Published online Jul 15, 2025. doi: 10.4251/wjgo.v17.i7.105403
Revised: April 20, 2025
Accepted: June 11, 2025
Published online: July 15, 2025
Processing time: 174 Days and 22.7 Hours
Locally advanced rectal cancer (LARC) carries a substantial risk of recurrence, prompting the use of neoadjuvant chemoradiotherapy (nCRT) to improve tumor resectability and long-term outcomes. However, individual treatment responses vary considerably, highlighting the need for robust predictive tools to guide clinical decision-making.
To develop a nomogram model integrating clinical characteristics and biomarkers to predict the likelihood of poor response to nCRT in LARC.
A retrospective analysis was performed on 178 patients with stage II-III LARC treated from January 2021 to December 2023. All patients underwent standar
A total of 178 patients were enrolled, with 36 (20.2%) achieving a good response and 142 (79.8%) exhibiting a poor response to nCRT. Baseline factors, including age and comorbidities, showed no significant differences. However, poor responders more frequently had lymph node metastasis, advanced tumor node metastasis/T stage, larger tumor diameter, and elevated CRP, IL-6, and CEA levels. Logistic regression confirmed CRP, IL-6, and CEA as independent predictors of poor response. The nomogram demonstrated high accuracy (area under the curve = 0.928), good calibration (Hosmer-Lemeshow P = 0.928), and a sensitivity of 88.1% with 82.6% specificity. Internal validation via bootstrap resampling (n = 1000) yielded an adjusted C-index of 0.716, and DCA confirmed substantial clinical utility.
A nomogram incorporating serum CRP, IL-6, and CEA accurately predicts poor nCRT response in patients with LARC. This model provides a valuable framework for individualized treatment planning, potentially improving clinical outcomes.
Core Tip: Despite advancements in the use of neoadjuvant chemoradiotherapy (nCRT) to improve resectability and outcomes in locally advanced rectal cancer, predicting treatment response remains challenging. We identified key biomarkers: C-reactive protein, interleukin-6, and carcinoembryonic antigen, as independent predictors of poor response to nCRT. Using these factors, we constructed a nomogram model that demonstrated high predictive accuracy (area under the curve = 0.928), good calibration, and strong clinical utility as confirmed by decision curve analysis.
- Citation: Guo QY, Zhang W, Fu L, Hu SS, Li L. Predictive value of a nomogram model for treatment response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. World J Gastrointest Oncol 2025; 17(7): 105403
- URL: https://www.wjgnet.com/1948-5204/full/v17/i7/105403.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i7.105403
Locally advanced rectal cancer (LARC) poses significant therapeutic challenges due to its high risk of recurrence and metastasis. Neoadjuvant chemoradiotherapy (nCRT) has become a cornerstone in the treatment of LARC, aiming to reduce tumor size, eradicate micrometastases, and facilitate surgical resection with improved outcomes. Tumor regression grade (TRG) following nCRT is widely used to evaluate treatment response, which correlates with prognosis and informs subsequent therapeutic decisions[1,2]. Global data specific to LARC remains scarce, with most cancer statistics focusing on overall colorectal cancer incidence[3]. However, it is estimated that LARC accounts for 5% to 10% of rectal cancer cases, and approximately 10% of rectal cancers relapse post-surgery, with half of these recurrences being limited to locoregional disease[4]. Colorectal cancer constitutes 9.6% of all cancers globally, with rectal cancer contributing significantly to this burden. The mortality rate for rectal cancer varies between 4 and 10 per 100000 annually, underscoring the clinical relevance of LARC globally[5]. Despite advancements in treatment, predicting individual responses to nCRT remains a complex, multifactorial challenge. Traditional predictive models typically rely on clinicopathological factors such as tumor stage, histological subtype, and baseline imaging findings; however, these alone lack sufficient accuracy and robustness. Recent developments in molecular biology and imaging technologies have introduced novel biomarkers, including inflammatory markers, tumor-associated antigens, and radiomic features, which may enhance predictive capacity. Nonetheless, integrating these diverse parameters into a clinically applicable predictive framework remains essential for advancing personalized treatment strategies[6-8].
Nomogram models, which integrate multiple predictors into a graphical format, have emerged as valuable tools in oncology for individualized risk assessment. By incorporating clinical, pathological, and molecular variables, nomograms offer probabilistic estimates of treatment response and long-term outcomes. Their utility has been well demonstrated in various malignancies, including gastric, lung, and breast cancers. However, despite these advancements, the development and validation of nomograms specifically tailored to patients with LARC undergoing nCRT remain limited[9,10]. In the setting of LARC, accurately predicting response to nCRT is crucial for optimizing therapeutic strategies. An effective predictive model can help identify patients most likely to benefit from treatment, potentially sparing poor responders from unnecessary toxicity and allowing for alternative interventions. Moreover, elucidating the determinants of treatment response may enhance our understanding of tumor biology and resistance mechanisms, thereby informing the development of novel therapeutic approaches[11-13].
This study aims to develop and internally validate a nomogram model for predicting response to nCRT in patients with LARC. The model incorporates a range of clinical, pathological, and biochemical parameters, including inflammatory markers and tumor-associated antigens, to enable comprehensive evaluation. Model performance is assessed through measures of calibration, discrimination, and clinical utility using internal validation techniques, receiver operating characteristic curves, calibration plots, and decision curve analysis.
A retrospective study was conducted at our hospital to develop a nomogram model predicting treatment response to nCRT in patients with LARC. The study included patients treated between January 2021 and December 2023. Eligibility criteria required a diagnosis of LARC (clinical stage II-III) confirmed by histopathological analysis and imaging studies, such as magnetic resonance imaging or computed tomography. Included patients underwent standardized nCRT followed by total mesorectal excision, with complete clinical, radiological, pathological, and follow-up data available for analysis. Exclusion criteria included the presence of distant metastases, prior pelvic radiotherapy, chemotherapy, or surgical interventions for rectal cancer before nCRT, concurrent malignancies or a history of malignancy within the past five years, non-completion of the prescribed chemoradiotherapy course, patients with incomplete clinical, imaging, pathological, or follow-up data were also excluded to ensure data integrity and consistency in analysis, and significant comorbidities that could interfere with treatment outcomes or follow-up. A total of 178 patients met these criteria, with 36 patients (20.22%) classified as having a good treatment response and 142 patients (79.78%) classified as having a poor treatment response to nCRT. The study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines[14] and received approval from the Ethics Committee of our hospital.
Patients underwent pre-operative three-dimensional conformal radiotherapy directed at the primary rectal lesion and pelvic lymph-node drainage regions. Clinical target volumes were delineated according to standardized guidelines and encompassed the mesorectum, presacral area, and internal iliac vessels; for T4 tumours infiltrating anterior structures, the external iliac nodes were additionally included. All patients received a uniform total dose of 50.4 Gy, delivered in 28 fractions over 38 days (1.8 Gy per fraction); dose escalation or hypofractionation was not permitted. Concurrent chemotherapy-initiated on day 1 of radiotherapy-consisted of oral capecitabine 550 mg m-2 twice daily, five days per week (manufactured by Shanghai Roche Pharmaceuticals Co., Ltd.). Supportive medications (acid suppressants, anti-emetics, and gastric-mucosal protectants) were provided as needed. Curative total mesorectal excision was scheduled approximately six weeks after completion of chemoradiotherapy.
Fasting venous blood samples (5 mL) were collected from the antecubital vein of each patient prior to the initiation of nCRT. Serum was separated by centrifugation and stored for subsequent analysis. Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) levels were quantified using enzyme-linked immunosorbent assay, while C-reactive protein (CRP) levels were measured via the turbidimetric scattering method. Additionally, a complete blood count was performed to record neutrophil, lymphocyte, and platelet counts. The neutrophil-to-lymphocyte ratio (NLR) was calculated as the absolute neutrophil count divided by the lymphocyte count, and the platelet-to-lymphocyte ratio (PLR) was derived similarly. Serum tumor markers, including carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), were measured using chemiluminescence immunoassay to explore their potential associations with treatment response and prognostic outcomes.
The response to nCRT was evaluated using Dworak’s TRG system[15]. This grading system classifies tumor regression as follows: (1) TRG 0: No regression of the tumor; (2) TRG 1: Minimal regression, with tumor regression not exceeding 25%; (3) TRG 2: Moderate regression, with fibrosis occupying 26% to 50% of the tumor area; (4) TRG 3: Good regression, with tumor regression exceeding 50%; and (5) TRG 4: Complete regression of the tumor. Patients were categorized into two groups based on their TRG: (1) Poor response group: TRG 0 to 2; and (2) Good response group: TRG 3 to 4.
Statistical analyses were performed using SPSS software (version 26.0) and R software (version 3.6.3). The normality of continuous variables was assessed using the Shapiro-Wilk test. The Shapiro-Wilk test confirmed that all continuous variables included in the analysis conformed to a normal distribution (all P > 0.05), justifying the use of parametric statistical tests. Variables that followed approximately normal distributions were expressed as mean ± SD and compared using independent-sample t-tests. Categorical variables were summarized as frequencies and percentages, and group comparisons were conducted using the χ2 test, continuity correction χ2 test, or Fisher’s exact test, as appropriate. To identify independent predictors of poor response to nCRT, stepwise multivariate logistic regression analysis was applied. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A predictive nomogram was constructed using the “rms” package in R based on significant variables. Model discrimination was evaluated via receiver operating characteristic curve analysis and concordance index (C-index). The optimal cutoff value was determined using the Youden index to derive sensitivity and specificity. Calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test and calibration plots. Internal validation was performed through 1000 bootstrap resamples, and the adjusted C-index was reported. Statistical significance was defined as P < 0.05.
The clinical characteristics of patients in the good response group (n = 36) and poor response group (n = 142) were compared to identify factors associated with the effectiveness of nCRT in LARC. No significant differences were observed in baseline characteristics, including age, gender, body mass index, history of hypertension, coronary artery disease, diabetes, smoking, or high-salt, high-fat dietary habits. Similarly, CEA levels and the distance from the anal verge showed no statistical differences between the groups (P > 0.05). However, several variables demonstrated significant associations with treatment response. Patients in the poor response group were more likely to exhibit lymph node metastasis (54.93% vs 33.33%, P = 0.021), lower tumor differentiation (P = 0.004), advanced tumor node metastasis (TNM)/T stage (stage 4, 72.54% vs 36.11%, P < 0.001), and larger maximum tumor diameters (6.7 cm ± 1.3 cm vs 5.3 cm ± 1.1 cm, P < 0.001). Although the pathological type (adenocarcinoma vs mucinous carcinoma) showed a trend toward significance (P = 0.077), it did not reach statistical significance (Table 1).
| Variable | Good response group (n = 36) | Poor response group (n = 142) | χ²/t value | P value |
| Age (years) | 55.1 ± 5.4 | 56.0 ± 6.3 | 0.787 | 0.433 |
| Gender (male/female) | 22/14 | 92/50 | 0.169 | 0.681 |
| BMI (kg/m²) | 23.4 ± 1.2 | 23.5 ± 1.5 | 0.371 | 0.711 |
| Hypertension | 12 (33.33) | 43 (30.28) | 0.125 | 0.723 |
| Coronary artery disease | 9 (25.00) | 28 (19.72) | 0.487 | 0.486 |
| CEA level (ng/mL) | 5.4 ± 1.2 | 5.6 ± 1.5 | 0.742 | 0.459 |
| Smoking | 17 (47.22) | 64 (45.07) | 0.054 | 0.817 |
| Diabetes | 9 (25.00) | 34 (23.94) | 0.017 | 0.895 |
| Family history of malignancy | 16 (44.44) | 54 (38.03) | 0.496 | 0.482 |
| High-salt, high-fat diet | 15 (41.67) | 58 (40.85) | 0.008 | 0.929 |
| Distance from the anal verge (cm) | 5.1 ± 1.2 | 5.3 ± 1.5 | 0.742 | 0.459 |
| Pathological type (adenocarcinoma/mucinous carcinoma) | 17/19 | 90/52 | 3.127 | 0.077 |
| Lymph node metastasis | 12 (33.33) | 78 (54.93) | 5.358 | 0.021 |
| Differentiation (moderate-high/low-poor) | 22/14 | 49/93 | 8.477 | 0.004 |
| TNM/T stage (stage 3/stage 4) | 23/13 | 39/103 | 16.79 | < 0.001 |
| Maximum tumor diameter (cm) | 5.3 ± 1.1 | 6.7 ± 1.3 | 5.941 | < 0.001 |
The comparison of inflammatory and tumor markers between the good response and poor response groups revealed significant differences in key biomarkers associated with nCRT outcomes in LARC. Patients in the poor response group demonstrated significantly higher levels of CRP (13.3 ± 2.8 mg/L vs 9.0 ± 1.4 mg/L, P < 0.001), IL-6 (18.7 ± 3.6 ng/L vs 12.5 ± 3.2 ng/L, P < 0.001), and TNF-α (41.9 ± 9.1 ng/L vs 37.0 ± 8.6 ng/L, P = 0.004), suggesting a stronger systemic inflammatory response in patients with suboptimal treatment outcomes. In addition, tumor markers such as CEA and CA19-9 were notably elevated in the poor response group compared to the good response group. Specifically, CEA levels were significantly higher (8.1 ± 1.3 μg/L vs 5.7 ± 1.1 μg/L, P < 0.001), as were CA19-9 levels (50.1 ± 10.1 U/mL vs 46.1 ± 9.0 U/mL, P = 0.032). Conversely, no statistically significant differences were observed in NLR or PLR between the two groups (P > 0.05) (Table 2).
| Variable | Good response group (n = 36) | Poor response group (n = 142) | t value | P value |
| CRP (mg/L) | 9.0 ± 1.4 | 13.3 ± 2.8 | 8.922 | < 0.001 |
| IL-6 (ng/L) | 12.5 ± 3.2 | 18.7 ± 3.6 | 9.428 | < 0.001 |
| TNF-α (ng/L) | 37.0 ± 8.6 | 41.9 ± 9.1 | 2.917 | 0.004 |
| CEA (μg/L) | 5.7 ± 1.1 | 8.1 ± 1.3 | 10.19 | < 0.001 |
| CA19-9 (U/mL) | 46.1 ± 9.0 | 50.1 ± 10.1 | 2.167 | 0.032 |
| NLR | 4.5 ± 0.7 | 4.7 ± 0.8 | 1.372 | 0.172 |
| PLR | 346.8 ± 55.9 | 357.1 ± 59.7 | 0.936 | 0.351 |
The logistic regression analysis identified several significant factors associated with poor response to nCRT in patients with LARC. Elevated levels of CRP (P = 0.009, OR = 2.571, 95%CI: 1.576-4.108) and IL-6 (P = 0.012, OR = 2.666, 95%CI: 1.507-4.305) were strongly correlated with suboptimal treatment outcomes. Similarly, elevated CEA levels demonstrated a significant association with poor response (P = 0.016, OR = 2.37, 95%CI: 1.263-3.921), underscoring the relevance of systemic inflammation and tumor burden as predictive markers. Although other factors, such as lymph node metastasis
| Factors | β | SE | Wald | OR | 95%CI for OR | P value |
| Elevated CA19-9 | 0.185 | 0.088 | 1.828 | 1.149 | 0.871-1.271 | 0.271 |
| Elevated TNF-α | 0.191 | 0.08 | 2.39 | 1.142 | 0.864-1.261 | 0.133 |
| Larger tumor diameter | 0.367 | 0.112 | 2.582 | 1.312 | 0.905-1.392 | 0.101 |
| Tumor poor differentiation | 0.341 | 0.119 | 2.843 | 1.372 | 0.798-1.471 | 0.085 |
| TNM/T stage 4 | 0.441 | 0.121 | 2.944 | 1.419 | 0.973-1.497 | 0.078 |
| Lymph node metastasis | 0.454 | 0.092 | 3.211 | 1.491 | 0.982-1.539 | 0.072 |
| Elevated CEA | 0.745 | 0.274 | 6.315 | 2.37 | 1.263-3.921 | 0.016 |
| Elevated IL-6 | 0.926 | 0.296 | 7.41 | 2.666 | 1.507-4.305 | 0.012 |
| Elevated CRP | 0.887 | 0.252 | 7.819 | 2.571 | 1.576-4.108 | 0.009 |
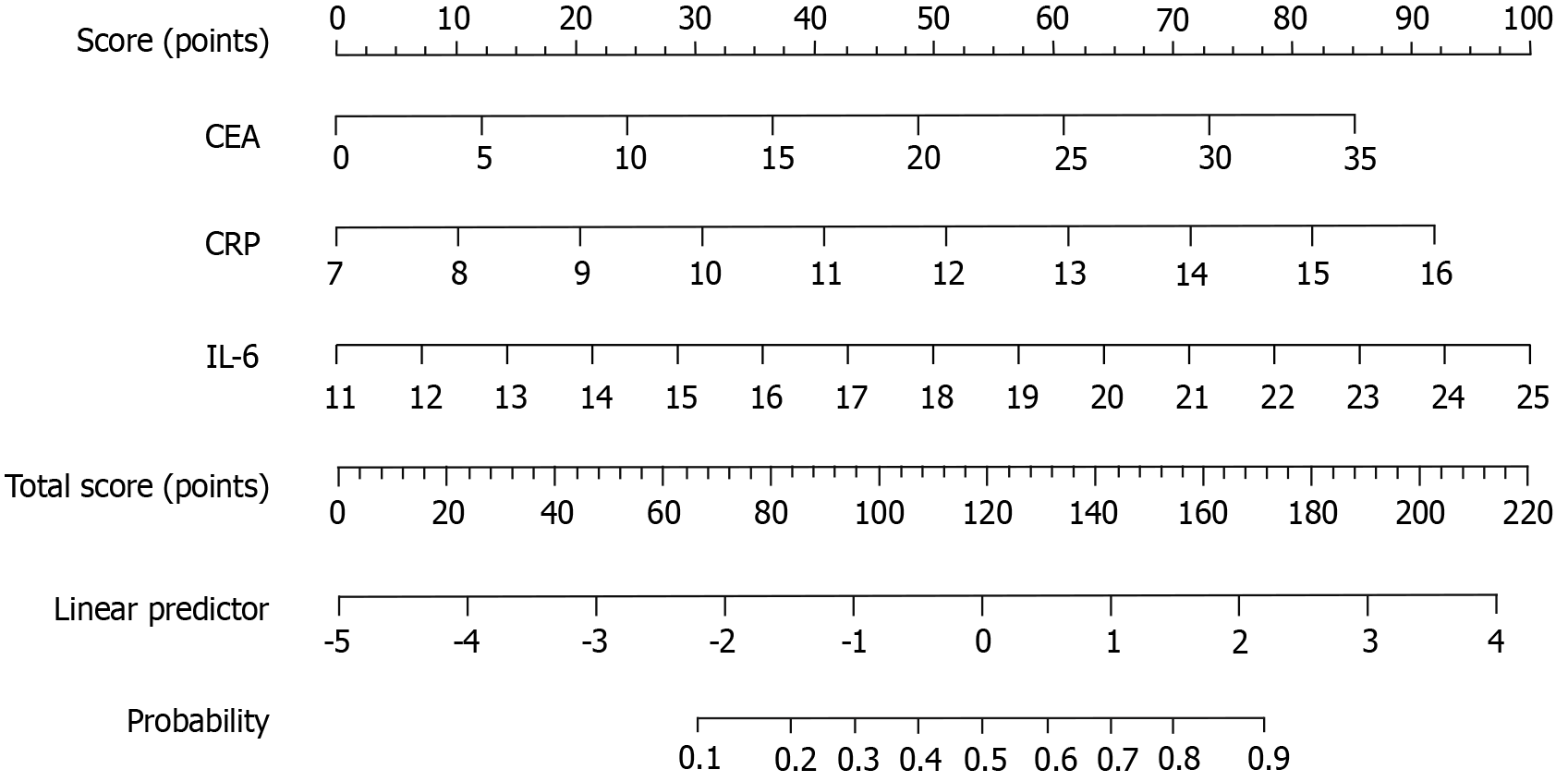
Based on the multivariate logistic regression analysis, three significant predictive factors were identified and used to construct a nomogram model for predicting the probability of poor response to nCRT in patients with LARC. These factors included elevated CRP, elevated IL-6, and elevated CEA, all of which demonstrated strong associations with treatment outcomes in the regression analysis. The nomogram assigns a specific score to each variable, reflecting its contribution to the likelihood of poor response. By summing the scores for all predictive factors, a total score is calculated for each patient. This total score corresponds to a value on the probability scale at the bottom of the nomogram, representing the individual risk of poor response to nCRT. The higher the total score, the greater the probability of a poor therapeutic outcome (Figure 1).
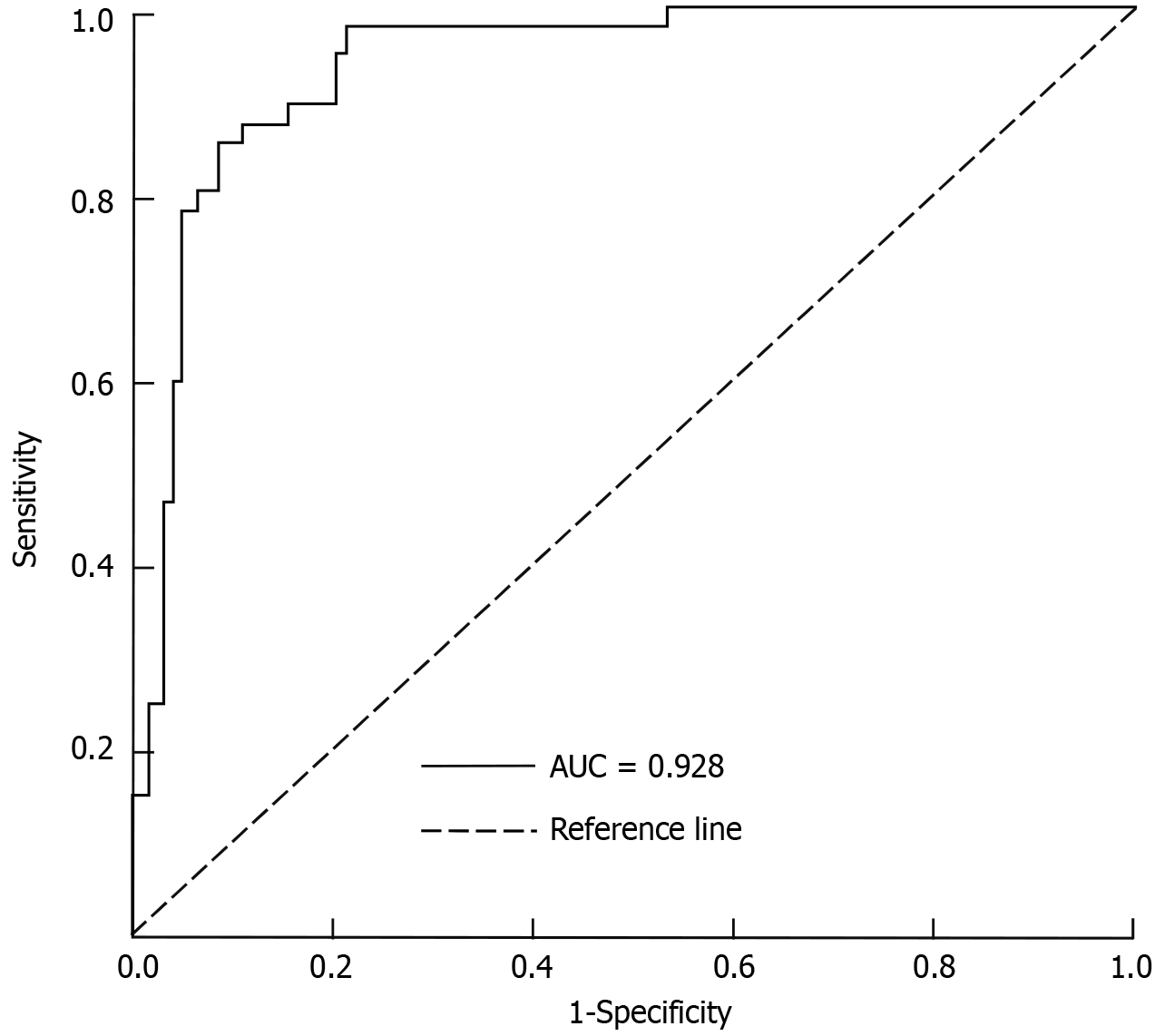
The predictive nomogram model for assessing the response to nCRT in patients with LARC demonstrated excellent discriminative ability. The area under the curve (AUC) was 0.928 (95%CI: 0.856-0.978), indicating a high level of accuracy in distinguishing between good and poor responders. Using the optimal cutoff point determined by the maximum Youden index, the model achieved a sensitivity of 88.1% and a specificity of 82.6%. Internal validation of the model was performed using Bootstrap resampling with 1000 iterations. The corrected C-index was calculated as 0.716, reflecting robust predictive performance. Additionally, the calibration curve showed good agreement between predicted probabilities and observed outcomes, with an average absolute error of 0.008 (Figure 2).
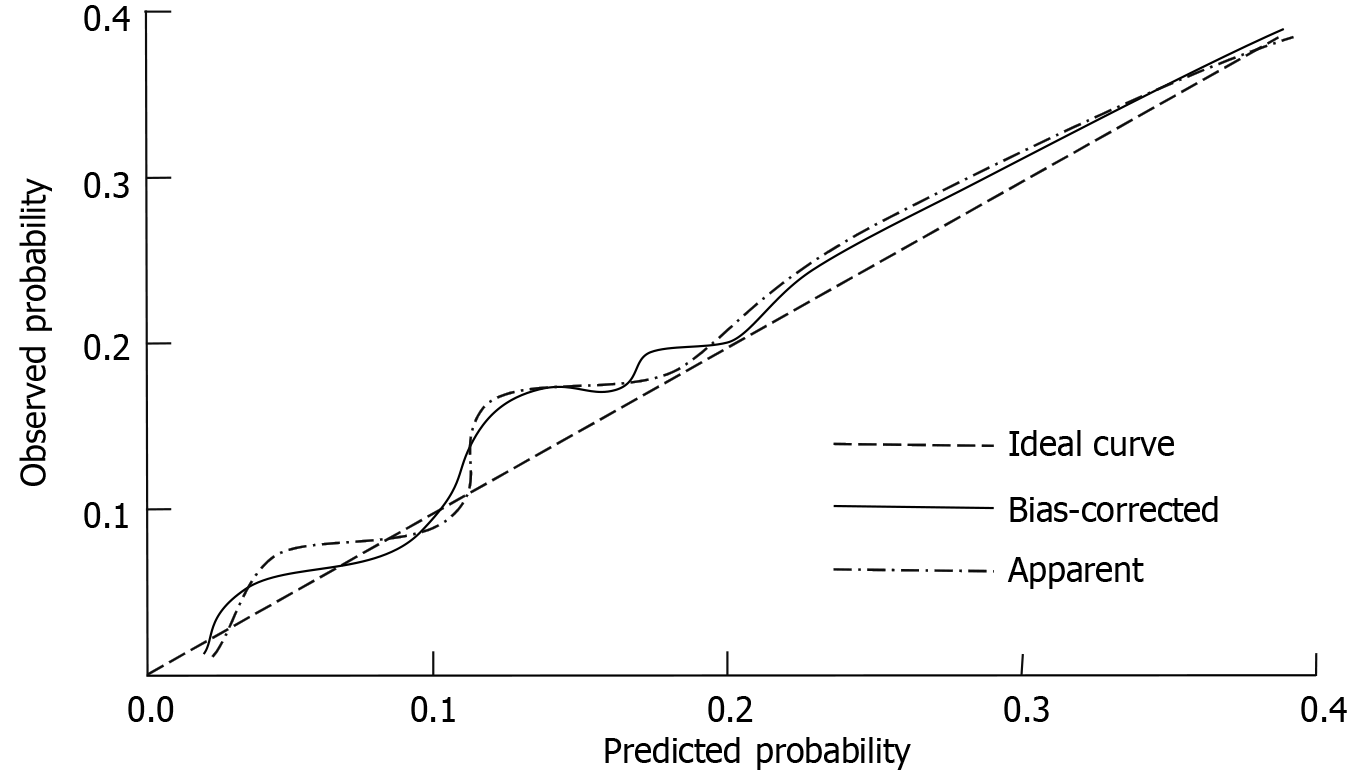
The calibration of the predictive nomogram model for assessing response to nCRT in LARC was evaluated using the Hosmer-Lemeshow goodness-of-fit test. The result (χ² = 2.911, P = 0.928) indicated no significant deviation between predicted and observed outcomes, suggesting that the model is well-calibrated (Figure 3).
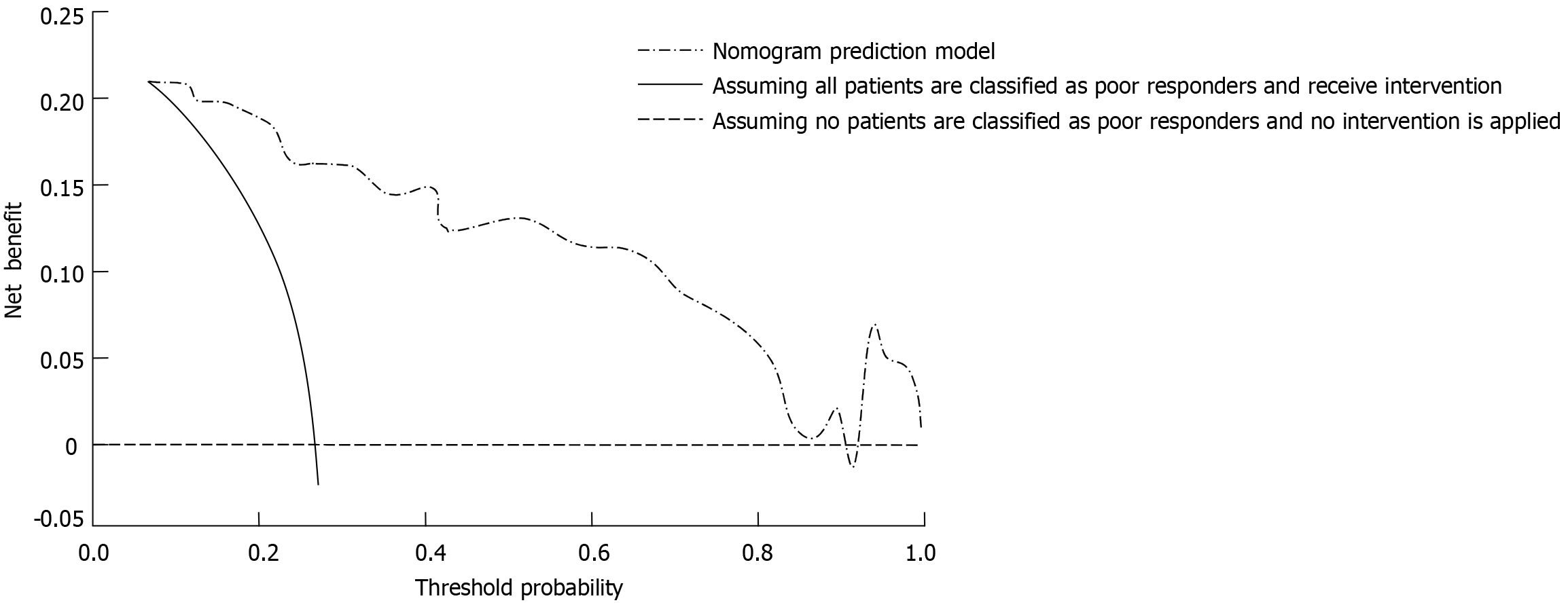
The clinical utility of the nomogram model for predicting the response to nCRT in LARC was evaluated using decision curve analysis. In the decision curve analysis curve, the horizontal line represents a scenario where all patients are classified as good responders without any intervention, yielding a net benefit of zero. Conversely, the diagonal line reflects a scenario where all patients are classified as poor responders and undergo intervention, resulting in a negative net benefit due to unnecessary treatments. The net benefit provided by the nomogram model was significantly higher across a wide range of threshold probabilities compared to these two extreme strategies. This indicates that the model effectively identifies patients who are likely to respond poorly to nCRT, enabling tailored interventions while minimizing unnecessary treatment-related risks (Figure 4).
LARC represents a significant therapeutic challenge due to its high risk of recurrence and metastasis. nCRT has become the standard of care for these patients, aiming to downstage tumors, improve resectability, and enhance long-term survival outcomes. Despite its widespread use, response to nCRT is highly variable, with a subset of patients demon
A key observation was the association between specific clinical characteristics and response to nCRT. While factors such as age, gender, and comorbidities did not differ significantly between good and poor responders, patients with poor response were more likely to present with lymph node metastasis, poor differentiation, advanced TNM/T stage, and larger tumor diameters. These findings are consistent with prior research indicating that aggressive tumor biology manifested as high-grade histological features, greater tumor burden, and nodal involvement can confer resistance to conventional chemoradiotherapy[19,20]. Mechanistically, larger tumors often harbor hypoxic regions that reduce radiosensitivity by limiting reactive oxygen species generation, whereas advanced nodal disease may reflect a heightened propensity for micro-metastatic spread. Poor tumor differentiation may also compromise treatment efficacy by fostering cellular heterogeneity and evasion of cytotoxic mechanisms, highlighting the importance of early and accurate staging to optimize therapeutic interventions[21,22].
Beyond these clinical features, the study underscores the pivotal role of systemic inflammation in treatment resistance. Elevated CRP, IL-6, and TNF-α levels in the poor response group reflect a heightened inflammatory environment, which can interfere with the efficacy of radiotherapy and chemotherapy. CRP activates the nuclear factor kappa-B pathway, which induces the expression of pro-inflammatory cytokines, survival factors, and genes involved in angiogenesis and epithelial-mesenchymal transition, promoting tumor progression and chemoresistance. IL-6, through activation of the Janus tyrosine kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, particularly STAT3, drives tumor cell survival, immune evasion, and angiogenesis, contributing to treatment resistance. Additionally, TNF-α modulates the tumor microenvironment by promoting inflammatory cell infiltration and vascular remodeling. These pathways collectively enhance cell proliferation, inhibit apoptosis, and foster an immune-suppressive environment, which diminishes the therapeutic impact of neoadjuvant therapies. Furthermore, natural products such as total glycosides of Cistanche deserticola have been shown to alleviate inflammatory bowel disease by regulating intestinal environmental homeostasis, suggesting that anti-inflammatory interventions may synergistically enhance chemoradiotherapy sensitivity[23]. This warrants further exploration. These findings support the potential for therapeutic intervention through anti-inflammatory or immunomodulatory strategies, which may target these specific pathways to improve outcomes for patients with LARC[24,25].
Tumor markers like CEA and CA19-9 have been found to be significant in predicting treatment response, with elevated CEA emerging as an independent predictor in multivariate analysis. High CEA levels reflect a higher tumor burden or more aggressive cellular phenotypes, which can facilitate cancer cell survival, thus compromising the success of nCRT[26-28]. While TNF-α demonstrated significance in univariate analysis (P = 0.004), its lack of significance in multivariate analysis (P = 0.133) may be explained by collinearity with other inflammatory markers such as CRP and IL-6. These markers share overlapping inflammatory pathways, which could reduce the independent effect of TNF-α in the multivariate model. Although CA19-9 was less predictive in this study, it is typically associated with mucinous differentiation or advanced disease, suggesting that different molecular subtypes of rectal cancer may respond differently to combined therapy. The interplay between CRP, IL-6, and CEA further contributes to treatment resistance. IL-6 activates the JAK/STAT pathway, particularly STAT3, which promotes tumor cell survival, immune evasion, and angiogenesis, potentially enhancing the effects of CRP and CEA in facilitating tumor progression. This biological network likely enhances treatment resistance by activating survival pathways and inhibiting apoptosis. To integrate these predictors, a nomogram was developed incorporating CRP, IL-6, and CEA, which demonstrated excellent discriminative power, with an AUC of 0.928. Calibration and decision curve analyses further confirmed the robustness and clinical relevance of the model, providing accurate risk estimations for poor response. This tool offers significant clinical benefit by allowing personalized treatment strategies, sparing low-risk individuals from excessive toxicity while guiding high-risk patients toward more aggressive or targeted therapies, such as dose escalation or molecular interventions.
Current predictive models for treatment response in LARC often rely on limited clinicopathological variables or focus on single biomarkers. These models typically show moderate accuracy, with some lacking external validation and the integration of comprehensive biomarkers, which hinders their generalizability and clinical applicability. Furthermore, many models do not account for the complex interplay between inflammatory markers and tumor markers, which may better capture the multifactorial nature of treatment resistance[19,29]. Our study addresses these gaps by incorporating a combination of clinical characteristics and key inflammatory markers (CRP, IL-6, CEA) in a nomogram model to predict poor response to nCRT. This approach improves predictive accuracy by integrating systemic inflammatory responses and tumor burden, which are known to significantly affect treatment outcomes. Additionally, our model underwent robust internal validation using bootstrap resampling and demonstrated high accuracy (AUC = 0.928), with external validation remaining a future direction. This comprehensive, data-driven tool is more applicable to personalized treatment planning in patients with LARC, addressing both clinical and biological factors that influence therapeutic responses.
Despite its strengths, this study has several limitations. As a retrospective analysis, it is subject to potential selection bias and unmeasured confounders, including treatment adherence and biomarker variability, which may affect the observed outcomes. Although the nomogram demonstrated strong internal validity through bootstrap resampling, external validation in larger, prospective, and more diverse cohorts is essential to confirm its generalizability. Additionally, the current model focuses on a limited set of clinical and inflammatory markers; integration of molecular signatures, radiomic features, or gut microbiota-related indicators may enhance predictive accuracy. Notably, natural compounds such as Dendrobium officinale oligosaccharides known to modulate inflammation and intestinal homeostasis represent a promising direction for overcoming chemoradiotherapy resistance via gut microenvironment regulation[30]. Prospective trials exploring these adjunctive strategies, along with immunotherapy or dose-escalation in high-risk patients, are warranted to further optimize treatment outcomes in LARC.
In conclusion, serum CEA, CRP, and IL-6 are independent predictors of nCRT response in patients with LARC. The nomogram model incorporating these three markers demonstrates robust predictive power and accuracy, making it a valuable tool for individualized treatment planning.
We would like to express our sincere gratitude to all the individuals and organizations who supported this study.
| 1. | Srivastava V, Goswami AG, Basu S, Shukla VK. Locally Advanced Rectal Cancer: What We Learned in the Last Two Decades and the Future Perspectives. J Gastrointest Cancer. 2023;54:188-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Koukourakis IM, Kouloulias V, Tiniakos D, Georgakopoulos I, Zygogianni A. Current status of locally advanced rectal cancer therapy and future prospects. Crit Rev Oncol Hematol. 2023;186:103992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64684] [Article Influence: 16171.0] [Reference Citation Analysis (177)] |
| 4. | Kokelaar RF, Evans MD, Davies M, Harris DA, Beynon J. Locally advanced rectal cancer: management challenges. Onco Targets Ther. 2016;9:6265-6272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Berardi R, Maccaroni E, Onofri A, Morgese F, Torniai M, Tiberi M, Ferrini C, Cascinu S. Locally advanced rectal cancer: the importance of a multidisciplinary approach. World J Gastroenterol. 2014;20:17279-17287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Liu S, Jiang T, Xiao L, Yang S, Liu Q, Gao Y, Chen G, Xiao W. Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. Oncologist. 2021;26:e1555-e1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Shen L, Wan J, Zhang H, Wu R, Wang J, Wang Y, Xu Y, Cai S, Zhang Z, Xia F. Neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer: A new era for anal preservation. Front Immunol. 2022;13:1067036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 8. | Body A, Prenen H, Lam M, Davies A, Tipping-Smith S, Lum C, Liow E, Segelov E. Neoadjuvant Therapy for Locally Advanced Rectal Cancer: Recent Advances and Ongoing Challenges. Clin Colorectal Cancer. 2021;20:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Saraf A, Roberts HJ, Wo JY, Parikh AR. Optimal Neoadjuvant Strategies for Locally Advanced Rectal Cancer by Risk Assessment and Tumor Location. J Natl Compr Canc Netw. 2022;20:1177-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, Ghidini M, Turati L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann Surg. 2020;271:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 11. | Wang H, Jia H, Gao Y, Zhang H, Fan J, Zhang L, Ren F, Yin Y, Cai Y, Zhu J, Zhu ZJ. Serum metabolic traits reveal therapeutic toxicities and responses of neoadjuvant chemoradiotherapy in patients with rectal cancer. Nat Commun. 2022;13:7802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Guan B, Xu M, Zheng R, Guan G, Xu B. Novel biomarkers to predict treatment response and prognosis in locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy. BMC Cancer. 2023;23:1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 13. | Li S, Wang D, Wei R, Yu G, Wang X, Jiang Z. Predictive Value of the Neutrophil-Lymphocyte Ratio for Tumor Regression Grade and Prognosis of Local Advanced Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiotherapy. Technol Cancer Res Treat. 2023;22:15330338231202611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5754] [Cited by in RCA: 9961] [Article Influence: 585.9] [Reference Citation Analysis (0)] |
| 15. | Erlandsson J, Lörinc E, Ahlberg M, Pettersson D, Holm T, Glimelius B, Martling A. Tumour regression after radiotherapy for rectal cancer - Results from the randomised Stockholm III trial. Radiother Oncol. 2019;135:178-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 16. | Li SF, Wei R, Yu GH, Jiang Z. Predictive value of indirect bilirubin before neoadjuvant chemoradiotherapy in evaluating prognosis of local advanced rectal cancer patients. World J Gastrointest Oncol. 2022;14:2224-2237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (0)] |
| 17. | Duque-Santana V, López-Campos F, Martin-Martin M, Valero M, Zafra-Martín J, Couñago F, Sancho S. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Factors in Locally Advanced Rectal Cancer. Oncology. 2023;101:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Nov P, Du K, Huang Z, Li Y, Gong M, Liu X, Li C, Li L, Wang D, Zhang Y, Wang C, Li J. A Meta-analysis of Total Neoadjuvant Therapies Combining Chemoradiotherapy with Induction or Consolidated Chemotherapy for Locally Advanced Rectal Cancer. J Gastrointest Cancer. 2023;54:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Yan L, Wu Y, Xu M, Liu X, Guan G. Worse treatment response to neoadjuvant chemoradiotherapy in young patients with locally advanced rectal cancer. BMC Cancer. 2020;20:854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Huang MY, Huang CW, Wang JY. Surgical treatment following neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Kaohsiung J Med Sci. 2020;36:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Molinari C, Matteucci F, Caroli P, Passardi A. Biomarkers and Molecular Imaging as Predictors of Response to Neoadjuvant Chemoradiotherapy in Patients With Locally Advanced Rectal Cancer. Clin Colorectal Cancer. 2015;14:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Kim NK, Hur H. New Perspectives on Predictive Biomarkers of Tumor Response and Their Clinical Application in Preoperative Chemoradiation Therapy for Rectal Cancer. Yonsei Med J. 2015;56:1461-1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Zhang S, Feng D, An J, Zhao J, Zhao J, Guo Y, Jiang Y, Yan W. Total Glycosides of Cistanche deserticola attenuates DSS-induced inflammatory bowel disease by regulating intestinal environmental homeostasis. Food Med Homol. 2025;2:9420048. [DOI] [Full Text] |
| 24. | Meng X, Huang Z, Wang R, Yu J. Prediction of response to preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Biosci Trends. 2014;8:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Park YA, Sohn SK, Seong J, Baik SH, Lee KY, Kim NK, Cho CW. Serum CEA as a predictor for the response to preoperative chemoradiation in rectal cancer. J Surg Oncol. 2006;93:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Park JW, Lim SB, Kim DY, Jung KH, Hong YS, Chang HJ, Choi HS, Jeong SY. Carcinoembryonic antigen as a predictor of pathologic response and a prognostic factor in locally advanced rectal cancer patients treated with preoperative chemoradiotherapy and surgery. Int J Radiat Oncol Biol Phys. 2009;74:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Wolff RA, Eng C, Krishnan S, Janjan NA, Crane CH. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer. 2007;109:1750-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Yoon SM, Kim DY, Kim TH, Jung KH, Chang HJ, Koom WS, Lim SB, Choi HS, Jeong SY, Park JG. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2007;69:1167-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Huang CM, Huang CW, Ma CJ, Yeh YS, Su WC, Chang TK, Tsai HL, Juo SH, Huang MY, Wang JY. Predictive Value of FOLFOX-Based Regimen, Long Interval, Hemoglobin Levels and Clinical Negative Nodal Status, and Postchemoradiotherapy CEA Levels for Pathological Complete Response in Patients with Locally Advanced Rectal Cancer after Neoadjuvant Chemoradiotherapy. J Oncol. 2020;2020:9437684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Shi D, Wang P, Xu L, Zhu H, Zhang W, Wu Q, Bu T, Tian B, Sun P, Cai M. Potential of Dendrobium officinale oligosaccharides to alleviate chronic colitis by modulating inflammation and gut microbiota. Food Med Homol. 2024;. [DOI] [Full Text] |