Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.107341
Revised: April 27, 2025
Accepted: June 6, 2025
Published online: July 15, 2025
Processing time: 116 Days and 0.9 Hours
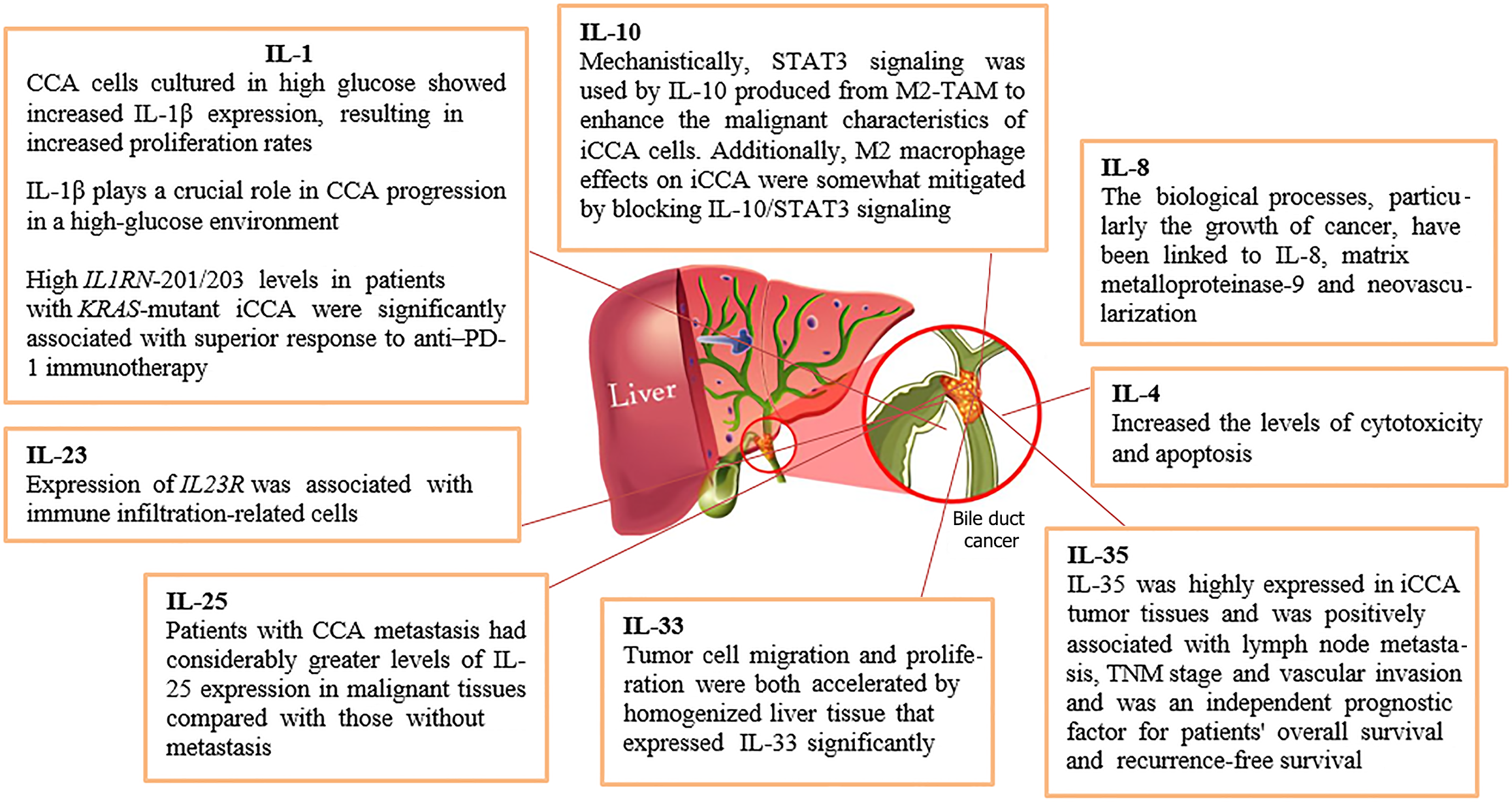
This article summarized the role of interleukins (ILs) in the pathogenesis of cholangiocarcinoma (CCA). It discovered a negative feedback mechanism wherein alternative splicing led to the upregulation of the IL 1 receptor antagonist (IL1RN) isoforms IL1RN-201 and IL1RN-203, which play a crucial anti-inflammatory role in KRAS-mutant intrahepatic CCA (iCCA). Higher levels of IL-4 receptor were associated with a worse survival rate in patients with CCA. In addition, elevated levels of serum IL-6 have been associated with the start and progression of CCA, a common cancer generated by inflammation. IL-8 was a useful predictor of human hilar CCA. Mechanistically, signal transducer and activator of transcription 3 signaling was used by IL-10 produced from M2-polarized tumor-associated macrophages to enhance the malignant characteristics of iCCA cells. It was suggested that IL-17A and IL-22 have an impact on the development of CCA associated with hepatic fluke infection. The most significant finding was the decreased expression of the IL-23 receptor, a prognostic gene, in iCCA. IL-25 may be a useful prognostic biomarker as aberrant expression of the protein in CCA tissue was linked to tumor spread and a poor prognosis. Tumor cell migration and proliferation were both accelerated by homogenized liver tissue that expressed IL-33 significantly. The correlation between high IL-35 expression and aggressiveness in iCCA highlights it as a useful biomarker for assessing the course and prognosis of iCCA in clinical settings. This article concluded that IL-1, IL-4, IL-6, IL-8, IL-10, IL-17, IL-23, IL-25, IL-33, and IL-35 play significant roles in the pathogenesis of CCA. Further research is required to find the association of other ILs such as IL-2, IL-3, IL-5, IL-7, IL-11, and more in the pathogenesis of CCA.
Core Tip: Interleukins (IL) contribute to cholangiocarcinoma (CCA) pathogenesis by promoting chronic inflammation, enhancing tumor cell survival, proliferation, and invasion, and shaping a tumor microenvironment that supports cancer growth and metastasis. ILs, which are a group of cytokines, play a crucial role in mediating the immune response and inflammation. This article concluded that IL-1, IL-4, IL-6, IL-8, IL-10, IL-17, IL-23, IL-25, IL-33, and IL-35 play significant roles in the pathogenesis of CCA. Further research is required to find the association of other ILs such as IL-2, IL-3, IL-5, IL-7, IL-11, and more in the pathogenesis of CCA.
- Citation: Rafaqat S, Hamid H, Asif R, Asif M, Tariq M, Saleem M, Abaid H. Role of interleukins in the pathogenesis of cholangiocarcinoma: A literature review. World J Gastrointest Oncol 2025; 17(7): 107341
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/107341.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.107341
Cholangiocarcinoma (CCA) is one of the most aggressive gastrointestinal cancers with a rising worldwide incidence over the last decade. Of all primary liver malignancies, CCA accounts for 10%-15% of cases, making it the second most frequent form of liver cancer. CCA, also known as bile duct cancer, is a type of cancer that forms in the bile ducts[1]. In recent decades, there has been a global increase in both the incidence and fatality rates associated with this illness[2]. Although most patients with CCA patients are discovered at an advanced stage and are thus not eligible for surgery, surgical resection is still a possible curative therapy. It is an extremely deadly hepatobiliary system adenocarcinoma with a 5-year overall survival (OS) rate of 5%-17%[3].
CCA can be categorized as intrahepatic CCA (iCCA or ICC), perihilar CCA, or distal CCA depending on the anatomy and surgery. iCCAs originate above the second-order bile ducts, perihilar CCAs originate above the cystic duct and below the second-order bile ducts, and distal CCAs originate below the cystic duct. Therefore, surgical techniques for each entity change dramatically[4]. Multiple types of complex biological processes underlie CCA and contribute to its aggressive nature and resistance to treatment. At the molecular level genetic changes like mutations are essential for encouraging unchecked cell proliferation and survival, especially in intrahepatic subtypes[5].
Epigenetic changes, such as DNA methylation and non-coding RNA dysregulation, further impair gene expression and aid in the development of tumors. A protumorigenic microenvironment is fostered by chronic inflammation, which activates important signaling pathways including nuclear factor kappa B and signal transducer and activator of transcription (STAT) 3. While immune evasion mechanisms, such as programmed death ligand 1 expression, enable CCA cells to evade immune monitoring, the epithelial-mesenchymal transition (EMT) pathway gives tumor cells invasive characteristics. The intricacy of CCA is highlighted by these interrelated biological processes, which also offer potential problems for focused treatments[5].
As such, it is imperative that novel biomarkers be found as soon as possible and that treatment plans be developed for this malignancy. Prolonged inflammation is a major factor in the development and progression of CCA, a frequent malignancy carried on by inflammation. A major risk factor for CCA is chronic inflammation of the biliary system brought on by primary sclerosing cholangitis (PSC), choledocholithiasis, or cholelithiasis[6]. Chronic inflammatory responses can also result from other risk factors for CCA, such as bile duct stones and infections (HBV, liver fluke, salmonella, etc.)[7].
With a 2% 5-year survival rate after metastasis, CCA, also known as cancer of the biliary epithelium, is an aggressive and very uncommon type of bile duct cancer. Although a variety of risk factors have been linked to the growth and progression of CCA, the most frequent causal relationship between the risk factors and the formation of CCA is an inflammatory environment close to the biliary tree[8]. The growth and metastasis of CCA are significantly influenced by the existence and maintenance of an inflammatory milieu at the location of the initial tumor, supporting the idea that inflammation predisposes afflicted persons to CCA[8]. Furthermore, CCA aggressiveness and metastasis are strongly influenced by processes that activate the tumor vasculature and promote angiogenesis and lymphangiogenesis[8].
Interleukins (ILs) are a group of cytokines (signaling proteins) that play critical roles in the immune system by regulating inflammation, immune responses, and hematopoiesis. The effect of ILs on the inflammatory response has led to their classifications in the scientific literature. Three unique groupings were produced by this categorization. IL-1, IL-4, IL-5, IL-6, IL-8, IL-9, IL-13, IL-14, and IL-15 are among the 22 inflammatory cytokines that make up the first and biggest category. IL-7, IL-10, IL-30, and IL-37 are among the 14 anti-inflammatory chemicals that make up the second group. IL-2, IL-3, IL-11, and IL-12 are examples of dual-function ILs that fall into the third category. These molecules might operate as both inflammatory and anti-inflammatory agents under certain conditions[9]. However, this review article aimed to summarize the knowledge of ILs in the pathogenesis of CCA as explained in Figure 1 and Table 1.
| Interleukins | Pathogenesis in cholangiocarcinoma |
| IL-6 | IL-6, IL-6R, CRP, gp130, and JAK2 were inversely correlated with vascular invasion, which is a risk factor for poor prognosis in patients with CCA |
| Plays a central role in tumorigenesis, angiogenesis, proliferation, and metastasis in biliary tract cancer | |
| Recently, IL-6 has been shown to induce PD-L1 expression through the mTOR pathway in iCCA | |
| The inflammatory cytokine such as IL-6 enhances tumor growth in cholangiocarcinoma by altered gene expression via autocrine mechanisms. IL-6 can regulate the activity of DNA methyltransferases, and aberrant DNA methylation can contribute to carcinogenesis | |
| Chronic inflammation may be a contributing factor in the development of cholangiocarcinomas since inflammation may provide survival signals to the lesion. It demonstrated the autocrine contribution of the inflammatory cytokine-like IL-6 to survival signals and the significant role played by myeloid cell leukaemia-1, an antiapoptotic member of the B-cell leukemia-2 family, in the tumor necrosis factor-related apoptosis-inducing ligand resistance in this neoplasm | |
| IL-8 | EMT-associated gene expression was significantly suppressed, and CD97 RNA interference reduced the IL-8-induced stimulation of cell migration and invasion |
| When siCXCR2 was used, it was demonstrated to dramatically reduce the carcinogenic effects of IL-8 by preventing the phosphorylation of PI3K/AKT, therefore indicating whether CXCR1 or CXCR2 are downstream effectors of IL-8 | |
| The stimulation of the PI3K pathway of CD97 expression was confirmed by the administration of the inhibitor LY294002 | |
| When CD97 was silenced in nude mice, the substantial effects of IL-8 injection on lung metastasis and tumor development were significantly reduced in vivo | |
| IL-17 | IL-17A expression and proliferation were revealed to be significantly correlated in both primary sclerosing cholangitis-CCA and random CCA by correlation analysis. Compared with sporadic CCA cells, PSC-CCA cells exhibit higher tumor cell proliferation |
| In vitro, IL-17A promoted the proliferation of CCA cells, which might be a factor in the high rate of proliferation seen in PSC-CCA in situ |
The literature review was conducted using databases such as Science Direct, PubMed, and Google Scholar on March 01, 2025, with specific keywords like “cholangiocarcinoma”, “intrahepatic cholangiocarcinoma”, “interleukins”, and “pathogenesis”. Current discoveries were prioritized, but no specific timeframe was imposed for clinical research. The English language was used for the selection of articles.
Interleukins play a significant role in the development and progression of cholangiocarcinoma by influencing various pathways involved in cell growth, proliferation, and survival. Another study explained that infection with Opisthorchis viverrini triggered chronic inflammation, and a small subset of those infected went on to develop advanced periductal fibrosis (APF) and cholangiocarcinoma. These biliary diseases may be related to inflammatory cytokines and/or gene polymorphisms encoding them[10]. Proinflammatory cytokines, including lymphotoxin-alpha (LT-α), tumor necrosis factor alpha (TNF-α), interferon-gamma (IFN-γ), IL-1β, and IL-6, were shown to be considerably higher in patients with CCA compared with non-CCA (APF- and APF+) instances.
PCR-restriction fragment length polymorphism or allele specific-PCR analyses were then used to look at polymorphisms in genes encoding IL-1β-511C/T, IL-6-174G/C, IFN-γ +874T/A, LT-α +252A/G, and TNF-α -308G/A. LT-α +252A/G and TNF-α -308G/A heterozygous and homozygous variants were found to exhibit considerably greater levels of these cytokines in the patients with CCA compared with the wildtype. While CCA was linked to IL-6 -174G/C polymorphisms. People infected with Opisthorchis viverrini who have many particular cytokine gene polymorphisms are vulnerable to developing fibrosis and CCA[10].
iCCA is an uncommon primary hepatic cancer with a poor prognosis and is poorly understood and understudied. Another study examined 11 genetic variants VEGF, EGF, EGFR, IL-1B, IL-6, CXCL8 (IL-8), IL-10, CXCR1, HIF1A, PTGS2, and COX-2 involved in tumor-promoting inflammation and their relationship to disease-free survival (DFS) and OS in patients undergoing curative-intent surgery for iCCA to identify prognostically relevant single-nucleotide poly
IL-1 is an inflammatory cytokine known for its wide range of physiological functions and pathological roles, playing a key part in both maintaining health and contributing to disease. IL-1 can start several physiological reactions, such as lymphocyte activation, production of acute-phase liver proteins, leukocyte infiltration at infection sites, fever, and anorexia. This highlights the critical function of cytokines in the innate immune response. The additional members of the IL-1 family, including IL-33, have demonstrated functions in inflammation and host defense since the discovery of IL-1α and IL-1β sequences more than 20 years ago. IL-1 family members are generated by macrophages as opposed to lymphocytes, which create IL-2. Blocking IL-1 receptor (IL-1R) is one way to treat autoinflammatory diseases that have been linked to IL-1 dysregulation in their pathophysiology[12-15].
KRAS mutations are key players in the development of tumors and are directly associated with protumor inflammation. Another study demonstrated that a particular landscape of alternative mRNA splicing linked to myeloid inflammation in iCCA was correlated with KRAS mutations based on multiomics data collected from a large group of patients. Next, it discovered a negative feedback mechanism by which KRAS-mutant iCCA benefits greatly from the overexpression of IL1RN-201/203 brought about by alternative splicing[16]. Both IL1RN-201/203 overexpression and anakinra therapy induced a noteworthy antitumor immune response in KRAS-mutant iCCA mice via modifying neutrophil recruitment and behaviors. In addition, anakinra therapy in KRAS-mutant iCCA mice synergistically improved anti-programmed death 1 therapy to activate intratumoral GZMB+ CD8+ T cells. Clinical research revealed a substantial correlation between a better response to anti-programmed death 1 immunotherapy and elevated IL1RN-201/203 levels in patients with KRAS-mutant iCCA[16].
CCA is more likely to develop and progress in people with diabetes mellitus (DM). It has been previously demonstrated that in CCA cells with unknown roles, high glucose levels upregulated IL-1β. With IL-1β being proposed as a communicative cytokine, another study aimed to explore molecular pathways connecting DM to the advancement of CCA. To simulate euglycemia and hyperglycemia, CCA cells were cultivated in conditions containing either normal (5.6 mmol/L) and high (25 mmol/L) glucose, respectively. Immunohistochemistry was utilized to investigate the expression of IL-1β and IL-1R in CCA tissues from people with and without DM[17].
Using siRNA and recombinant human IL-1R antagonist (rhIL-1RA), functional studies of IL-1β were carried out, and the effectiveness of the knockdown was examined using western blots. To study the effects of hyperglycemia on CCA development and the anti-tumor effects of IL-1RA, CCA xenografts were transplanted into BALB/c Rag-2 -/-Jak3 -/- mice. Compared with mice without DM, those with hyperglycemia had CCA tumors with considerably greater levels of IL-1β expression, and there was a positive correlation between IL-1β and fasting blood glucose levels. When CCA cells were cultivated in high glucose, they expressed more IL-1β, which led to faster rates of cell proliferation[17].
In vitro CCA cell proliferation was markedly inhibited by si-IL-1β or recombinant human IL-1RA-induced suppression of IL-1β signaling. In vivo, the synthetic IL-1RA anakinra demonstrated noteworthy anti-tumor actions and dramatically mitigated the growth-inducing effects of hyperglycemia in CCA xenografts. In a high-glucose milieu, IL-1β is essential to the advancement of CCA. Thus, targeting IL-1β may aid in improving the therapeutic results of CCA in patients with DM and hyperglycemia[17].
IL-4 is recognized as a cytokine that holds a central position in regulating the immune response. Investigations into the signal transduction of cytokines have elucidated the mechanism through which IL-4 carries out its functions. The two cytoplasmic proteins that are active during IL-4 signal transduction are IL-4-induced 4 phosphorylated substrate/insulin receptor substrate 2 and STAT6[18]. STAT6 plays a crucial role in modulating the biological activities of IL-4 as demonstrated by recent investigations employing STAT6-deficient animals. Moreover, cytokine IL-13, which is connected to IL-4, requires STAT6 to perform its tasks. Evidence suggests that both IL-4 and IL-13 can promote the synthesis of immunoglobulin E, a key mediator of allergic reactions[18].
Numerous cancer cells have overexpressed the IL-4 receptor (IL-4R), which is essential for the growth of tumors and the development of medication resistance. Phage display allowed for the identification of IL-4R targeting peptide 1 (IL4RPep-1), an IL-4R-binding peptide that is utilized to target tumors[19]. It utilized IL4RPep-1 to direct the delivery of a proapoptotic peptide to chemoresistant CCA that is unique to the tumor, therefore preventing the development of the tumor. Higher levels of IL-4R are associated with a worse survival rate in patients with CCA according to immunohistochemistry analysis of human primary CCA tissues. It revealed that IL-4R levels were elevated in moderately to poorly differentiated types. It was shown that IL4RPep-1 bonded more strongly to human CCA cells with high IL-4R expression (KKU-213 cells) than it did to KKU-055 cells with low IL-4R expression[19].
In KKU-213 cells, a combination of IL4RPep-1 and the proapoptotic peptide IL4RPep-1–KLA increased the levels of cytotoxicity and apoptosis induced by 5-fluorouracil. After internalizing in the cells, IL4RPep-1–KLA colocalized with mitochondria. The homing of IL4RPep-1–KLA and IL4RPep-1 to the KKU-213 tumor in mice was shown by whole-body fluorescence imaging and immunohistochemistry examination of tumor tissues[19].
Treatment with 5-fluorouracil alone did not significantly suppress tumor development in mice, but systemic delivery of IL4RPep-1–KLA effectively inhibited KKU-213 tumor growth. During peptide therapy, mice did not exhibit any notable systemic adverse effects, such as immunotoxicity or liver toxicity. These results imply that IL4RPep-1–KLA has promise as a targeted therapeutic agent to treat CCA that is resistant to chemotherapy[19].
Interleukin-4 receptor α (IL-4Rα) is known to be abundantly expressed on the surface of several types of solid tumors in humans. Previously, a novel IL-4Rα-lytic hybrid peptide that was composed of cell-lytic peptide and binding peptide to IL-4Rα was designed. The designed IL-4Rα-lytic hybrid peptide demonstrated both in vitro and in vivo antitumor activity against human pancreatic cancer cells that expressed IL-4Rα[20].
As a new molecular targeted treatment for human biliary tract cancer (BTC), the anticancer effect of the IL-4Rα-lytic hybrid peptide was assessed. Six BTC cell lines were exposed to cytotoxic action from the IL-4Rα-lytic hybrid peptide at concentrations as low as 5 μM, which was shown to kill 50% of all cells[20]. Additionally, it demonstrated that gemcitabine and IL-4Rα-lytic hybrid peptides had synergistic cytotoxic action in vitro. Furthermore, in a human BTC xenograft model in vivo, intravenous injection of IL-4Rα-lytic hybrid peptide markedly suppressed tumor development. These findings suggested that the IL-4Rα-lytic hybrid peptide was a powerful medication that may provide patients with BTC a new kind of treatment[20].
IL-6 is a type I transmembrane receptor that is activated by the binding of the IL-6 ligand to the coreceptor glycoprotein 130 (gp130) and the IL-6 receptor (IL-6R). Furthermore, soluble forms of the IL-6R can attach to IL-6, allowing it to continue binding and activating the coreceptor gp130[21]. This process is known as transient IL-6 signaling. Autophosphorylation and Janus kinase (JAK) activation are started when the ligand binds to the receptor. Activated JAKs subsequently phosphorylate and trigger the transcription factor STAT3. The active STAT3 factors then go to the nucleus, dimerize, and bind to DNA to control transcription. Most IL-6-dependent effects are ascribed to changes in gene expression regulated by the transcriptional regulatory function of STAT3[21].
BTC is a highly malignant tumor that starts from bile duct epithelium. The production of inflammatory cytokines as a result of persistent infection created an inflammatory milieu that affects BTC carcinogenesis. A key player in BTC carcinogenesis, angiogenesis, proliferation, and metastasis is IL-6, which is a multifunctional cytokine that is released by Kupffer cells, tumor-associated macrophages, cancer-associated fibroblasts, and cancer cells[22].
Furthermore, BTC is monitored, diagnosed, and prognosed using IL-6 as a clinical biomarker. Preclinical data suggested that IL-6 antibodies may increase the susceptibility of tumor immune checkpoint inhibitors by modulating the number of immune cells that infiltrate the tumor and controlling the expression of immunological checkpoints in the tumor microenvironment. It has recently been demonstrated that IL-6 induces the expression of programmed death ligand 1 in iCCA via the mTOR (mammalian target of rapamycin) pathway. Nevertheless, there is not enough data to conclude that IL-6 antibodies might strengthen immune responses and possibly overcome resistance in BTC to immune checkpoint inhibitors[22].
In the biliary system where ongoing inflammation significantly predisposes to CCA, the relationship between chronic inflammation and the onset and spread of cancer is well illustrated. Through altered gene expression through autocrine processes, inflammatory cytokines such as IL-6 promote tumor development in CCA[23].
Additionally, abnormal DNA methylation can contribute to carcinogenesis. IL-6 can influence the activity of DNA methyltransferases. Thus, long-term exposure to IL-6 affected methylation-dependent gene expression and altered cell development in CCA in humans. Malignant cholangiocytes that had been stably transfected to overexpress IL-6 were used to evaluate the connection between autocrine IL-6 pathways, DNA methylation, and altered cell proliferation[23].
The methylcytosine concentration, growth in soft agar, and cell proliferation of malignant cholangiocytes were all reduced when the DNA methylation inhibitor 5-aza-2’-deoxycytidine was applied. On the other hand, IL-6-overexpressing cells did not exhibit this impact. The overexpression of IL-6 led to modifications in the expression and promoter methylation of many genes, including epidermal growth factor receptor (EGFR). IL-6 enhanced gene and protein expression while decreasing EGFR promoter methylation[23].
Because IL-6 modifies the promoter methylation and gene expression of growth-regulatory pathways, including EGFR, it can thus contribute to the evolution of tumors through epigenetic control of gene expression. Additionally, increased IL-6 expression could make tumor cells less sensitive to methylation inhibitor-based therapeutic interventions. These observations have important implications for cancer treatment and provide a mechanism by which persistent cytokine stimulation can promote tumor growth[23].
Elevated levels of serum IL-6 have been associated with the start and progression of CCA. By employing multiplex immunofluorescence, it was possible to identify the expression of IL-6, IL-6R, gp130, C-reactive protein, JAK2, and STAT3 in CCA tissue microarray. It showed that in tumor tissues compared with normal tissues STAT3 expression was greater and IL-6 expression was lower[24].
The expression of genes involved in the IL-6 pathway was usually downregulated, particularly in the tumor microenvironment. It is significant to note that gp130 and JAK2 showed a substantial correlation in tumor tissues but just a modest correlation in normal tissue. IL-6, IL-6R, C-reactive protein, gp130, and JAK2 were inversely connected with vascular invasion, which is a risk factor for a poor prognosis in patients with CCA, even though the expression of the genes was directly linked to OS or DFS. The IL-6 signaling pathway can be useful in predicting the prognosis of CCA[24].
BTC includes CCA and gallbladder carcinoma (GBC). Because of delayed diagnosis and fast disease development, the 5-year survival rate for BTC is 5%-18%. One major risk factor for CCA and GBC is chronic inflammation. When IL-6 is released from the body, it can do so through soluble forms (IL-6 trans-signaling) or by a membrane-bound receptor alpha-chain (IL-6 classic signaling). Still, not much is understood about how IL-6 trans-signaling affects the cellular responses of BTC. It included searching The Cancer Genome Atlas database and analyzing original tumors as entire sections and tissue microarrays[25].
The OS of the patients was linked with the downregulation of IL-6Rα in GBC compared with non-inflammatory, non-neoplastic gallbladder tissue. Moreover, several CCA cell lines and substances for IL-6 traditional signaling and trans-signaling activation (IL-6 and hyper-IL-6) or inhibition (tocilizumab and sgp130Fc) were employed to ascertain their impacts on cellular processes between the two IL-6 signaling modalities. When sgp130Fc inhibited IL-6 trans-signaling, CCA cell line survival and apoptosis decreased while migration and proliferation increased. For GBC IL-6Rα expression is a favorable prognostic indicator. Additionally, tumor promotion can be achieved by activating IL-6 classic signaling and suppressing IL-6 trans-signaling. These results support excluding individuals receiving IL-6R inhibitor medication if they have GBC or other cancers linked to bile metabolism[25].
Patients with hepatobiliary carcinoma had higher levels of IL-6 than healthy controls. Increased pain ratings, a worse prognosis, and subpar performance status in patients were all linked to higher levels of IL-6. A correlation between high IL-6 levels and portal vein thrombosis around the time of cancer diagnosis was discovered[26].
CCA cells exhibit abnormal persistent phosphorylation (activation) of STAT3, which leads to increased myeloid cell leukemia-1 (Mcl-1) expression and resistance to apoptosis. This activation is mediated by IL-6. Another study investigated suppressor of cytokine signaling 3 (SOCS-3) regulation in human CCA because SOCS regulates the IL-6/STAT3 signaling pathway through a traditional feedback loops. The Mz-ChA-1 and CCLP1 human CCA cell lines as well as human CCA tissue were used to measure SOCS-3 expression. In CCA there was an inverse relationship found between the expression of SOCS-3 protein and phospho-STAT3[27].
The SOCS-3 promoter showed significant methylation in the tumor but not in the corresponding non-tumor tissue in those tumors that did not express SOCS-3. Similarly, it was shown that two CCA cell lines had methylation of the SOCS-3 promoter. Treatment with 5-aza-2’-deoxycytidine, a demethylating agent, ended the phospho-STAT3 response, decreased the amount of Mcl-1 in cells, and restored the induction of SOCS-3 by IL-6. The induction of Mcl-1 and phospho-STAT3 by IL-6 was likewise inhibited by enforced SOCS-3 expression. The cells were rendered more susceptible to TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis by either 5-aza-2’-deoxycytidine treatment or induced SOCS-3 expression. Extended IL-6/STAT3 signaling and elevated Mcl-1 expressions in CCA are caused by SOCS-3 epigenetic silencing[27].
Due to inadequate diagnosis methods and limited clinical value, iCCA is a deadly bile system malignancy with a poor prognosis. Peripheral blood indices and cytokine levels were examined to diagnose iCCA. Both the lymphocyte-to-monocyte ratio and IL-6 can predict iCCA. Individuals are susceptible to developing iCCA if their lymphocyte-to-monocyte ratio is less than 7.2 (odds ratio = 58.08, P < 0.001) or if their IL-6 concentration is more than 11.635 pg/mL (odds ratio = 23.33, P < 0.001)[28]. Since the calculation is based on a standard laboratory test that is accessible in the majority of hospitals, LMR is advised for iCCA screening.
The poor prognosis and lack of effective therapies associated with BTCs pose problems. Recent research has demonstrated the growth factor status of IL-6 in human bile duct epithelium and its association with tumor burden. After photodynamic treatment the change in IL-6 blood levels in patients with CCA was assessed. Patients with bile duct cancer had higher blood levels of IL-6. Furthermore, it indicated that the function of IL-6 was a tumor marker following photodynamic treatment[29]. Chronic inflammation may be a contributing factor in the development of CCAs since inflammation may provide survival signals to the malignancy[30].
The autocrine contribution of the IL-6 to survival signals and the significant role played by Mcl-1 in the TRAIL resistance in this neoplasm was demonstrated. Another study assessed whether Mcl-1 overexpression in CCA was caused in part by IL-6 signaling. Using immunohistochemistry, the expression of Mcl-1 and protein kinase B (AKT) in human tissue was evaluated. In cell lines the connection between IL-6 signaling, AKT activity, and Mcl-1 expression was investigated[30].
The preneoplastic bile duct inflammatory illness PSC and human CCA tissues both have significant expression of the serine/threonine kinases AKT and Mcl-1. Three human CCA lines had constitutively phosphorylated AKT as shown by immunoblotting. Subsequent investigation revealed that anti-IL-6-neutralizing antiserum therapy resulted in decreased Mcl-1 expression, lower AKT phosphorylation, and increased TRAIL sensitivity. Similarly, reduced AKT pathway activation, reduced Mcl-1 expression, and increased TRAIL-mediated apoptosis were seen with the AKT inhibitor A443654.3. These results demonstrated that Mcl-1 expression was elevated in CCA due to an autocrine IL-6/AKT signaling pathway, and they offered a potential method to overcome the apoptosis resistance[30].
Rat thioacetamide (TAA)-induced iCCA shares histological and molecular similarities with human iCCA. In TAA-CCAs activation of the EGFR and IL-6 pathways as well as important genes involved in cancer-related glucose metabolic reprogramming were confirmed. A further epigenetic protumorigenic writer, G9a, was upregulated in TAA-CCAs. It demonstrated that both mutant KRASG12D and EGFR signaling may stimulate IL-6 production in CCA cells. Moreover, there was an upregulation of phosphoglycerate dehydrogenase (PHGDH), the rate-limiting enzyme in the serine-glycine pathway, in human iCCA, and it was associated with the expression of G9a[31].
KRASG12D enhanced PHGDH expression, glucose flow towards serine synthesis, and CCA cell survival in a way that was reliant on G9a activity. Compared with controls, KRASG12D CAA cells were more susceptible to PHGDH and G9a inhibition. G9a pharmacological targeting decreased PHGDH expression in mouse iCCA. Novel protumorigenic pathways in CCA were discovered: The serine-glycine pathway was activated during glucose metabolism reprogramming in insulin-producing cancers (iCCA); mutant KRAS drove PHGDH production in a G9a-dependent manner; PHGDH and G9a emerged as therapeutic targets in iCCA; and EGFR signaling activation or KRAS mutation promoted IL-6 expression in tumor cells[31].
Of the specialized cytokines produced and released by different normal and malignant human cell types, IL-8 or CXCL8 is a member of the chemokine family. Its ability to cause leukocytes to migrate in a specific direction is what makes these cytokines unique. Their secretion usually occurs in reaction to pathophysiological circumstances, inflammatory cytokines, and growth factors[32-34]. IL-8 is a chemokine distinguished by its CXC amino acid motif and originally identified for its ability to attract leukocytes. It is now also recognized for its tumor-promoting and proangiogenic functions. As a chemotactic agent generated by activated monocytes and macrophages, IL-8 was one of the first chemokines to be discovered. It promoted the directed migration of neutrophils, basophils, and T lymphocytes. Subsequently, it was revealed that IL-8 plays a significant role in autoimmune, inflammatory, and infectious diseases. Due to its strong proinflammatory characteristics, tight regulation is in place, resulting in low or undetectable expression in normal tissues. Monocytes and macrophages produce angiogenic factors that contribute to the development of chronic inflammatory diseases marked by sustained angiogenesis. Researchers investigated whether IL-8, a cytokine known for attracting lymphocytes and neutrophils, also exhibited angiogenic activity[35-39].
The 5-year survival rate for iCCA is 20%-40% following surgery. It is still unclear how IL-8 contributes to the development of iCCA. The prometastatic gene CD97 and important EMT components vimentin and E-cadherin were first shown to be significantly upregulated using a transcriptome technique based on IL-8 stimulation. They are both independent predictors of iCCA prognosis according to multivariate Cox regression. In QBE and QBC-939 cells, IL-8 administration mechanistically produced CD97 expression at 50 ng/mL and 100 ng/mL, respectively[40].
Furthermore, EMT-associated gene expression was significantly suppressed, and CD97 RNA interference reduced the IL-8-induced stimulation of cell migration and invasion. When siCXCR2 was used, it was demonstrated to dramatically reduce the carcinogenic effects of IL-8 by preventing the phosphorylation of PI3K/AKT, therefore indicating whether CXCR1 or CXCR2 are downstream effectors of IL-8. Ultimately, the stimulation of the PI3K pathway of CD97 expression was confirmed by the administration of the inhibitor LY294002[40].
When CD97 was silenced in nude mice, the substantial effects of IL-8 injection on lung metastasis and tumor development were significantly reduced in vivo. The work as a whole provided a unique mechanism of the IL-8-CXCR2-PI3K/AKT axis in controlling CD97 expression, which mostly results in EMT-mediated iCCA metastasis. Targeting the tumor microenvironment in metastatic iCCA may have different options[40].
The biological processes, particularly the growth of cancer, have been linked to IL-8, matrix metalloproteinase (MMP-9), and neovascularization. Nevertheless, not much research has been done on the function of IL-8 in human hilar CCA. The clinicopathological importance and prognostic usefulness of IL-8, MMP-9, and microvessel density (MVD) in hilar CCA were investigated. It examined the relationship between IL-8 and MMP-9 expression, MVD, clinicopathological characteristics, and patient survival time[41].
A significant correlation was found between the expression of IL-8 in 56.5% of tumors and the advanced tumor, node, metastasis (TNM) stage as well as tumor recurrence. MVD and MMP-9 expression showed a promising connection with IL-8. Additionally, patient OS was considerably lower in those with high IL-8 expression compared with those with low expression. IL-8 was validated as an independent prognostic factor by multivariate analysis. In summary, MVD and IL-8 expression showed a substantial correlation, and IL-8 was a useful predictor of human hilar CCA[41].
Together with TGF-β and IL-35, IL-10 is an essential anti-inflammatory cytokine. The primary producers of IL-10 are activated immune cells, namely monocytes/macrophages and T cell subsets like regulatory T cells and helper T (Th) cells. Working via a transmembrane receptor complex made up of IL-10 receptor 1 and IL-10 receptor 2, IL-10 efficiently controls the activities of different immune cells. In monocytes/macrophages, IL-10 promotes antigen absorption while inhibiting the generation of inflammatory mediators and impeding antigen presentation. Moreover, IL-10 is important for the biological functions of T and B cells[42].
It has been demonstrated that the development of certain malignancies, including iCCA, is correlated with M2-polarized tumor-associated macrophages (M2-TAMs). Nevertheless, it is unclear exactly how M2 macrophages and iCCA interact with one another. Another study aimed to ascertain if and how M2 macrophages contribute to the malignancy of iCCA. In both of the mouse models, it was discovered that the iCCA tissues had a notably larger concentration of M2 macrophages than the normal bile ducts. Compared with intratumoral regions and normal liver, there was a significant increase in M2 macrophage infiltration in peritumoral areas[43].
The M2-TAM phenotype was produced by iCCA cells in macrophages, and the in vitro proliferation, invasion, and EMT of iCCA cells were enhanced by coculturing with these M2 macrophages. Mechanistically, STAT3 signaling was used by IL-10 produced from M2-TAM to enhance the malignant characteristics of iCCA cells. Additionally, M2 macrophage effects on iCCA were somewhat mitigated by blocking IL-10/STAT3 signaling[43].
One important cytokine that connects T cell activation to neutrophil mobilization and activation is IL-17 (often referred to as IL-17A). Therefore, IL-17 may either have a role in the pathophysiology of inflammatory illnesses like psoriasis and rheumatoid arthritis or mediate beneficial innate immunity against pathogens. According to a growing body of research using both human and animal models, IL-17 signaling ultimately contributes to the development of illness. IL-17 has strong pro-osteoclastogenic effects in addition to stimulating neutrophilic inflammation, which may aid in the etiology of rheumatoid arthritis, periodontitis, and other disorders involving bone immunopathology[44].
PSC is a chronic inflammatory disease of the biliary tree and a risk factor for development of CCA. It is believed that a chronic inflammatory milieu plays a crucial role in the pathogenesis of CCA associated with PSC, but this is not entirely understood. Another study investigated how the proliferation rate of cancer cells was affected by cytokines associated with inflammation in PSC. For this Ki-67 immunohistochemistry was used to calculate the proliferation index in PSC-CCA and spontaneous CCA[45]. Compared with random CCA, PSC-CCA had a considerably greater percentage of Ki-67 positive cancer cells. Patient-derived CCA organoids were exposed to five PSC-related cytokines IL-1β, IL-6, IL-17A, IFN-γ, and TNF-α to determine whether cytokines in the inflammatory milieu could induce cancer cell growth. In CCA organoids the organoid size increased by 45.9% ± 16.4% (P < 0.01) and proliferation rate by 38% ± 16% (P < 0.05) in response to IL-17A being the only stimulant that showed a meaningful effect on cell proliferation. According to IL-17A immunohistochemistry, PSC-CCA may express higher IL-17A than random CCA[45].
Furthermore, IL-17A expression and proliferation were revealed to be significantly correlated in both PSC-CCA and random CCA by correlation analysis. In summary compared with sporadic CCA cells, PSC-CCA cells exhibit higher tumor cell proliferation. In vitro, IL-17A promotes the proliferation of CCA cells, which might be a factor in the high rate of proliferation seen in PSC-CCA in situ. Thus, IL-17A is a novel target for possible therapy in (PSC-)CCA that will be investigated in upcoming studies[45].
It is uncertain if the proliferative cytokine IL-22 and the proinflammatory cytokine IL-17A are involved in the inflammatory and immunological mechanisms that cause CCA linked to liver fluke infection. In this instance the quantities of Th cells that produced IL-22 and IL-17A as well as the levels of cytokines in 30 patients with chronic liver fluke infection and CCA, 40 patients with hepatic fluke infection but no CCA, and 16 healthy controls were compared. When liver fluke infection was present in patients with CCA, immunohistochemical labeling revealed lower expression of IL-22 and IL-17A (P < 0.01). CCA patients had a considerably higher median percentage of IL-22-producing Th cells (2.2%) according to flow cytometry than did patients without it (0.69%) or controls (0.4%, P < 0.001)[46].
For Th cells that produced IL-17A, similar outcomes were seen. It demonstrated that plasma concentrations of IL-22 were 4.6 times higher in controls and 1.3 times higher in patients with CCA than in those without it (P < 0.001). IL-17A plasma concentrations were 2.5 times higher in patients with CCA than in patients without CCA and 21 times higher in controls[46].
Patients with CCA had considerably greater blood levels of IL-22 and IL-17A mRNA than the other two groups. Plasma concentrations of IL-22 were associated with those of IL-17A as were the percentage of CD4+CD45RO+ T cells generating IL-22 and IL-17A. It was suggested that IL-17A and IL-22 have an impact on the development of CCA associated with hepatic fluke infection[46].
IL-23 is a cytokine with proinflammatory characteristics and belongs to the IL-12 family. Its capacity to significantly boost Th17 cell proliferation suggests that it is the cause of several inflammatory autoimmune reactions. Recent research indicates that IL-23 plays a pivotal role in central control of the inflammatory cell pathways. Through Th17 cells IL-23 and IL-17 establish a novel axis that has developed in response to human illnesses such as bacterial or viral infections, chronic inflammation, and immunopathology. One possible treatment strategy for autoimmune illnesses, such as psoriasis, multiple sclerosis, inflammatory bowel disease, and rheumatoid arthritis, is to target IL-23, the IL-23 receptor (IL-23R), or the IL-23 axis[47].
iCCA has an extremely bad prognosis while having a low incidence. Cancer incidence and development are correlated with the expression level of the IL-23R. The Gene Expression Omnibus database provided the mRNA, microRNA (miRNA), and circular RNA (circRNA) datasets. Functional enrichment analysis was performed using the Kyoto Encyclopedia of Genes and Genomes and Gene Ontology, and the results were confirmed using gene set enrichment analysis software[48].
Clinical data were sourced from The Cancer Genome Atlas, and the DriverDBv3 database and Gene Expression Profiling Interactive Analysis website were utilized for survival analysis. The immune cell infiltration investigation of IL-23R was obtained from the TIMER2.0 database. Verification of IL-23R expression was done by real-time quantitative PCR or reverse transcription-quantitative PCR. Differentially expressed mRNAs were shown to be more abundant in the immune-related tumor microenvironment, amino acid metabolism, and the phosphoinositide 3-kinase-serine/threonine kinase signaling pathway. Moreover, cells linked to immune infiltration were linked to the expression of IL-23R[48].
Moreover, networks of circRNA-miRNA-IL-23R and IL-23R protein-protein interactions were created. The most significant finding was the decreased expression of IL-23R, a prognostic gene, in iCCA. Further investigation into the potential prognostic and immune-related biomarkers of IL-23R in iCCA is warranted as evidenced by the identification of a circRNA-miRNA-IL-23R network[48].
The cytokine family IL-17 includes IL-25, commonly referred to as IL-17E, which is a crucial modulator of the type 2 immune response. A growing body of research indicates that IL-25 has a complex role in many organ systems and interacts with a variety of immune and non-immune cells. The physiology and pathophysiology of the gut have been somewhat explored concerning IL-25. Since the gut is a major source of epithelial cells, IL-25 is involved in intestinal immune responses and is linked to the development of cancer tumors, autoimmune disorders, and incorrect allergy reactions[49].
Tumor growth and carcinogenesis are influenced by a variety of cytokines. Certain tumor cells can manufacture cytokines on their own. APEX-1 was shown to be highly expressed in CCA cell lines by secretome profiling. It was discovered through this secretome analysis that CCA cell lines overexpressed IL-25 and other associated cytokines. The current work determined that CCA cell lines overexpressed IL-25 by performing detailed secretome analysis on cytokines and associated chemicals in CCA cell lines. The expression of IL-25 in the cancer tissues of 20 patients with CCA in Northeastern Thailand was then examined utilizing immunohistochemistry techniques[50].
The clinical characteristics of the patients and the levels of IL-25 expression were correlated. The findings demonstrated that compared to normal bile ducts and surrounding tissues, malignant tissues had considerably greater levels of IL-25 expression. Western blot analysis was used to confirm that the IL-25 protein was overexpressed in CCA tissue. Furthermore, patients with CCA metastasis had considerably greater levels of IL-25 expression in malignant tissues compared with those without metastasis[50].
A higher expression of IL-25 was linked to a shorter survival period for patients with CCA according to survival analysis. It has been suggested that IL-25 may be a useful prognostic biomarker as aberrant expression of the protein in CCA tissue was linked to tumor spread and a poor prognosis. Future research on the biological functions of IL-25 in the origin and development of CCA is warranted[50].
As an alarmin cytokine, IL-33, a member of the IL-1 family, has critical roles in cancer, viral infection, allergic and non-allergic inflammation, tissue homeostasis and repair, and type 2 immunity. The nuclei of tissue-derived cells, such as blood vessel endothelial cells, barrier tissue epithelial cells, and fibroblastic stromal cells from diverse tissues, are rich in IL-33. When cells express the ST2/IL-1RL1 receptor, IL-33 is produced in response to cell damage or tissue injury, activating signaling pathways that are dependent on Myd88. Studies conducted in murine models and analysis of human samples confirmed the critical role that IL-33/ST2/IL-1RL1 signaling plays in allergic inflammation in a variety of tissues and illnesses. Among the most often repeated susceptibility loci for asthma are IL33 and ST2/IL-1RL1. However, the IL-33/ST2 pathway also plays a significant role in non-allergic inflammation[51].
CCA develops more easily in a mouse model when IL-33 is present. The relationship between IL-33 and human CCA is not entirely understood though. During hepatectomy for CCA, IL-33 is produced and then promotes the growth of subclinical CCA and ultimately results in recurrent illness. The expression of IL-33 in a range of human and mouse samples was evaluated, including resected liver and matched plasma samples taken during and after hepatectomy, and found that it had an impact on recurrent disease and prognosis[52].
When homogenized human liver tissues with either high or low IL-33 expression were introduced to the growth media of the human CCA cells, the alterations in migration and proliferation were assessed. To investigate the impact of blocking the hepatectomy-induced release of IL–33, murine CCA cells were syngraft transplanted into C57BL/6J mice either with or without IL–33 blockage. There was a correlation between the baseline liver expression and the quantity of IL-33 released into the plasma following hepatectomy[52].
An independent risk factor for recurrence was the high expression of IL-33 in the liver. Tumor cell migration and proliferation were both accelerated by homogenized liver tissue that expressed IL-33 significantly. Hepatectomy-affected mice showed CCA development in the remaining liver, although tumor progression was prevented by blocking IL-33 during the procedure. Consequently, a curative hepatectomy for CCA promoted the release of IL–33, which in turn made CCA recurrence easier, and hepatectomy anti–IL–33 treatment may lower the chance of CCA recurrence[52].
Immunoregulatory cell populations and immunosuppressive cytokines sustain the equilibrium between inflammatory and anti-inflammatory immune responses. An inhibitory cytokine that is a member of the IL-12 family, IL-35 can cause induced regulatory T cells to produce IL-35 and effectively reduce T cell proliferation. This helps to control inflammatory reactions. A growing body of research conducted in the last 10 years has shown that IL-35 is crucial in regulating immune-related conditions, such as cancer, infectious illnesses, and autoimmune diseases[53].
Though its precise effect on iCCA is unknown, IL-35 is linked to carcinogenesis. Examining the precise impact of IL-35 on patient prognosis was the goal of the current investigation. Furthermore, following curative resection, it developed an efficient prognostic nomogram for iCCA patients. To investigate IL-35 and IL-35 receptor expression in 102 patients with iCCA, immunohistochemistry was used. IL-35 was substantially expressed in iCCA tumor tissues and was correlated with lymph node metastasis, TNM stage, and vascular invasion, and served as a stand-alone predictor of patient OS and recurrence-free survival[54].
The iCCA cancer tissues exhibited elevated expression of IL-35 receptor (gp130 and IL-12 receptor β2). However, just gp130 exhibited independent prognostic significance for OS and recurrence-free survival and was essential for the IL-35-mediated prognosis of iCCA. When compared with the TNM stage for OS, the nomogram containing carcinoembryonic antigen, lymph node metastasis, IL-35, and gp130 expression had a higher predictive accuracy. This finding validated the correlation between high IL-35 expression and aggressiveness in iCCA, highlighting it as a useful biomarker for assessing the course and prognosis of iCCA in clinical settings[54].
In the pathophysiology of CCA, cytokines such as IL-1, IL-4, IL-6, IL-8, IL-10, IL-17, IL-23, IL-25, IL-33, and IL-35 play a crucial role by coordinating a complex interaction between inflammation, immune evasion, tumor microenvironment modification, and tumor growth. Through processes including angiogenesis, chronic inflammation, and the EMT, proinflammatory cytokines such as IL-1, IL-6, IL-8, IL-17, IL-23, and IL-33 encourage the development and spread of tumors. Cytokines such as IL-4, IL-10, IL-25, and IL-35 on the other hand help create an immunosuppressive milieu that promotes tumor immune escape and treatment resistance. New approaches to treatments become possible when the precise functions and interconnections of these cytokines are understood.
Targeting cytokine signaling pathways may provide new techniques to break the inflammatory environment that supports tumor growth and reestablish efficient anti-tumor immunity. Future studies should concentrate on creating cytokine-based biomarkers for early diagnosis and prognosis, creating selective cytokine inhibitors, and creating combination therapies that incorporate cytokine modulation with currently used treatments including immunotherapy, chemotherapy, and targeted medicines. To maximize the timing and specificity of therapies, it will also be crucial to clarify the temporal and spatial expression patterns of these cytokines during cholangiocarcinogenesis. Ultimately, a deeper molecular understanding of cytokine networks in CCA will pave the way toward more personalized and effective treatment strategies, improving patient outcomes in this aggressive malignancy.
CCA is a type of cancer that originates in the bile ducts, and its pathogenesis is influenced by various factors, including chronic inflammation. ILs, which are a group of cytokines, play a crucial role in mediating the immune response and inflammation. This article concluded that IL-1, IL-4, IL-6, IL-8, IL-10, IL-17, IL-23, IL-25, IL-33, and IL-35 play significant roles in the pathogenesis of CCA as explained in Figure 1 and Table 1. Further research is required to find the association of other ILs such as IL-2, IL-3, IL-5, IL-7, IL-11, and more in the pathogenesis of CCA. ILs contribute to CCA pathogenesis by promoting chronic inflammation, enhancing tumor cell survival, proliferation, and invasion, and shaping a tumor microenvironment that supports cancer growth and metastasis. Controlling the pathogenesis of ILs in CCA requires a multifaceted approach, including targeted therapies, immunotherapies, and lifestyle modifications. By inhibiting the signaling pathways and reducing the inflammatory responses mediated by ILs, it is possible to slow or prevent the progression of CCA.
| 1. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Ilyas SI, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1530] [Article Influence: 306.0] [Reference Citation Analysis (0)] |
| 2. | Izquierdo-Sanchez L, Lamarca A, La Casta A, Buettner S, Utpatel K, Klümpen HJ, Adeva J, Vogel A, Lleo A, Fabris L, Ponz-Sarvise M, Brustia R, Cardinale V, Braconi C, Vidili G, Jamieson NB, Macias RI, Jonas JP, Marzioni M, Hołówko W, Folseraas T, Kupčinskas J, Sparchez Z, Krawczyk M, Krupa Ł, Scripcariu V, Grazi GL, Landa-Magdalena A, Ijzermans JN, Evert K, Erdmann JI, López-López F, Saborowski A, Scheiter A, Santos-Laso A, Carpino G, Andersen JB, Marin JJ, Alvaro D, Bujanda L, Forner A, Valle JW, Koerkamp BG, Banales JM. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J Hepatol. 2022;76:1109-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 198] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 3. | Squadroni M, Tondulli L, Gatta G, Mosconi S, Beretta G, Labianca R. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2017;116:11-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 494] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 5. | Cigliano A, Chen X, Calvisi DF. Current challenges to underpinning the genetic basis for cholangiocarcinoma. Expert Rev Gastroenterol Hepatol. 2021;15:511-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, Teh BT, Wongkham S, Gores GJ. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 460] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 7. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1378] [Article Influence: 125.3] [Reference Citation Analysis (1)] |
| 8. | Roy S, Glaser S, Chakraborty S. Inflammation and Progression of Cholangiocarcinoma: Role of Angiogenic and Lymphangiogenic Mechanisms. Front Med (Lausanne). 2019;6:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Mertowska P, Mertowski S, Smarz-Widelska I, Grywalska E. Biological Role, Mechanism of Action and the Importance of Interleukins in Kidney Diseases. Int J Mol Sci. 2022;23:647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 10. | Surapaitoon A, Suttiprapa S, Mairiang E, Khuntikeo N, Pairojkul C, Bethony J, Brindley PJ, Sripa B. Subsets of Inflammatory Cytokine Gene Polymorphisms are Associated with Risk of Carcinogenic Liver Fluke Opisthorchis viverrini-Associated Advanced Periductal Fibrosis and Cholangiocarcinoma. Korean J Parasitol. 2017;55:295-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Lurje I, Gaisa NT, Dahl E, Knüchel R, Strnad P, Trautwein C, Tacke F, Neumann UP, Czigany Z, Lurje G. Genetic polymorphisms in interleukin-1β (rs1143634) and interleukin-8 (rs4073) are associated with survival after resection of intrahepatic cholangiocarcinoma. Sci Rep. 2023;13:12283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 13. | Lomedico PT, Gubler U, Hellmann CP, Dukovich M, Giri JG, Pan YC, Collier K, Semionow R, Chua AO, Mizel SB. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature. 1984;312:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 630] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984;81:7907-7911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 705] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 15. | Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2564] [Cited by in RCA: 2892] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 16. | Zhang M, Huang Y, Pan J, Sang C, Lin Y, Dong L, Shen X, Wu Y, Song G, Ji S, Liu F, Wang M, Zheng Y, Zhang S, Wang Z, Ren J, Gao D, Zhou J, Fan J, Wei W, Lin J, Gao Q. An Inflammatory Checkpoint Generated by IL1RN Splicing Offers Therapeutic Opportunity for KRAS-Mutant Intrahepatic Cholangiocarcinoma. Cancer Discov. 2023;13:2248-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 17. | Khawkhiaw K, Chomphoo S, Kunprom W, Thithuan K, Sorin S, Yueangchantuek P, Chiu CF, Umezawa K, Panaampon J, Okada S, Wongkham S, Saengboonmee C. Involvement of interleukin-1β in high glucose-activated proliferation of cholangiocarcinoma. Transl Gastroenterol Hepatol. 2024;9:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Takeda K, Kishimoto T, Akira S. STAT6: its role in interleukin 4-mediated biological functions. J Mol Med (Berl). 1997;75:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Permpoon U, Khan F, Vadevoo SMP, Gurung S, Gunassekaran GR, Kim MJ, Kim SH, Thuwajit P, Lee B. Inhibition of Tumor Growth against Chemoresistant Cholangiocarcinoma by a Proapoptotic Peptide Targeting Interleukin-4 Receptor. Mol Pharm. 2020;17:4077-4088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Seto K, Shoda J, Horibe T, Warabi E, Kohno M, Yanagawa T, Bukawa H, Nakanuma Y, Kawakami K. Targeting interleukin-4 receptor alpha by hybrid Peptide for novel biliary tract cancer therapy. Int J Hepatol. 2014;2014:584650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 532] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 22. | Zhou M, Na R, Lai S, Guo Y, Shi J, Nie J, Zhang S, Wang Y, Zheng T. The present roles and future perspectives of Interleukin-6 in biliary tract cancer. Cytokine. 2023;169:156271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Wehbe H, Henson R, Meng F, Mize-Berge J, Patel T. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006;66:10517-10524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Gu D, Zhao X, Song J, Xiao J, Zhang L, Deng G, Li D. Expression and clinical significance of interleukin-6 pathway in cholangiocarcinoma. Front Immunol. 2024;15:1374967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Kleinegger F, Hofer E, Wodlej C, Golob-Schwarzl N, Birkl-Toeglhofer AM, Stallinger A, Petzold J, Orlova A, Krassnig S, Reihs R, Niedrist T, Mangge H, Park YN, Thalhammer M, Aigelsreiter A, Lax S, Garbers C, Fickert P, Rose-John S, Moriggl R, Rinner B, Haybaeck J. Pharmacologic IL-6Rα inhibition in cholangiocarcinoma promotes cancer cell growth and survival. Biochim Biophys Acta Mol Basis Dis. 2019;1865:308-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Gosain R, Anwar S, Miller A, Iyer R, Mukherjee S. Interleukin-6 as a biomarker in patients with hepatobiliary cancers. J Gastrointest Oncol. 2019;10:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, Gores GJ. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 28. | Saeheng T, Karbwang J, Na-Bangchang K. Interleukin-6 and Lymphocyte-to-Monocyte Ratio Indices Identify Patients with Intrahepatic Cholangiocarcinoma. Biomedicines. 2024;12:844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Cheon YK, Cho YD, Moon JH, Lee MS, Kim YS, Lee JS, Shim CS. The Change of Serum Interleukin-6 in Patients with Bile Duct Cancer after Photodynamic Therapy Validation of Utility as a Clinical Marker. Gastrointest Endosc. 2005;61:AB201. [DOI] [Full Text] |
| 30. | Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 31. | Colyn L, Alvarez-Sola G, Latasa MU, Uriarte I, Herranz JM, Arechederra M, Vlachogiannis G, Rae C, Pineda-Lucena A, Casadei-Gardini A, Pedica F, Aldrighetti L, López-López A, López-Gonzálvez A, Barbas C, Ciordia S, Van Liempd SM, Falcón-Pérez JM, Urman J, Sangro B, Vicent S, Iraburu MJ, Prosper F, Nelson LJ, Banales JM, Martinez-Chantar ML, Marin JJG, Braconi C, Trautwein C, Corrales FJ, Cubero FJ, Berasain C, Fernandez-Barrena MG, Avila MA. New molecular mechanisms in cholangiocarcinoma: signals triggering interleukin-6 production in tumor cells and KRAS co-opted epigenetic mediators driving metabolic reprogramming. J Exp Clin Cancer Res. 2022;41:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989;1:2-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 420] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 33. | Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 355] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987;84:9233-9237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 710] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 35. | Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1441] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 36. | Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 567] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 37. | Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 771] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 38. | Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1497] [Cited by in RCA: 1507] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 39. | Smyth MJ, Zachariae CO, Norihisa Y, Ortaldo JR, Hishinuma A, Matsushima K. IL-8 gene expression and production in human peripheral blood lymphocyte subsets. J Immunol. 1991;146:3815-3823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 40. | Meng ZW, Zhang L, Cai XR, Wang X, She FF, Chen YL. IL-8 is a novel prometastatic chemokine in intrahepatic cholangiocarcinoma that induces CXCR2-PI3K/AKT signaling upon CD97 activation. Sci Rep. 2023;13:18711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 41. | Sun Q, Li F, Sun F, Niu J. Interleukin-8 is a prognostic indicator in human hilar cholangiocarcinoma. Int J Clin Exp Pathol. 2015;8:8376-8384. [PubMed] |
| 42. | Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 780] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 43. | Yuan H, Lin Z, Liu Y, Jiang Y, Liu K, Tu M, Yao N, Qu C, Hong J. Intrahepatic cholangiocarcinoma induced M2-polarized tumor-associated macrophages facilitate tumor growth and invasiveness. Cancer Cell Int. 2020;20:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 44. | Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000. 2015;69:142-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 321] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 45. | Lieshout R, Kamp EJCA, Verstegen MMA, Doukas M, Dinjens WNM, Köten K, IJzermans JNM, Bruno MJ, Peppelenbosch MP, van der Laan LJW, de Vries AC. Cholangiocarcinoma cell proliferation is enhanced in primary sclerosing cholangitis: A role for IL-17A. Int J Cancer. 2023;152:2607-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 46. | Su SB, Zhang JF, Huang FF, Cen Y, Jiang HX. Large numbers of interleukins-22- and -17A-producing T helper cells in cholangiocarcinoma related to liver fluke infection. Microbiol Immunol. 2017;61:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Tang C, Chen S, Qian H, Huang W. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 48. | Zhang LT, Yang YF, Chen XM, Wang SB, Tong GL. IL23R as an indicator of immune infiltration and poor prognosis in intrahepatic cholangiocarcinoma: a bioinformatics analysis. Transl Cancer Res. 2023;12:2461-2476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Jiang W, Wang Z, Zhang J, Li M. Interleukin 25 and its biological features and function in intestinal diseases. Cent Eur J Immunol. 2022;47:362-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Kaewsarabhumi S, Proungvitaya T, Limpaiboon T, Tippayawat P, Tummanatsakun D, Titapun A, Sa-Ngaimwibool P, Proungvitaya S. Interleukin 25 (IL-25) expression in cholangiocarcinoma. Mol Clin Oncol. 2020;13:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Cayrol C, Girard JP. Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine. 2022;156:155891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 154] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 52. | Nagaoka S, Yamada D, Eguchi H, Yokota Y, Iwagami Y, Asaoka T, Noda T, Kawamoto K, Gotoh K, Kobayashi S, Miyoshi E, Doki Y, Mori M. The blockade of interleukin-33 released by hepatectomy would be a promising treatment option for cholangiocarcinoma. Cancer Sci. 2021;112:347-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Ye C, Yano H, Workman CJ, Vignali DAA. Interleukin-35: Structure, Function and Its Impact on Immune-Related Diseases. J Interferon Cytokine Res. 2021;41:391-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 54. | Zhang MX, Gan W, Jing CY, Zheng SS, Zhang J, Shen HJ, Xu X, Lin JJ, Zhang BH, Qiu SJ. Overexpression of interleukin-35 in intrahepatic cholangiocarcinoma is a prognostic indicator after curative resection. Cancer Sci. 2018;109:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |