA methyltransferase inhibitors (DNMTis) are hence the subject of active pharmacological research. The use of DNMTis in conjunction with routine chemotherapy has been shown to correct aberrant gene regulation, enhancing the receptivity of cancer cells to treatment and decreasing their resistance to medications. In gastric cancer patients, for example, the combination of 5-fluorouracil and decitabine results in the over-methylation and sensitization of TFAP2E. A phase I clinical trial in which azacitidine was used to eliminate methyl groups in patients with advanced gastric cancer prior to neoadjuvant chemotherapy was conducted by researchers in the United States. They administered 5-azacitidine to gastric cancer patients prior to initiating neoadjuvant chemotherapy with the EOX (epirubicin, oxaliplatin, capecitabine) regimen. They discovered that some tumor-related genes, such as HPP1, TIMP3, CDKN2A, ESR1, and MGMT, had an excessive number of methyl groups attached, and preliminary findings showed that patients generally tolerated the neoadjuvant VEOX regimen. The effectiveness of chemotherapy may be increased by 5-azacitidine prechemotherapy. Of course, further neoadjuvant regimens and high-quality randomized studies are needed to further ascertain whether the combination of chemotherapy with neoadjuvant regimens is superior to chemotherapy alone. Adjuvant radiation therapy for stomach cancer has been controversial until recently, and it is unclear which patients may benefit from it. It is feasible to predict the impact of adjuvant radiation on the basis of the DNA methylation state of specific genes because studies[161-163] have demonstrated that radio-resistance in gastric cancer cells may result from the hypermethylation and inactivation of certain cancer-related genes. Furthermore, recent research has suggested that particular DNMTis can be utilized as radiation sensitizers to treat specific forms of gastric cancer and increase the radiosensitivity of gastric cancer cells[164]. DNMTis work in concert with radiotherapy and chemotherapy medications, and they can be utilized as sensitizers for both treatments. Therefore, in the future, DNMTs that target DNA methylation may be employed to cure and enhance multidrug resistance in gastric cancer. To prevent or reverse aberrant epigenetic changes early in disease treatment, the emerging therapeutic discipline of drug epigenetics focuses on important epigenetic targets. Clinical and preclinical trials[147,148,165] are currently being conducted to examine a growing number of medications that target DNMTis, such as the novel DNMT inhibitor zebularine. Histone methylation medications are still in their infancy as potential targeted therapy avenues; however, they are comparable to DNMTis. Additionally, research has shown that the CRISPR-Cas9 system is a promising targeted therapeutic approach for DNA methylation. In light of this, directed methylation editing might be more promising than DNMTis, which is more consistent with the idea of precision medicine.
Few prospective or clinical trials exist, and the majority of the studies that are now available are retrospective and small sample studies, which often lead to selection bias. Therefore, there is an urgent need for high-quality, large-scale, in-depth research (Figure 5).
Early detection can greatly increase the survival rate and quality of life for patients with stomach cancer; nevertheless, patients with middle- and advanced-stage gastric cancer must weigh the relative benefits of various treatments. DNMTis can increase the efficacy of conventional chemotherapy regimens, address multidrug resistance, and predict treatment response, adjuvant therapy regimens, and drug resistance[166]. They also have special advantages in these areas. Furthermore, DNA methylation might be associated with the tumor microenvironment, which could predict how well adjuvant immunotherapy and even gastric cancer immunotherapy would work (Figure 6). However, many chromatin-modifying enzymes have more varied activities and do not constantly evolve in the way we anticipate, and these methylating medications and regulators still carry the potential for off-target harm. For future epigenetic therapies to be successful, comprehending the underlying mechanisms of DNA methylation and finding new targets and medications are crucial.
To anticipate therapy response, track relapses, and conduct early illness screening, interdisciplinary collaboration is essential. The use of AI to help find and validate markers in the process of creating screening models, for example, could accelerate the process and even increase the precision of prediction models. However, few papers on DNA methylation-based biomarker AI models exist. A single biomarker may also have limited accuracy and specificity; future multimodal, multi-sample combination markers may be able to reach higher sensitivity and specificity. Without a doubt, epigenetics is a new area of study for gastric cancer. As more potent and affordable technologies and high-caliber clinical research become available, epigenetic indicators for gastric cancer may become more comprehensive and objective. To routinely use epigenetic markers for early identification, recurrence monitoring, and therapy effect prediction, more information is needed.
| 1. | Cao QX, Yan L, Hou NY, Chen JF, Yu S, Lu HJ, Dan ZJ, Pang MH. [Progress of circulating tumor DNA methylation for gastric cancer screening and management]. Zhonghua Wei Chang Wai Ke Za Zhi. 2024;27:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Seo SY, Youn SH, Bae JH, Lee SH, Lee SY. Detection and Characterization of Methylated Circulating Tumor DNA in Gastric Cancer. Int J Mol Sci. 2024;25:7377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Saliminejad K, Soleymani Fard S, Khorram Khorshid HR, Yaghmaie M, Mahmoodzadeh H, Mousavi SA, Ghaffari SH. Methylation Analysis of P16, RASSF1A, RPRM, and RUNX3 in Circulating Cell-Free DNA for Detection of Gastric Cancer: A Validation Study. Avicenna J Med Biotechnol. 2020;12:99-106. [PubMed] |
| 4. | Xiong D, Han T, Li Y, Hong Y, Li S, Li X, Tao W, Huang YS, Chen W, Li C. TOTEM: a multi-cancer detection and localization approach using circulating tumor DNA methylation markers. BMC Cancer. 2024;24:840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Yuan R, Wang G, Xu Z, Zhao H, Chen H, Han Y, Wang B, Zhou J, Hu H, Guo Z, Shen H, Xue X. Up-regulated Circulating miR-106a by DNA Methylation Promised a Potential Diagnostic and Prognostic Marker for Gastric Cancer. Anticancer Agents Med Chem. 2016;16:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Fu B, Yan P, Zhang S, Lu Y, Pan L, Tang W, Chen S, Chen S, Zhang A, Liu W. Cell-Free Circulating Methylated SEPT9 for Noninvasive Diagnosis and Monitoring of Colorectal Cancer. Dis Markers. 2018;2018:6437104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Balgkouranidou I, Matthaios D, Karayiannakis A, Bolanaki H, Michailidis P, Xenidis N, Amarantidis K, Chelis L, Trypsianis G, Chatzaki E, Lianidou ES, Kakolyris S. Prognostic role of APC and RASSF1A promoter methylation status in cell free circulating DNA of operable gastric cancer patients. Mutat Res. 2015;778:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Hu XY, Ling ZN, Hong LL, Yu QM, Li P, Ling ZQ. Circulating methylated THBS1 DNAs as a novel marker for predicting peritoneal dissemination in gastric cancer. J Clin Lab Anal. 2021;35:e23936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Hideura E, Suehiro Y, Nishikawa J, Shuto T, Fujimura H, Ito S, Goto A, Hamabe K, Saeki I, Okamoto T, Higaki S, Fujii I, Suzuki C, Hoshida T, Matsumoto T, Takami T, Sakaida I, Yamasaki T. Blood Free-Circulating DNA Testing of Methylated RUNX3 Is Useful for Diagnosing Early Gastric Cancer. Cancers (Basel). 2020;12:789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Ling ZQ, Lv P, Lu XX, Yu JL, Han J, Ying LS, Zhu X, Zhu WY, Fang XH, Wang S, Wu YC. Circulating Methylated XAF1 DNA Indicates Poor Prognosis for Gastric Cancer. PLoS One. 2013;8:e67195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Han J, Lv P, Yu JL, Wu YC, Zhu X, Hong LL, Zhu WY, Yu QM, Wang XB, Li P, Ling ZQ. Circulating methylated MINT2 promoter DNA is a potential poor prognostic factor in gastric cancer. Dig Dis Sci. 2014;59:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Sakakura C, Hamada T, Miyagawa K, Nishio M, Miyashita A, Nagata H, Ida H, Yazumi S, Otsuji E, Chiba T, Ito K, Ito Y. Quantitative analysis of tumor-derived methylated RUNX3 sequences in the serum of gastric cancer patients. Anticancer Res. 2009;29:2619-2625. [PubMed] |
| 13. | Paschold L, Binder M. Circulating Tumor DNA in Gastric and Gastroesophageal Junction Cancer. Curr Oncol. 2022;29:1430-1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Chen W, Yan H, Li X, Ge K, Wu J. Circulating tumor DNA detection and its application status in gastric cancer: a narrative review. Transl Cancer Res. 2021;10:529-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Campos-Carrillo A, Weitzel JN, Sahoo P, Rockne R, Mokhnatkin JV, Murtaza M, Gray SW, Goetz L, Goel A, Schork N, Slavin TP. Circulating tumor DNA as an early cancer detection tool. Pharmacol Ther. 2020;207:107458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 16. | Li JH, Zhang DY, Zhu JM, Dong L. Clinical applications and perspectives of circulating tumor DNA in gastric cancer. Cancer Cell Int. 2024;24:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 17. | Shaker F, Razi S, Rezaei N. Circulating miRNA and circulating tumor DNA application as liquid biopsy markers in gastric cancer. Clin Biochem. 2024;129:110767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Yuan SQ, Nie RC, Huang YS, Chen YB, Wang SY, Sun XW, Li YF, Liu ZK, Chen YX, Yao YC, Xu Y, Qiu HB, Liang Y, Wang W, Liu ZX, Zhao Q, Xu RH, Zhou ZW, Wang F. Residual circulating tumor DNA after adjuvant chemotherapy effectively predicts recurrence of stage II-III gastric cancer. Cancer Commun (Lond). 2023;43:1312-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 19. | Grizzi G, Salati M, Bonomi M, Ratti M, Holladay L, De Grandis MC, Spada D, Baiocchi GL, Ghidini M. Circulating Tumor DNA in Gastric Adenocarcinoma: Future Clinical Applications and Perspectives. Int J Mol Sci. 2023;24:9421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 409] [Reference Citation Analysis (5)] |
| 21. | Zhang M, Yang H, Fu T, Meng M, Feng Y, Qu C, Li Z, Xing X, Li W, Ye M, Li S, Bu Z, Jia S. Liquid biopsy: circulating tumor DNA monitors neoadjuvant chemotherapy response and prognosis in stage II/III gastric cancer. Mol Oncol. 2023;17:1930-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Zhou H, Liu H, Li J, Wang J, Fu X, Li Y, Mao S, Du J. Postoperative circulating tumor DNA detection and CBLB mutations are prognostic biomarkers for gastric cancer. Genes Genomics. 2023;45:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Mi J, Wang R, Han X, Ma R, Li H. Circulating tumor DNA predicts recurrence and assesses prognosis in operable gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2023;102:e36228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Yasuda T, Wang YA. Gastric cancer immunosuppressive microenvironment heterogeneity: implications for therapy development. Trends Cancer. 2024;10:627-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 46] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 25. | van Velzen MJM, Creemers A, van den Ende T, Schokker S, Krausz S, Reinten RJ, Dijk F, van Noesel CJM, Halfwerk H, Meijer SL, Mearadji B, Derks S, Bijlsma MF, van Laarhoven HWM. Circulating tumor DNA predicts outcome in metastatic gastroesophageal cancer. Gastric Cancer. 2022;25:906-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Wu R, Shi C, Chen Q, Wu F, Li Q. Detection of circulating tumor cell DNA for monitoring advanced gastric cancer. Int J Clin Exp Pathol. 2020;13:203-211. [PubMed] |
| 27. | Wang Y, Zhao C, Chang L, Jia R, Liu R, Zhang Y, Gao X, Li J, Chen R, Xia X, Bulbul A, Husain H, Guan Y, Yi X, Xu J. Circulating tumor DNA analyses predict progressive disease and indicate trastuzumab-resistant mechanism in advanced gastric cancer. EBioMedicine. 2019;43:261-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 28. | Huffman BM, Aushev VN, Budde GL, Chao J, Dayyani F, Hanna D, Botta GP, Catenacci DVT, Maron SB, Krinshpun S, Sharma S, George GV, Malhotra M, Jurdi A, Moshkevich S, Aleshin A, Kasi PM, Klempner SJ. Analysis of Circulating Tumor DNA to Predict Risk of Recurrence in Patients With Esophageal and Gastric Cancers. JCO Precis Oncol. 2022;6:e2200420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Min L, Chen J, Yu M, Yang K, Liu D. Utilizing circulating tumour DNA as a prognostic predictor of gastric cancer: a meta-analysis. Biomarkers. 2023;28:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Seo SH, Park YS, Nam SK, Lee HS, Park DJ, Park KU. Concordance of circulating tumor DNA and matched formalin-fixed paraffin-embedded tumor tissue in gastric cancer as a predictor of recurrence. Korean J Clin Oncol. 2023;19:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Chakrabarti S, Mahipal A. Circulating Tumor DNA-Guided Recurrence Risk Assessment in Esophageal and Gastric Cancer: Assay Holds the Key. JCO Precis Oncol. 2023;7:e2200710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | He W, Yang J, Sun X, Jiang S, Jiang J, Liu M, Mu T, Li Y, Zhang X, Duan J, Xu R. Advantages and Limitations of Monitoring Circulating Tumor DNA Levels to Predict the Prognosis of Patients Diagnosed With Gastric Cancer. Biomark Insights. 2022;17:11772719221141525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Zhang H, Jin T, Peng Y, Luan S, Li X, Xiao X, Yuan Y. Association between plasma circulating tumor DNA and the prognosis of esophageal cancer patients: a meta-analysis. Int J Surg. 2024;110:4370-4381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Li J, Li Z, Ding Y, Xu Y, Zhu X, Cao N, Huang C, Qin M, Liu F, Zhao A. TP53 mutation and MET amplification in circulating tumor DNA analysis predict disease progression in patients with advanced gastric cancer. PeerJ. 2021;9:e11146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Yang J, Gong Y, Lam VK, Shi Y, Guan Y, Zhang Y, Ji L, Chen Y, Zhao Y, Qian F, Chen J, Li P, Zhang F, Wang J, Zhang X, Yang L, Kopetz S, Futreal PA, Zhang J, Yi X, Xia X, Yu P. Deep sequencing of circulating tumor DNA detects molecular residual disease and predicts recurrence in gastric cancer. Cell Death Dis. 2020;11:346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 36. | Cao X, Ge S, Hua W, Zhou X, Lu W, Gu Y, Li Z, Qian Y. A pump-free and high-throughput microfluidic chip for highly sensitive SERS assay of gastric cancer-related circulating tumor DNA via a cascade signal amplification strategy. J Nanobiotechnology. 2022;20:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Yu P, Zhu S, Luo Y, Li G, Pu Y, Cai B, Zhang C. Application of Circulating Tumor Cells and Circulating Free DNA from Peripheral Blood in the Prognosis of Advanced Gastric Cancer. J Oncol. 2022;2022:9635218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 38. | Hyung J, Lee JY, Kim JE, Yoon S, Yoo C, Hong YS, Jeong JH, Kim TW, Jeon S, Jun HR, Jung CK, Jang JP, Kim J, Chun SM, Ahn JH. Safety and efficacy of trastuzumab biosimilar plus irinotecan or gemcitabine in patients with previously treated HER2 (ERBB2)-positive non-breast/non-gastric solid tumors: a phase II basket trial with circulating tumor DNA analysis. ESMO Open. 2023;8:101583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Suzuki T, Suzuki T, Yoshimura Y, Yahata M, Yew PY, Nakamura T, Nakamura Y, Park JH, Matsuo R. Detection of circulating tumor DNA in patients of operative colorectal and gastric cancers. Oncotarget. 2020;11:3198-3207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Catenacci DV, Kang YK, Uronis HE, Lee KW, Ng MC, Enzinger PC, Park SH, Gold PJ, Lacy J, Hochster HS, Oh SC, Kim YH, Marrone KA, Kelly RJ, Juergens RA, Kim JG, Alcindor T, Sym SJ, Song EK, Chee CE, Chao Y, Kim S, Oh DY, Yen J, Odegaard JI, Lagow E, Li D, Sun J, Kaminker P, Moore PA, Rosales MK, Park H. Circulating Tumor DNA as a Predictive Biomarker for Clinical Outcomes With Margetuximab and Pembrolizumab in Pretreated HER2-Positive Gastric/Gastroesophageal Adenocarcinoma. Oncology (Williston Park). 2023;37:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Iqbal M, Roberts A, Starr J, Mody K, Kasi PM. Feasibility and clinical value of circulating tumor DNA testing in patients with gastric adenocarcinomas. J Gastrointest Oncol. 2019;10:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Xi W, Zhou C, Xu F, Sun D, Wang S, Chen Y, Ji J, Ma T, Wu J, Shangguan C, Zhu Z, Zhang J. Molecular evolutionary process of advanced gastric cancer during sequential chemotherapy detected by circulating tumor DNA. J Transl Med. 2022;20:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 43. | Sasaki N, Iwaya T, Chiba T, Fujita M, Ju Z, Endo F, Yaegashi M, Hachiya T, Sugimoto R, Sugai T, Siwak DR, Liotta LA, Lu Y, Mills GB, Nakagawa H, Nishizuka SS. Analysis of mutational and proteomic heterogeneity of gastric cancer suggests an effective pipeline to monitor post-treatment tumor burden using circulating tumor DNA. PLoS One. 2020;15:e0239966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Ren J, Lu P, Zhou X, Liao Y, Liu X, Li J, Wang W, Wang J, Wen L, Fu W, Tang F. Genome-Scale Methylation Analysis of Circulating Cell-Free DNA in Gastric Cancer Patients. Clin Chem. 2022;68:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 45. | Jiang R, Cheng X, Li P, Meng E, Wu X, Wu H. Plasma circulating tumor DNA unveils the efficacy of PD-1 inhibitors and chemotherapy in advanced gastric cancer. Sci Rep. 2024;14:14027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 46. | Singh H, Klempner SJ, Melnitchouk N, Chander DP, Negrea OG, Patel AK, Schlechter BL, Rubinson DA, Huffman BM, Nambiar C, Remland J, Andrews E, Leahy ME, Brais LK, Enzinger PC, Mamon HJ, Giannakis M, Meyerhardt JA, Ng K, Perez KJ, Aguirre AJ, Clark JW, Cleary JM, Wolpin BM. Highly Sensitive Circulating Tumor DNA Assay Aids Clinical Management of Radiographically Occult Isolated Peritoneal Metastases in Patients With GI Cancer. JCO Precis Oncol. 2023;7:e2200572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 47. | Chen Z, Zhang C, Zhang M, Li B, Niu Y, Chen L, Yang J, Lu S, Gao J, Shen L. Chromosomal instability of circulating tumor DNA reflect therapeutic responses in advanced gastric cancer. Cell Death Dis. 2019;10:697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Jogo T, Nakamura Y, Shitara K, Bando H, Yasui H, Esaki T, Terazawa T, Satoh T, Shinozaki E, Nishina T, Sunakawa Y, Komatsu Y, Hara H, Oki E, Matsuhashi N, Ohta T, Kato T, Ohtsubo K, Kawakami T, Okano N, Yamamoto Y, Yamada T, Tsuji A, Odegaard JI, Taniguchi H, Doi T, Fujii S, Yoshino T. Circulating Tumor DNA Analysis Detects FGFR2 Amplification and Concurrent Genomic Alterations Associated with FGFR Inhibitor Efficacy in Advanced Gastric Cancer. Clin Cancer Res. 2021;27:5619-5627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | He HC, Han R, Xu BH, Huang C, Li MM, He CY, Lin WQ. Circulating Epstein-Barr virus DNA associated with hepatic impairment and its diagnostic and prognostic role in patients with gastric cancer. Front Med (Lausanne). 2022;9:1028033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Kim YW, Kim YH, Song Y, Kim HS, Sim HW, Poojan S, Eom BW, Kook MC, Joo J, Hong KM. Monitoring circulating tumor DNA by analyzing personalized cancer-specific rearrangements to detect recurrence in gastric cancer. Exp Mol Med. 2019;51:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Jin Y, Chen DL, Wang F, Yang CP, Chen XX, You JQ, Huang JS, Shao Y, Zhu DQ, Ouyang YM, Luo HY, Wang ZQ, Wang FH, Li YH, Xu RH, Zhang DS. The predicting role of circulating tumor DNA landscape in gastric cancer patients treated with immune checkpoint inhibitors. Mol Cancer. 2020;19:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 52. | Qi Z, Li Y, Wang Z, Tan X, Zhou Y, Li Z, Zhao W, Zheng X, Yao J, Li F, Wang W, Wang Z, Pang F, Wang G, Gu W. Monitoring of gastrointestinal carcinoma via molecular residual disease with circulating tumor DNA using a tumor-informed assay. Cancer Med. 2023;12:16687-16696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 53. | Yuan H, Xu F, Wang S, Liu D, Zhang H, Zhang J, Shi M, Yan C, Zhu Z. Analysis of circulating tumor DNA identifies distinct therapeutic response to intraperitoneal and intravenous paclitaxel plus S-1 in gastric cancer patients with peritoneal metastasis. Ther Adv Med Oncol. 2024;16:17588359231225038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Li J, Jiang W, Wei J, Zhang J, Cai L, Luo M, Wang Z, Sun W, Wang S, Wang C, Dai C, Liu J, Wang G, Wang J, Xu Q, Deng Y. Patient specific circulating tumor DNA fingerprints to monitor treatment response across multiple tumors. J Transl Med. 2020;18:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 55. | Onidani K, Shoji H, Kakizaki T, Yoshimoto S, Okaya S, Miura N, Sekikawa S, Furuta K, Lim CT, Shibahara T, Boku N, Kato K, Honda K. Monitoring of cancer patients via next-generation sequencing of patient-derived circulating tumor cells and tumor DNA. Cancer Sci. 2019;110:2590-2599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 56. | Cabel L, Decraene C, Bieche I, Pierga JY, Bennamoun M, Fuks D, Ferraz JM, Lefevre M, Baulande S, Bernard V, Vacher S, Mariani P, Proudhon C, Bidard FC, Louvet C. Limited Sensitivity of Circulating Tumor DNA Detection by Droplet Digital PCR in Non-Metastatic Operable Gastric Cancer Patients. Cancers (Basel). 2019;11:396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Shahjehan F, Kamatham S, Kasi PM. Role of Circulating Tumor DNA in Gastrointestinal Cancers: Update From Abstracts and Sessions at ASCO 2018. Front Oncol. 2019;9:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Lan J, Lu Y, Guan Y, Chang L, Yu Z, Qian H. Identification of circulating tumor DNA using a targeted 545-gene next generation sequencing panel in patients with gastric cancer. Oncol Lett. 2020;19:2251-2257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Chen X, Bai K, Zhang Y, Xu Y, Huo Y, Wang S, Zou Y, Qi X, Guo R, Ou Q, Liu D, Yin S, Chen S, Bu H. Genomic alterations of cerebrospinal fluid cell-free DNA in leptomeningeal metastases of gastric cancer. J Transl Med. 2023;21:296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 60. | Yu P, Chen P, Wu M, Ding G, Bao H, Du Y, Xu Z, Yang L, Fang J, Huang X, Lai Q, Wei J, Yan J, Yang S, He P, Wu X, Shao Y, Su D, Cheng X. Multi-dimensional cell-free DNA-based liquid biopsy for sensitive early detection of gastric cancer. Genome Med. 2024;16:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Wu L, Zheng Y, Liu J, Luo R, Wu D, Xu P, Wu D, Li X. Comprehensive evaluation of the efficacy and safety of LPV/r drugs in the treatment of SARS and MERS to provide potential treatment options for COVID-19. Aging (Albany NY). 2021;13:10833-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 62. | Nevisi F, Yaghmaie M, Pashaiefar H, Alimoghaddam K, Iravani M, Javadi G, Ghavamzadeh A. Correlation of HER2, MDM2, c-MYC, c-MET, and TP53 Copy Number Alterations in Circulating Tumor Cells with Tissue in Gastric Cancer Patients: A Pilot Study. Iran Biomed J. 2020;24:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Gerratana L, Movarek M, Wehbe F, Katam N, Mahalingam D, Donahue J, Shah A, Chae YK, Mulcahy M, Tsarwhas D, Villaflor V, Kalyan A, Hussein M, Patel J, Chandra S, Platanias LC, Gradishar W, Cristofanilli M, Behdad A. Genomic Landscape of Advanced Solid Tumors in Circulating Tumor DNA and Correlation With Tissue Sequencing: A Single Institution's Experience. JCO Precis Oncol. 2022;6:e2100289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Yukawa N, Yamada T, Aoyama T, Woo T, Ueda K, Mastuda A, Hara K, Kazama K, Tamagawa H, Sato T, Oshima T, Suzuki A, Aburatani H, Ishikawa S, Saito A, Masuda M, Yoshida H, Rino Y. Tumor DNA in Peritoneal Lavage as a Novel Biomarker for Predicting Peritoneal Recurrence in Patients With Gastric Cancer. Anticancer Res. 2023;43:2069-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 65. | Slagter AE, Vollebergh MA, Caspers IA, van Sandick JW, Sikorska K, Lind P, Nordsmark M, Putter H, Braak JPBM, Meershoek-Klein Kranenbarg E, van de Velde CJH, Jansen EPM, Cats A, van Laarhoven HWM, van Grieken NCT, Verheij M. Prognostic value of tumor markers and ctDNA in patients with resectable gastric cancer receiving perioperative treatment: results from the CRITICS trial. Gastric Cancer. 2022;25:401-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 66. | Wu L, Zhong Y, Wu D, Xu P, Ruan X, Yan J, Liu J, Li X. Immunomodulatory Factor TIM3 of Cytolytic Active Genes Affected the Survival and Prognosis of Lung Adenocarcinoma Patients by Multi-Omics Analysis. Biomedicines. 2022;10:2248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 67. | Al Zoughbi W, Fox J, Beg S, Papp E, Hissong E, Ohara K, Keefer L, Sigouros M, Kane T, Bockelman D, Nichol D, Patchell E, Bareja R, Karandikar A, Alnajar H, Cerqueira G, Guthrie VB, Verner E, Manohar J, Greco N, Wilkes D, Tagawa S, Malbari MS, Holcomb K, Eng KW, Shah M, Altorki NK, Sboner A, Nanus D, Faltas B, Sternberg CN, Simmons J, Houvras Y, Molina AM, Angiuoli S, Elemento O, Mosquera JM. Validation of a Circulating Tumor DNA-Based Next-Generation Sequencing Assay in a Cohort of Patients with Solid tumors: A Proposed Solution for Decentralized Plasma Testing. Oncologist. 2021;26:e1971-e1981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 68. | Rahman M, Niu J, Cui X, Zhou C, Tang N, Jin H, Cui D. Electrochemical Biosensor Based on l-Arginine and rGO-AuNSs Deposited on the Electrode Combined with DNA Probes for Ultrasensitive Detection of the Gastric Cancer-Related PIK3CA Gene of ctDNA. ACS Appl Bio Mater. 2022;5:5094-5103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Karamitrousis EI, Balgkouranidou I, Xenidis N, Amarantidis K, Biziota E, Koukaki T, Trypsianis G, Karayiannakis A, Bolanaki H, Kolios G, Lianidou E, Kakolyris S. Prognostic Role of RASSF1A, SOX17 and Wif-1 Promoter Methylation Status in Cell-Free DNA of Advanced Gastric Cancer Patients. Technol Cancer Res Treat. 2021;20:1533033820973279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Wu L, Liu Q, Ruan X, Luan X, Zhong Y, Liu J, Yan J, Li X. Multiple Omics Analysis of the Role of RBM10 Gene Instability in Immune Regulation and Drug Sensitivity in Patients with Lung Adenocarcinoma (LUAD). Biomedicines. 2023;11:1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 71. | Alarcón MA, Olivares W, Córdova-Delgado M, Muñoz-Medel M, de Mayo T, Carrasco-Aviño G, Wichmann I, Landeros N, Amigo J, Norero E, Villarroel-Espíndola F, Riquelme A, Garrido M, Owen GI, Corvalán AH. The Reprimo-Like Gene Is an Epigenetic-Mediated Tumor Suppressor and a Candidate Biomarker for the Non-Invasive Detection of Gastric Cancer. Int J Mol Sci. 2020;21:9472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Fu Y, Jiang J, Wu Y, Cao D, Jia Z, Zhang Y, Li D, Cui Y, Zhang Y, Cao X. Genome-wide 5-hydroxymethylcytosines in circulating cell-free DNA as noninvasive diagnostic markers for gastric cancer. Gastric Cancer. 2024;27:735-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Openshaw MR, Suwaidan AA, Ottolini B, Fernandez-Garcia D, Richards CJ, Page K, Guttery DS, Thomas AL, Shaw JA. Longitudinal monitoring of circulating tumour DNA improves prognostication and relapse detection in gastroesophageal adenocarcinoma. Br J Cancer. 2020;123:1271-1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 74. | Zhang C, Chen Z, Chong X, Chen Y, Wang Z, Yu R, Sun T, Chen X, Shao Y, Zhang X, Gao J, Shen L. Clinical implications of plasma ctDNA features and dynamics in gastric cancer treated with HER2-targeted therapies. Clin Transl Med. 2020;10:e254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Huang X, Zhao Q, An X, Pan J, Zhao L, Shen L, Xu Y, Yuan D. The Ratio of ssDNA to dsDNA in Circulating Cell-Free DNA Extract is a Stable Indicator for Diagnosis of Gastric Cancer. Pathol Oncol Res. 2020;26:2621-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 572] [Article Influence: 95.3] [Reference Citation Analysis (1)] |
| 77. | Deng Q, Jiang B, Yan H, Wu J, Cao Z. Circulating tumor cells in gastric cancer: developments and clinical applications. Clin Exp Med. 2023;23:4385-4399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 78. | Wu L, Zhong Y, Yu X, Wu D, Xu P, Lv L, Ruan X, Liu Q, Feng Y, Liu J, Li X. Selective poly adenylation predicts the efficacy of immunotherapy in patients with lung adenocarcinoma by multiple omics research. Anticancer Drugs. 2022;33:943-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 79. | Wu L, Li H, Liu Y, Fan Z, Xu J, Li N, Qian X, Lin Z, Li X, Yan J. Research progress of 3D-bioprinted functional pancreas and in vitro tumor models. Int J Bioprinting. 2024;10:1256. [DOI] [Full Text] |
| 80. | Guo T, Tang XH, Gao XY, Zhou Y, Jin B, Deng ZQ, Hu Y, Xing XF, Li ZY, Ji JF. A liquid biopsy signature of circulating exosome-derived mRNAs, miRNAs and lncRNAs predict therapeutic efficacy to neoadjuvant chemotherapy in patients with advanced gastric cancer. Mol Cancer. 2022;21:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 81. | Wu L, Li X, Qian X, Wang S, Liu J, Yan J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines (Basel). 2024;12:186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 59] [Reference Citation Analysis (0)] |
| 82. | Conti CB, Agnesi S, Scaravaglio M, Masseria P, Dinelli ME, Oldani M, Uggeri F. Early Gastric Cancer: Update on Prevention, Diagnosis and Treatment. Int J Environ Res Public Health. 2023;20:2149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 78] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 83. | Aalami AH, Aalami F, Sahebkar A. Gastric Cancer and Circulating microRNAs: An Updated Systematic Review and Diagnostic Meta-Analysis. Curr Med Chem. 2023;30:3798-3814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 84. | Deng D, Zhang Y, Zhang R, Yi J, Dong J, Sha L, Yan M. Circulating Proteins and Metabolite Biomarkers in Gastric Cancer: A Systematic Review and Meta-analysis. Arch Med Res. 2023;54:124-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 85. | Wu L, Chen X, Zeng Q, Lai Z, Fan Z, Ruan X, Li X, Yan J. NR5A2 gene affects the overall survival of LUAD patients by regulating the activity of CSCs through SNP pathway by OCLR algorithm and immune score. Heliyon. 2024;10:e28282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 86. | Jhi JH, Kim GH, Park SJ, Kim DU, Lee MW, Lee BE, Kwon CH, Cho YK. Circulating Tumor Cells and TWIST Expression in Patients with Metastatic Gastric Cancer: A Preliminary Study. J Clin Med. 2021;10:4481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Thanh Huong P, Gurshaney S, Thanh Binh N, Gia Pham A, Hoang Nguyen H, Thanh Nguyen X, Pham-The H, Tran PT, Truong Vu K, Xuan Duong N, Pelucchi C, La Vecchia C, Boffetta P, Nguyen HD, Luu HN. Emerging Role of Circulating Tumor Cells in Gastric Cancer. Cancers (Basel). 2020;12:695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 88. | Wu L, Li X, Yan J. Commentary: Machine learning developed an intratumor heterogeneity signature for predicting prognosis and immunotherapy benefits in cholangiocarcinoma. Transl Oncol. 2024;45:101995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 89. | Cheng R, Peng Y, Sun X, Zhang S, Li P. Circulating Tumor Cells as Diagnostic Markers of Early Gastric Cancer and Gastric Precancerous Lesions. Oncology. 2023;101:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Lee MW, Kim GH, Jeon HK, Park SJ. Clinical Application of Circulating Tumor Cells in Gastric Cancer. Gut Liver. 2019;13:394-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 91. | Sugano K, Moss SF, Kuipers EJ. Gastric Intestinal Metaplasia: Real Culprit or Innocent Bystander as a Precancerous Condition for Gastric Cancer? Gastroenterology. 2023;165:1352-1366.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 92. | Hou W, Zhao Y, Zhu H. Predictive Biomarkers for Immunotherapy in Gastric Cancer: Current Status and Emerging Prospects. Int J Mol Sci. 2023;24:15321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 93. | Li Z, Song M, Han S, Jin C, Yang J. The prognostic role of circulating tumor cells in gastric cancer: A meta-analysis. Front Oncol. 2022;12:963091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Zhou C, Guo L, Cai Q, Xi W, Yuan F, Zhang H, Yan C, Huang L, Zhu Z, Zhang J. Circulating neutrophils activated by cancer cells and M2 macrophages promote gastric cancer progression during PD-1 antibody-based immunotherapy. Front Mol Biosci. 2023;10:1081762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 95. | Yi-Wen W, Long-Long L, Ming L, Hao L, Kong-Wang H. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Arch Med Sci. 2022;18:1297-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 96. | Li C, Yang S, Li R, Gong S, Huang M, Sun Y, Xiong G, Wu D, Ji M, Chen Y, Gao C, Yu Y. Dual-Aptamer-Targeted Immunomagnetic Nanoparticles to Accurately Explore the Correlations between Circulating Tumor Cells and Gastric Cancer. ACS Appl Mater Interfaces. 2022;14:7646-7658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 97. | Zhang Y, Yao J, Feng J, Wang S, Yang Z, Huang W, Da M. Relationship between PRRX1, circulating tumor cells, and clinicopathological parameter in patients with gastric cancer. J BUON. 2020;25:1455-1462. [PubMed] |
| 98. | Fan L, Chong X, Zhao M, Jia F, Wang Z, Zhou Y, Lu X, Huang Q, Li P, Yang Y, Hu Z, Li Q, Zhang X, Shen L. Ultrasensitive Gastric Cancer Circulating Tumor Cellular CLDN18.2 RNA Detection Based on a Molecular Beacon. Anal Chem. 2021;93:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Miki Y, Yashiro M, Kuroda K, Okuno T, Togano S, Masuda G, Kasashima H, Ohira M. Circulating CEA-positive and EpCAM-negative tumor cells might be a predictive biomarker for recurrence in patients with gastric cancer. Cancer Med. 2021;10:521-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 100. | Li H, Zhu YZ, Xu L, Han T, Luan J, Li X, Liu Y, Wang Z, Liu Q, Kong X, Zou C, Su L, Hou Y, Chen X, Chen L, Wang R, Xu Z, Zhao M. Exploring new frontiers: cell surface vimentin as an emerging marker for circulating tumor cells and a promising therapeutic target in advanced gastric Cancer. J Exp Clin Cancer Res. 2024;43:129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 101. | Zeng CDD, Jin CC, Gao C, Xiao AT, Tong YX, Zhang S. Preoperative Folate Receptor-Positive Circulating Tumor Cells Are Associated With Occult Peritoneal Metastasis and Early Recurrence in Gastric Cancer Patients: A Prospective Cohort Study. Front Oncol. 2022;12:769203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Abdallah EA, Braun AC, Flores BCTCP, Senda L, Urvanegia AC, Calsavara V, Fonseca de Jesus VH, Almeida MFA, Begnami MD, Coimbra FJF, da Costa WL Jr, Nunes DN, Dias-Neto E, Chinen LTD. The Potential Clinical Implications of Circulating Tumor Cells and Circulating Tumor Microemboli in Gastric Cancer. Oncologist. 2019;24:e854-e863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 103. | Chen Y, Yuan J, Li Y, Li X, Yang Y, Li J, Li Y, Shen L. Profiling heterogenous sizes of circulating tumor microemboli to track therapeutic resistance and prognosis in advanced gastric cancer. Hum Cell. 2021;34:1446-1454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 104. | Ning D, Cui K, Liu M, Ou Y, Wang Z, Zou B, Shen Y, Lu X, Li S, Li P. Comparison of CellSearch and Circulating Tumor Cells (CTC)-Biopsy Systems in Detecting Peripheral Blood Circulating Tumor Cells in Patients with Gastric Cancer. Med Sci Monit. 2021;27:e926565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 105. | Zuo Y, Xia Y, Lu W, Li Y, Xiao Y, Gao S, Zhou Z, Xu H, Feng X, Li C, Yu Y. A multifunctional black phosphorus nanosheet-based immunomagnetic bio-interface for heterogeneous circulating tumor cell capture and simultaneous self-identification in gastric cancer patients. Nanoscale. 2023;15:3872-3883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Chen Y, Li Y, Qi C, Zhang C, Liu D, Deng Y, Fu Y, Khadka VS, Wang DD, Tan S, Liu S, Peng Z, Gong J, Lin PP, Zhang X, Li J, Li Y, Shen L. Dysregulated KRAS gene-signaling axis and abnormal chromatin remodeling drive therapeutic resistance in heterogeneous-sized circulating tumor cells in gastric cancer patients. Cancer Lett. 2021;517:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 107. | Negishi R, Yamakawa H, Kobayashi T, Horikawa M, Shimoyama T, Koizumi F, Sawada T, Oboki K, Omuro Y, Funasaka C, Kageyama A, Kanemasa Y, Tanaka T, Matsunaga T, Yoshino T. Transcriptomic profiling of single circulating tumor cells provides insight into human metastatic gastric cancer. Commun Biol. 2022;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 108. | Gu X, Zhang Q, Zhang W, Zhu L. Curcumin inhibits liver metastasis of gastric cancer through reducing circulating tumor cells. Aging (Albany NY). 2019;11:1501-1509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 109. | Xiang A, Xue M, Ren F, Wang L, Ye Z, Li D, Ji Q, Ji G, Lu Z. High‑throughput and continuous flow isolation of rare circulating tumor cells and clusters in gastric cancer from human whole blood samples using electromagnetic vibration‑based filtration. Oncol Rep. 2020;43:1975-1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Zhu Y, Chen N, Chen M, Cui X, Yang H, Zhu X, Dai J, Gong Y, Gu D, Huo X, Huang H, Tang C. Circulating tumor cells: A surrogate to predict the effect of treatment and overall survival in gastric adenocarcinoma. Int J Biol Markers. 2021;36:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 111. | Cho JH, Kim SY, Lee J, Park SH, Park JO, Park YS, Lim HY, Kang WK, Kim ST. Detection of circulating tumor cells (CTCs) in cerebrospinal fluid of a patient with HER2-overexpressing gastric cancer and single cell analysis of intra-patient heterogeneity of CTCs. Transl Cancer Res. 2019;8:2107-2112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 112. | Matsushita D, Uenosono Y, Arigami T, Yanagita S, Okubo K, Kijima T, Miyazono F, Hamanoue M, Hokita S, Nakashima S, Ohtsuka T, Natsugoe S. Clinical significance of circulating tumor cells in the response to trastuzumab for HER2-negative metastatic gastric cancer. Cancer Chemother Pharmacol. 2021;87:789-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | Ishiguro Y, Sakihama H, Yoshida T, Ichikawa N, Homma S, Fukai M, Kawamura H, Takahashi N, Taketomi A. Prognostic Significance of Circulating Tumor Cells with Mesenchymal Phenotypes in Patients with Gastric Cancer: A Prospective Study. Ann Surg Oncol. 2021;28:1178-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 114. | Qiu Y, Zhang X, Deng X, Zhang R, Cai Z, Zhang Z, Liu H. Circulating tumor cell-associated white blood cell cluster is associated with poor survival of patients with gastric cancer following radical gastrectomy. Eur J Surg Oncol. 2022;48:1039-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 115. | Song Q, Lv X, Ru Y, Dong J, Chang R, Wu D, Chen L, Wang X, Guo X. Circulating exosomal gastric cancer-associated long noncoding RNA1 as a noninvasive biomarker for predicting chemotherapy response and prognosis of advanced gastric cancer: A multi-cohort, multi-phase study. EBioMedicine. 2022;78:103971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 116. | Liu M, Wang R, Sun X, Liu Y, Wang Z, Yan J, Kong X, Liang S, Liu Q, Zhao T, Ji X, Wang G, Wang F, Wang G, Chen L, Zhang Q, Lv W, Li H, Sun M. Prognostic significance of PD-L1 expression on cell-surface vimentin-positive circulating tumor cells in gastric cancer patients. Mol Oncol. 2020;14:865-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 117. | Becerril-Rico J, Grandvallet-Contreras J, Ruíz-León MP, Dorantes-Cano S, Ramírez-Vidal L, Tinajero-Rodríguez JM, Ortiz-Sánchez E. Circulating Gastric Cancer Stem Cells as Blood Screening and Prognosis Factor in Gastric Cancer. Stem Cells Int. 2024;2024:9999155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | ZiaSarabi P, Sorayayi S, Hesari A, Ghasemi F. Circulating microRNA-133, microRNA-17 and microRNA-25 in serum and its potential diagnostic value in gastric cancer. J Cell Biochem. 2019;120:12376-12381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 119. | Cheng J, Yang A, Cheng S, Feng L, Wu X, Lu X, Zu M, Cui J, Yu H, Zou L. Circulating miR-19a-3p and miR-483-5p as Novel Diagnostic Biomarkers for the Early Diagnosis of Gastric Cancer. Med Sci Monit. 2020;26:e923444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 120. | Zhou H, Shen W, Zou H, Lv Q, Shao P. Circulating exosomal long non-coding RNA H19 as a potential novel diagnostic and prognostic biomarker for gastric cancer. J Int Med Res. 2020;48:300060520934297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 121. | Guo X, Lv X, Ru Y, Zhou F, Wang N, Xi H, Zhang K, Li J, Chang R, Xie T, Wang X, Li B, Chen Y, Yang Y, Chen L, Chen L. Circulating Exosomal Gastric Cancer-Associated Long Noncoding RNA1 as a Biomarker for Early Detection and Monitoring Progression of Gastric Cancer: A Multiphase Study. JAMA Surg. 2020;155:572-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 122. | Yuan F, Cheng L, Zhao Z, Wang Y, Jia B, Lei W. Expression of circulating RNA (hsa_circ_0074854) in human gastric cancer and its clinical significance. Panminerva Med. 2022;64:124-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 123. | Nanishi K, Konishi H, Shoda K, Arita T, Kosuga T, Komatsu S, Shiozaki A, Kubota T, Fujiwara H, Okamoto K, Ichikawa D, Otsuji E. Circulating circERBB2 as a potential prognostic biomarker for gastric cancer: An investigative study. Cancer Sci. 2020;111:4177-4186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 124. | Xia F, Zhang Q, Ndhlovu E, Zhang M, Zou Y. A Novel Nomogram to Predict Resectable Gastric Cancer Based on Preoperative Circulating Tumor Cell. Clin Transl Gastroenterol. 2024;15:e00561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Ge L, Zhang N, Li D, Wu Y, Wang H, Wang J. Circulating exosomal small RNAs are promising non-invasive diagnostic biomarkers for gastric cancer. J Cell Mol Med. 2020;24:14502-14513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 126. | Shu X, Cai H, Lan Q, Cai Q, Ji BT, Zheng W, Shu XO. A Prospective Investigation of Circulating Metabolome Identifies Potential Biomarkers for Gastric Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2021;30:1634-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 127. | Cao B, Liu L, Zhang R, Dong H, Shen J. Sensitivity and specificity of folate receptor α-positive circulating tumour cells in gastric cancer. Postgrad Med J. 2024;100:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 128. | Li W, Zhang X, Yang Y, Lin J, Zhou K, Sun R, Dang C, Diao D. Circulating tumor cells are a good predictor of tumor recurrence in clinical patients with gastric cancer. Sci Rep. 2024;14:12758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 129. | Lu R, Chen Q, Liu X, Shen S, Pan Z, Shi C. Detection of circulating stage III-IV gastric cancer tumor cells based on isolation by size of epithelial tumor: using the circulating tumor cell biopsy technology. Transl Cancer Res. 2019;8:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 130. | Palle J, Hirsch L, Lapeyre-Prost A, Malka D, Bourhis M, Pernot S, Marcheteau E, Voron T, Castan F, Lacotte A, Benhamouda N, Tanchot C, François E, Ghiringhelli F, de la Fouchardière C, Zaanan A, Tartour E, Taieb J, Terme M. Targeting HGF/c-Met Axis Decreases Circulating Regulatory T Cells Accumulation in Gastric Cancer Patients. Cancers (Basel). 2021;13:5562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 131. | Mohamed WA, Schaalan MF, Ramadan B. The expression profiling of circulating miR-204, miR-182, and lncRNA H19 as novel potential biomarkers for the progression of peptic ulcer to gastric cancer. J Cell Biochem. 2019;120:13464-13477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 132. | Ju Y, Seol YM, Kim J, Jin H, Choi GE, Jang A. Expression Profiles of Circulating MicroRNAs in XELOX-Chemotherapy-Induced Peripheral Neuropathy in Patients with Advanced Gastric Cancer. Int J Mol Sci. 2022;23:6041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 133. | Wang JB, Gao YX, Ye YH, Lin TX, Li P, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Lin JL, Huang ZN, Zheng HL, Xie JW, Zheng CH, Huang CM. CDK5RAP3 acts as a tumour suppressor in gastric cancer through the infiltration and polarization of tumour-associated macrophages. Cancer Gene Ther. 2023;30:22-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 134. | Wu L, Zheng Y, Ruan X, Wu D, Xu P, Liu J, Wu D, Li X. Long-chain noncoding ribonucleic acids affect the survival and prognosis of patients with esophageal adenocarcinoma through the autophagy pathway: construction of a prognostic model. Anticancer Drugs 2022; 33: e590-e603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 135. | Cho JH, Sim MH, Kim SY, Kim K, Lee T, Lee J, Kang WK, Kim ST. Analysis of intrapatient heterogeneity of circulating tumor cells at the single-cell level in the cerebrospinal fluid of a patient with metastatic gastric cancer. J Cancer Res Ther. 2021;17:1047-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 136. | Zhou Y, Sun X, Zhou L, Zhang X. pH-Sensitive and Long-Circulation Nanoparticles for Near-Infrared Fluorescence Imaging-Monitored and Chemo-Photothermal Synergistic Treatment Against Gastric Cancer. Front Pharmacol. 2020;11:610883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 137. | Jiang H, Guo S, Zhao Y, Wang Y, Piao HY, Wu Y, Zhang J. Circulating long non-coding RNA PCGEM1 as a novel biomarker for gastric cancer diagnosis. Pathol Res Pract. 2019;215:152569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 138. | Wei H, Pu K, Liu XG, Li BX, Zhang HS, Wang H, Wang H, Sun WM, Wang YP. The diagnostic value of circulating microRNAs as a biomarker for gastric cancer: A metaanalysis. Oncol Rep. 2019;41:87-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 139. | Necula L, Matei L, Dragu D, Pitica I, Neagu AI, Bleotu C, Dima S, Popescu I, Diaconu CC, Chivu-Economescu M. High plasma levels of COL10A1 are associated with advanced tumor stage in gastric cancer patients. World J Gastroenterol. 2020;26:3024-3033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 140. | Lu X, Lu J, Wang S, Zhang Y, Ding Y, Shen X, Jing R, Ju S, Chen H, Cong H. Circulating serum exosomal miR-92a-3p as a novel biomarker for early diagnosis of gastric cancer. Future Oncol. 2021;17:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 141. | Wada Y, Nishi M, Yoshikawa K, Takasu C, Tokunaga T, Nakao T, Kashihara H, Yoshimoto T, Shimada M. Circulating Exosomal MicroRNA Signature Predicts Peritoneal Metastasis in Patients with Advanced Gastric Cancer. Ann Surg Oncol. 2024;31:5997-6006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 142. | Hu B, Tian X, Li Y, Liu Y, Yang T, Han Z, An J, Kong L, Li Y. Epithelial-mesenchymal transition may be involved in the immune evasion of circulating gastric tumor cells via downregulation of ULBP1. Cancer Med. 2020;9:2686-2697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 143. | Liu X, Chu KM. Exosomal miRNAs as circulating biomarkers for prediction of development of haematogenous metastasis after surgery for stage II/III gastric cancer. J Cell Mol Med. 2020;24:6220-6232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 144. | Cao F, Hu Y, Chen Z, Han W, Lu W, Xu J, Ding H, Shen X. Circulating long noncoding RNAs as potential biomarkers for stomach cancer: a systematic review and meta-analysis. World J Surg Oncol. 2021;19:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 145. | Wu L, Yang L, Qian X, Hu W, Wang S, Yan J. Mannan-Decorated Lipid Calcium Phosphate Nanoparticle Vaccine Increased the Antitumor Immune Response by Modulating the Tumor Microenvironment. J Funct Biomater. 2024;15:229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 146. | Saito H, Shimizu S, Kono Y, Murakami Y, Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y, Ashida K, Fujiwara Y. PD-1 Expression on Circulating CD8(+) T-Cells as a Prognostic Marker for Patients With Gastric Cancer. Anticancer Res. 2019;39:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Ko K, Kananazawa Y, Yamada T, Kakinuma D, Matsuno K, Ando F, Kuriyama S, Matsuda A, Yoshida H. Methylation status and long-fragment cell-free DNA are prognostic biomarkers for gastric cancer. Cancer Med. 2021;10:2003-2012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 148. | Guo X, Wang Y, Zha L, Li H, Qian K. DNA methylation-related lncRNAs predict prognosis and immunotherapy response in gastric cancer. J Cancer Res Clin Oncol. 2023;149:14745-14760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 149. | Chong X, Li Y, Lu J, Feng X, Li Y, Zhang X. Tracking circulating PD-L1-positive cells to monitor the outcome of patients with gastric cancer receiving anti-HER2 plus anti-PD-1 therapy. Hum Cell. 2024;37:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 150. | Yang S, Liu XL, Guo XL, Song B, Li SZ, Sun XF, Feng Y. Solitary metastasis to the skin and colon from gastric cancer after curative gastrectomy and chemotherapy: A case report. Medicine (Baltimore). 2020;99:e21532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 151. | Shin HM, Kim G, Kim S, Sim JH, Choi J, Kim M, Kwon M, Ye SK, Lee DS, Cho SW, Kim ST, Lee J, Kim HR. Chromatin accessibility of circulating CD8(+) T cells predicts treatment response to PD-1 blockade in patients with gastric cancer. Nat Commun. 2021;12:975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 152. | Chen Y, Zheng J, Chen J. Preoperative Circulating MiR-210, a Risk Factor for Postoperative Delirium Among Elderly Patients with Gastric Cancer Undergoing Curative Resection. Curr Pharm Des. 2020;26:5213-5219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 153. | Minikes AM, Song Y, Feng Y, Yoon C, Yoon SS, Jiang X. E-cadherin is a biomarker for ferroptosis sensitivity in diffuse gastric cancer. Oncogene. 2023;42:848-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 154. | Zhang J, Qiu W, Zhang W, Chen Y, Shen H, Zhu H, Liang X, Shen Z. Tracking of trastuzumab resistance in patients with HER2-positive metastatic gastric cancer by CTC liquid biopsy. Am J Cancer Res. 2023;13:5684-5697. [PubMed] |
| 155. | Busada JT, Ramamoorthy S, Cain DW, Xu X, Cook DN, Cidlowski JA. Endogenous glucocorticoids prevent gastric metaplasia by suppressing spontaneous inflammation. J Clin Invest. 2019;129:1345-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 156. | Zhou S, Xu B, Qi L, Zhu D, Liu B, Wei J. Next-generation sequencing reveals mutational accordance between cell-free DNA from plasma, malignant pleural effusion and ascites and directs targeted therapy in a gastric cancer patient. Cancer Biol Ther. 2019;20:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 157. | Shao JP, Su F, Zhang SP, Chen HK, Li ZJ, Xing GQ, Liu HJ, Li YY. miR-212 as potential biomarker suppresses the proliferation of gastric cancer via targeting SOX4. J Clin Lab Anal. 2020;34:e23511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 158. | Chen W, Yu J, Zhang Y, Wang W, Yu W, Shi X, Xu Z, Liu H. First-line application of apatinib combined with S-1 based on peripheral circulating tumor cell screening to treat advanced gastric adenocarcinoma: a case report. Ann Transl Med. 2019;7:181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 159. | Gęca K, Rawicz-Pruszyński K, Mielko J, Mlak R, Sędłak K, Polkowski WP. Rapid Detection of Free Cancer Cells in Intraoperative Peritoneal Lavage Using One-Step Nucleic Acid Amplification (OSNA) in Gastric Cancer Patients. Cells. 2020;9:2168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 160. | Chung KY, Quek JM, Neo SH, Too HP. Polymer-Based Precipitation of Extracellular Vesicular miRNAs from Serum Improve Gastric Cancer miRNA Biomarker Performance. J Mol Diagn. 2020;22:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 161. | van Zweeden AA, Opperman RCM, Honeywell RJ, Peters GJ, Verheul HMW, van der Vliet HJ, Poel D. The prognostic impact of circulating miRNAs in patients with advanced esophagogastric cancer during palliative chemotherapy. Cancer Treat Res Commun. 2021;27:100371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 162. | Hu Y, Chen C, Tong X, Chen S, Hu X, Pan B, Sun X, Chen Z, Shi X, Hu Y, Shen X, Xue X, Lu M. NSUN2 modified by SUMO-2/3 promotes gastric cancer progression and regulates mRNA m5C methylation. Cell Death Dis. 2021;12:842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 163. | Xu W, Lai Y, Pan Y, Tan M, Ma Y, Sheng H, Wang J. m6A RNA methylation-mediated NDUFA4 promotes cell proliferation and metabolism in gastric cancer. Cell Death Dis. 2022;13:715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 164. | Zhang B, Wu Q, Li B, Wang D, Wang L, Zhou YL. m(6)A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer. 2020;19:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 737] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 165. | Wang P, Zhao L, Rui Y, Ding Y. SMYD3 regulates gastric cancer progression and macrophage polarization through EZH2 methylation. Cancer Gene Ther. 2023;30:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 166. | Nie Y, Gao X, Cai X, Wu Z, Liang Q, Xu G, Liu N, Gao P, Deng J, Xu H, Shen Z, Cao C, Chen F, Zhang N, Song Y, Sun M, Liu C, Zhou G, Han W, Dou J, Xie H, Yao L, Liu Z, Ji G, Wang X, Zhao Q, Shang L, Fan D, Han X, Ren J, Liang H, Wang Z, Wang J, Wu Q, Yu J, Wu K; MAGIS Study Group. Combining methylated SEPTIN9 and RNF180 plasma markers for diagnosis and early detection of gastric cancer. Cancer Commun (Lond). 2023;43:1275-1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |