Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.107919
Revised: April 27, 2025
Accepted: May 21, 2025
Published online: June 15, 2025
Processing time: 73 Days and 5.3 Hours
The expression level of Ki-67 and the degree of differentiation in pancreatic cancer determine tumor aggressiveness and patient prognosis, which holds significant implications for clinical decision-making. A major challenge in preoperative pancreatic ductal adenocarcinoma management is predicting tumor malignancy. Contrast-enhanced ultrasound (CEUS), a dynamic imaging technique based on blood pool visualization, can reveal lesion vasculature and provide quantitative perfusion data reflecting angiogenesis. By tracking contrast agent kinetics, CEUS offers non-invasive insights into tumor vascularization, helping assess malig
To investigate the correlation between Ki-67 and pancreatic cancer differentiation using CEUS quantitative parameters and evaluated their diagnostic accuracy.
This retrospective study analyzed pancreatic cancer patients who underwent CEUS and pathological confirmation. Pathological differentiation, clinical data, and quantitative CEUS parameters [maximum intensity (IMAX), rise time (RT), rise slope 50% (Rs50), rise slope 10%-90% (Rs1090), etc.] were collected. Based on Ki-67 expression (< 50% vs ≥ 50%), patients were divided into low- and high-expression groups. The study evaluated correlations between Ki-67 expression, differentiation degree, and CEUS quantitative parameters to assess tumor aggressiveness.
Among 54 patients (25 high Ki-67, 29 low Ki-67), significant differences (P < 0.05) were observed in Rs50, IMAX, wash-out area under the curve (WoutAUC), wash-in and out area under curve, and Rs1090 between high and low Ki-67 groups. High-expression patients showed elevated Rs50, IMAX, WoutAUC, and area under the curve (AUC), while RT and falling slope 50% (Fs50) were lower. Rs1090 demonstrated the highest diagnostic accuracy (AUC = 0.863, sensitivity = 0.92, specificity = 0.759). Fs50 was effective in low Ki-67 detection (AUC = 0.838). No correlation was found between enhancement patterns and Ki-67 or differentiation.
CEUS parameters (Rs50, IMAX, WoutAUC, Rs1090) strongly correlate with Ki-67, aiding non-invasive pancreatic cancer assessment. Rs1090/IMAX predict high Ki-67; Fs50 identifies low Ki-67, supporting CEUS for tumor aggressiveness evaluation.
Core Tip: This study systematically integrates contrast-enhanced ultrasound quantitative parameters with pathological markers (Ki-67 index and tumor grade) in pancreatic cancer for the first time. By combining ultrasonography, tumor pathology, and statistical methods, it provides a noninvasive approach to assess tumor proliferation and aggressiveness. The findings may enable more precise treatment decisions (e.g., surgery, chemotherapy, or targeted therapy), potentially improving clinical outcomes.
- Citation: Yang ZY, Wan WN, Zhao L, Li SN, Liu Z, Sang L. Noninvasive prediction of Ki-67 expression in pancreatic cancer via contrast-enhanced ultrasound quantitative parameters: A diagnostic model study. World J Gastrointest Oncol 2025; 17(6): 107919
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/107919.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.107919
Pancreatic cancer, often referred to as the “king of cancers”, is characterized by its high malignancy and an alarmingly low five-year survival rate. In 2022, China reported 118700 new cases of pancreatic cancer, with 106,300 deaths attributed to the disease[1]. From 2019 to 2021, the 5-year survival rate of pancreatic cancer in China was only 8.5% (95% confidence interval: 8.2-8.7)[2]. Surgical resection remains the sole curative option for pancreatic cancer. However, pancreatic cancer surgery is highly complex, with a resection rate of only approximately 20% to 30%. The insidious nature of early symptoms and the lack of effective screening methods often result in delayed diagnosis, with most patients being diagnosed at an advanced stage and thus ineligible for surgery. Furthermore, the complexity of pancreatic cancer surgery is compounded by the limited efficacy of radiotherapy and chemotherapy, which further complicates treatment outcomes[3].
The expression of Ki-67 in tumor tissues serves as a marker of tumor proliferation. It is highly expressed in most malignant cells but is rarely detected in normal cells. As a result, Ki-67 has garnered increasing attention as a potential prognostic and predictive biomarker and as a therapeutic target for various malignancies, including bladder cancer, breast cancer, and cervical cancer. Consequently, it is considered as a promising predictor in oncology[4-7]. Recent studies have demonstrated that Ki-67 plays a critical role in the grading and classification of pancreatic neuroendocrine tumors, as outlined by the World Health Organization (WHO) 2017 and ENETS criteria. Notably, Ki-67 is recognized as an independent predictor of the survival of patients with pancreatic cancer[8]. The degree of pathological differentiation of pancreatic cancer is a critical factor influencing its prognosis. Accurate prediction of the differentiation degree should facilitate the formulation of more suitable treatment plans for patients. Current studies indicate that for patients with poorly differentiated pancreatic ductal adenocarcinoma (PDAC), the survival period following neoadjuvant therapy is significantly longer compared to surgery alone[9,10]. Histological differentiation significantly influences prognosis in pancreatic cancer. Both Ki-67 index and tumor differentiation are assessed via immunohistochemistry of biopsy specimens, with ultrasound-guided pancreatic biopsy providing high diagnostic accuracy as the minimally invasive gold standard[11]. However, the anatomical location of the pancreas, which is situated behind the peritoneum and in close proximity to major blood vessels, poses challenges in the confirmation of a safe puncture pathway and significantly increases the associated risk.
Contrast-enhanced ultrasound (CEUS) is a noninvasive imaging technique based on blood pool imaging. It enables visualization of both the major blood vessels and the microvascular system within the pancreas and has been endorsed by clinical guidelines for characterizing solid pancreatic lesions[12]. Quantitative analysis of CEUS involves deriving quantitative parameters of tissue or tumor blood flow perfusion during the CEUS process through the application of specific models and functions. These parameters can be utilized for the early diagnosis of tumors and the dynamic evaluation of antitumor treatment efficacy. In the context of pancreatic cancer, quantitative CEUS analysis enables the differentiation of pancreatic adenocarcinoma from focal pancreatitis and can be used to distinguish between malignant and benign pancreatic hypovascular diseases[13,14]. In addition to assisting in differential diagnosis, quantitative analysis of CEUS results can be performed to predict the expression of molecular markers in tumor tissues. For example, CEUS features can predict different subtypes of breast cancer, and can predict microvascular invasion, pathological grading, and Ki-67 index of hepatocellular carcinoma[15,16]. Dynamic CEUS enables the understanding of tissue perfusion and angiogenesis in lesions via quantitative analysis. Previous studies have demonstrated that CEUS can be used to assess the microvessel density (MVD) of pancreatic tumors[17]. In previous studies, two-dimensional ultrasound has been used to predict Ki-67 in breast cancers[18], but CEUS quantitative analysis has not yet been applied to assess Ki-67 expression and the pathological differentiation in pancreatic cancer.
This study aims to explore the correlation between the quantitative parameters of CEUS and the Ki-67 index as well as the pathological differentiation degree of pancreatic cancer. By analyzing the expression levels of CEUS quantitative parameters, we attempt to establish a noninvasive prediction model to evaluate the Ki-67 index and pathological stage of pancreatic cancer. This research not only helps to reduce the risks associated with invasive examinations for patients but also provides a more accurate basis for clinical treatment decisions and prognosis assessment, thereby improving the treatment outcomes and quality of life of patients.
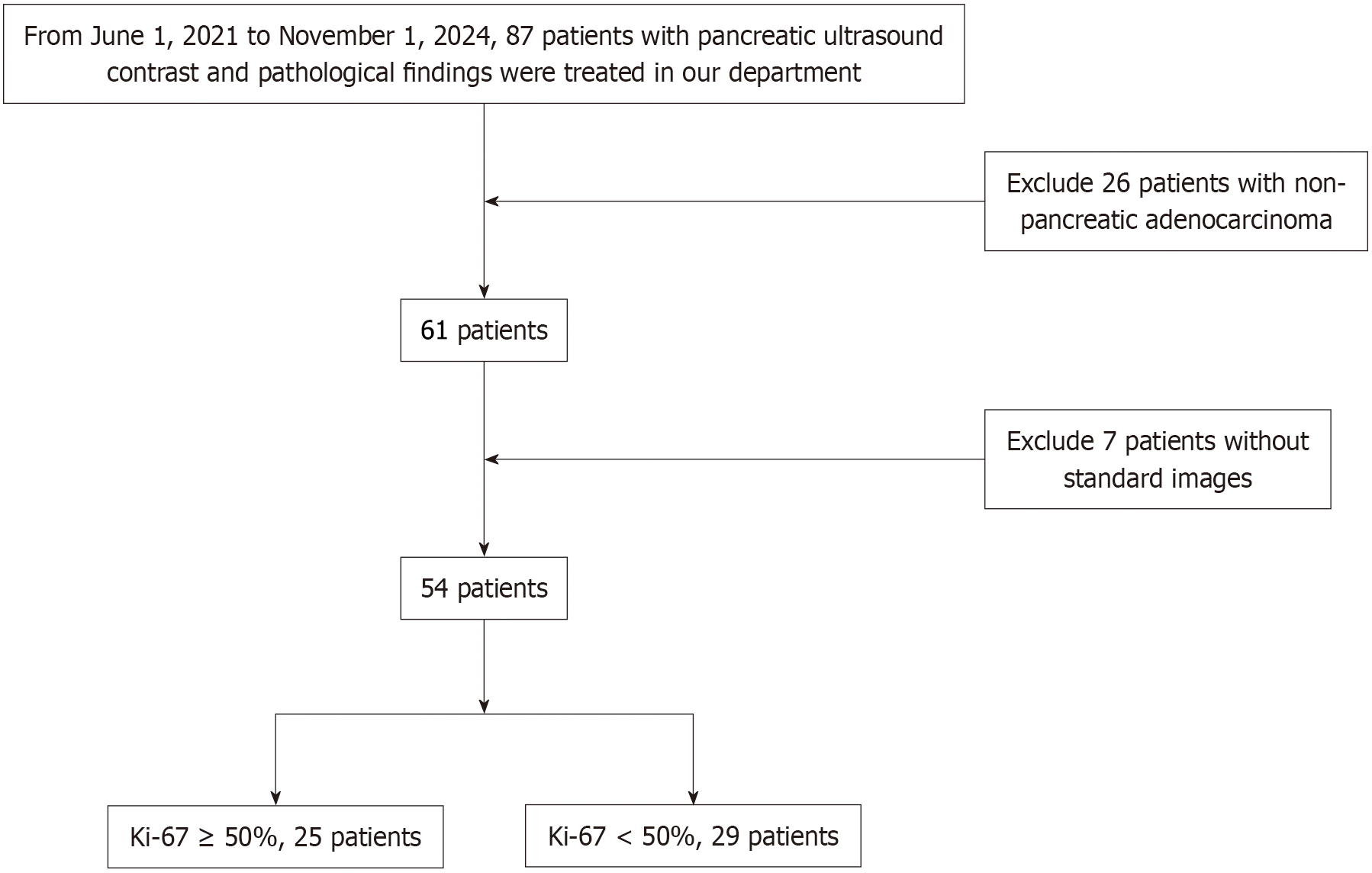
This study was approved by the Ethics Committee of the First Hospital Affiliated to China Medical University (No. AF-SOP-07-1.2-01). Due to its retrospective nature, the requirement for written informed consent was waived. The study enrolled patients who underwent CEUS for pancreatic masses in the Department of Ultrasonography at our hospital between June 1, 2021, and November 1, 2024, and who subsequently underwent surgery or core needle biopsy with pathological confirmation of pancreatic adenocarcinoma.
Inclusion criteria: (1) Complete medical history, preoperative examinations, hospitalization records, and puncture pathology results for all patients; (2) Confirmation of malignancy through puncture pathology or surgical results; (3) Pathological diagnosis of adenocarcinoma with available Ki-67 immunohistochemistry results and pathological differentiation degree; and (4) Availability of CEUS images in digital imaging and communications in medicine (DICOM) format.
Exclusion criteria: (1) Pathological confirmation of benign conditions; (2) Pathological diagnosis of conditions other than pancreatic adenocarcinoma; (3) Prior history of radiotherapy or chemotherapy; (4) Pancreatic lesions identified as metastatic foci; and (5) Insufficient duration or poor quality of CEUS videos.
Finally, 54 patients with pancreatic cancer, comprising a total of 54 masses, were included in the study. Data on age, serum carbohydrate antigen (CA) 19-9, size and location of pancreatic lesions, and clinical staging (based on the latest version of Journal of the American College of Cardiology) were retrospectively collected for all patients. Additionally, CEUS videos and the immunohistochemical expression of Ki-67 protein in pancreatic cancer tissues were obtained. Using 50% as the cut-off value for Ki-67 expression, the tissues were categorized into two groups: A high-expression group (Ki-67 ≥ 50%) and a low-expression group (Ki-67 < 50%). The specific workflow is illustrated in Figure 1.
The patients fasted and lay in a supine position to fully expose the upper abdomen. The study used Siemens ACUSON Sequoia (Munich, Germany) and Mindray Neuwa R9 Exp (Shenzhen, China) ultrasound machines, both equipped with an abdominal convex array probe (1-5 MHz). The second-generation contrast agent, SonoVue (Bracco, Italy), was mixed with 5 mL of normal saline to form a suspension. Conventional ultrasound was first performed to localize pancreatic lesions. The contrast mode was then activated, and a 2.4 mL suspension was rapidly injected into the superficial elbow vein within 2-3 seconds, followed by a 5 mL saline flush. Timing began at the moment of injection. Real-time observation of lesion enhancement and regression patterns was conducted, ensuring the probe remained stable to avoid distortion. Observation lasted over 2 minutes, and images were saved.
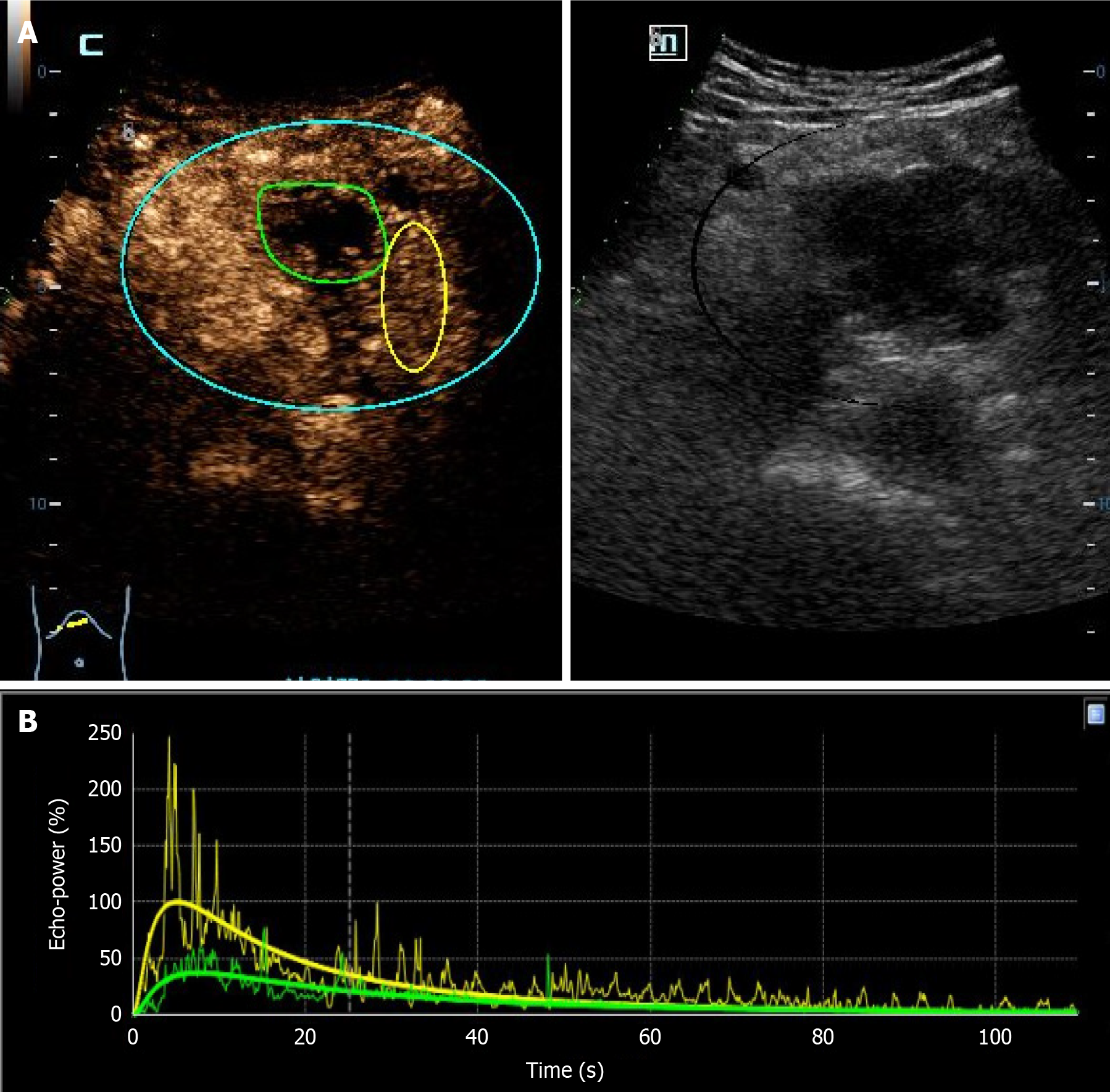
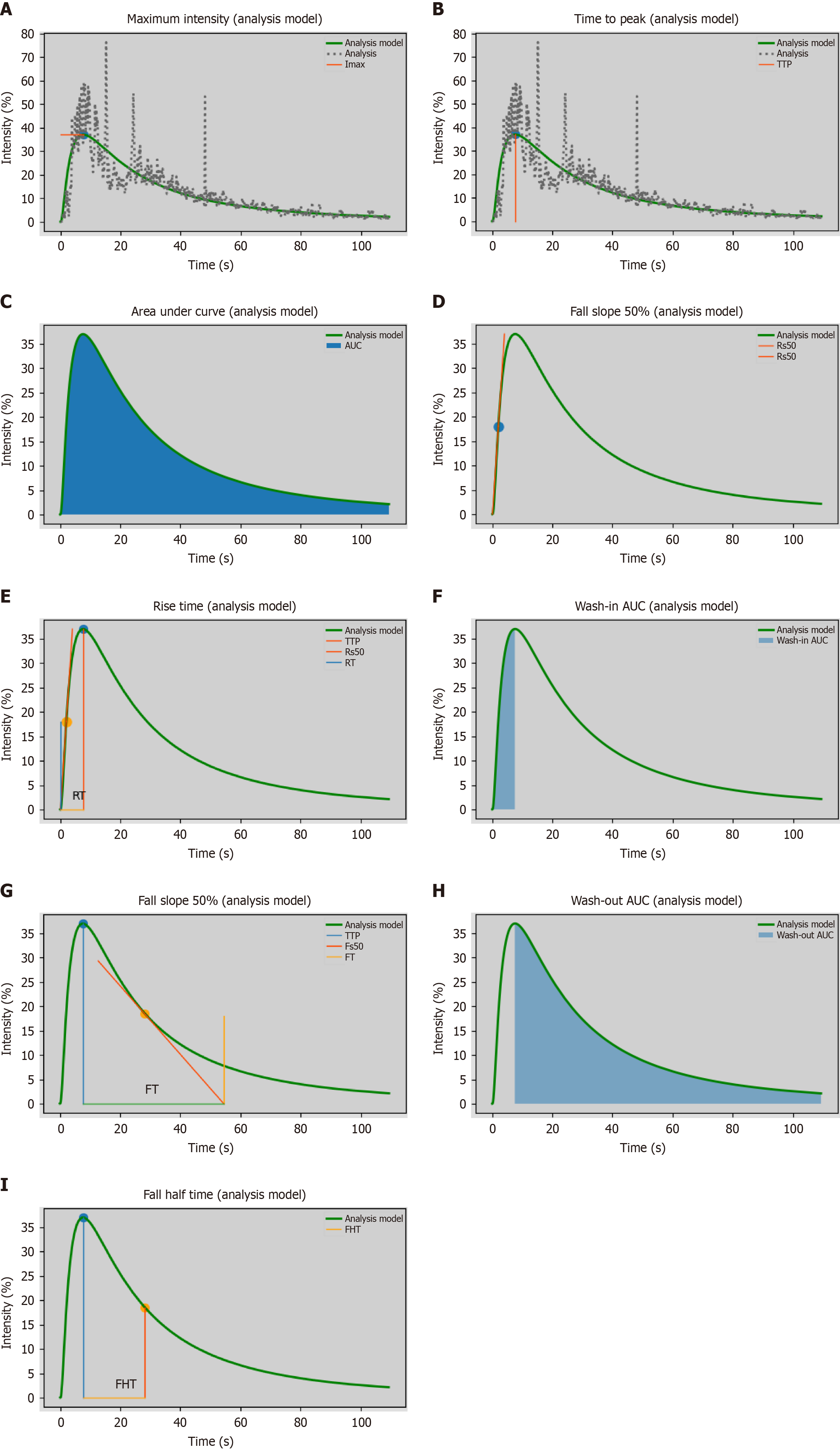
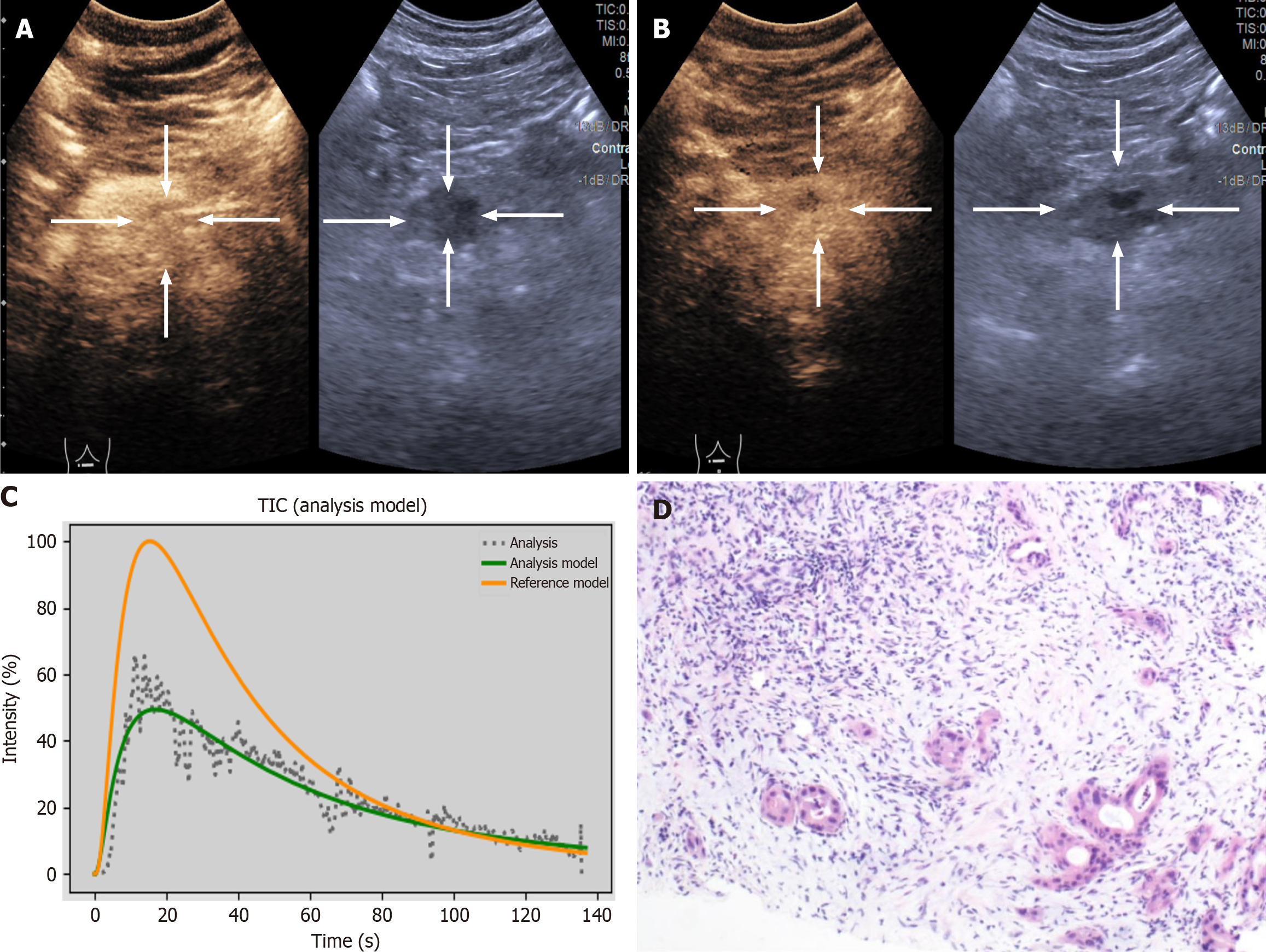
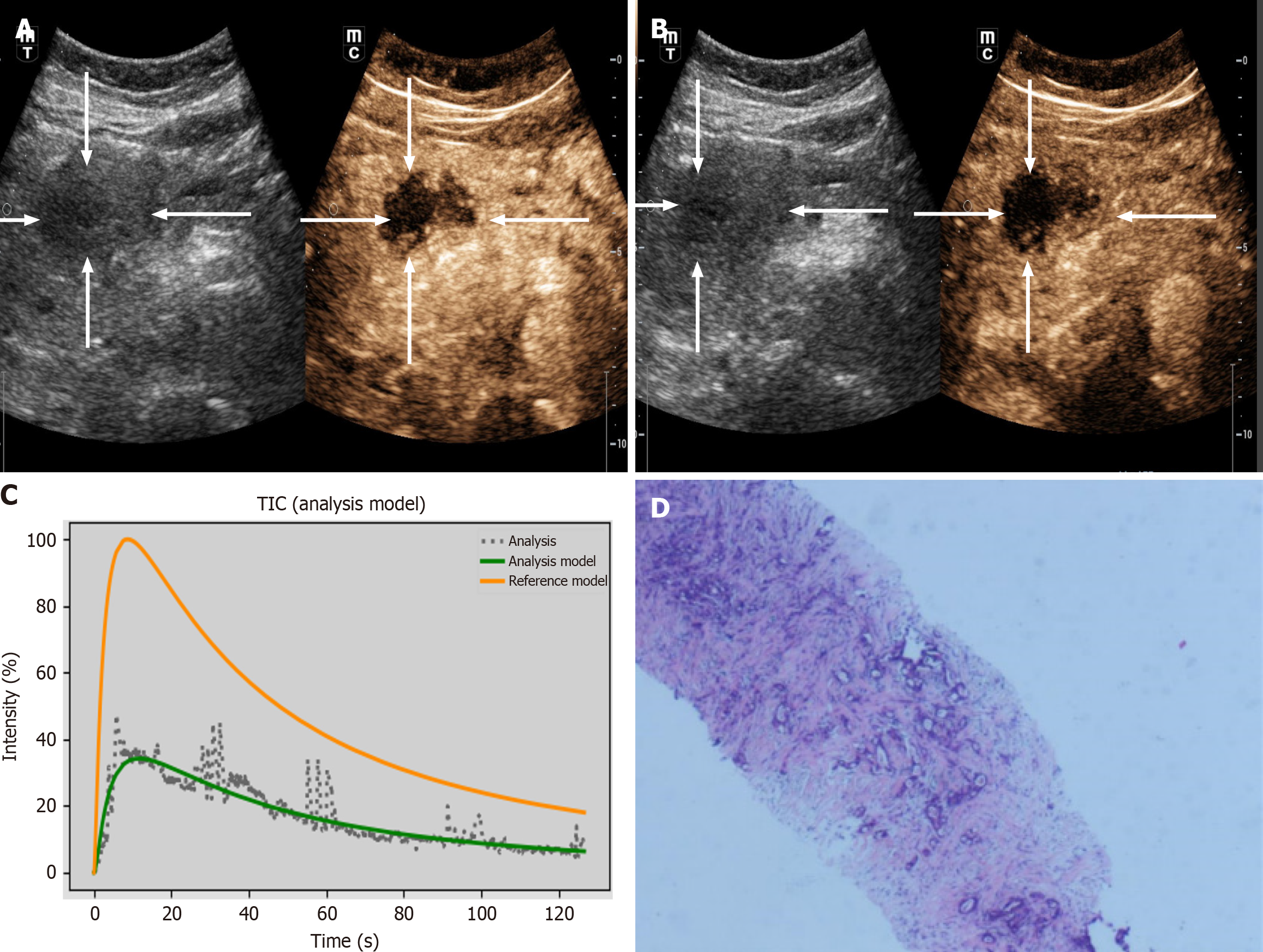
The DICOM format of the dynamic CEUS images was exported from the machine. Using SonoLiver software (Tomtec, Munich, Germany), the region of interest (ROI) was delineated, covering both the lesions and the surrounding normal tissues, as illustrated in Figure 2. The person performing the ROI delineation had over 10 years of experience in abdominal ultrasound. After delineation, quantitative analysis was conducted to generate the time-intensity curve (TIC). Through quantitative analysis of the curve, we derived several key parameters, including: Maximum intensity (IMAX), rise time (RT), time to peak (TTP), mean transit time (mTT), rise slope 50% (Rs50), rise slope 10%-90% (Rs1090), falling time (FT), falling slope 50% (Fs50), falling half time (FHT), area under the curve (AUC), wash-in AUC (WinAUC) (calculated between RT and TTP), wash-out AUC (WoutAUC) (calculated between TTP and FT), wash-in and out AUC (WioAUC), wash-in rate (WinR), and wash-out rate (WoutR). These parameters are visually represented in Figure 3. To maintain study quality, we established a quality of fit threshold of ≥ 75% for the TIC.
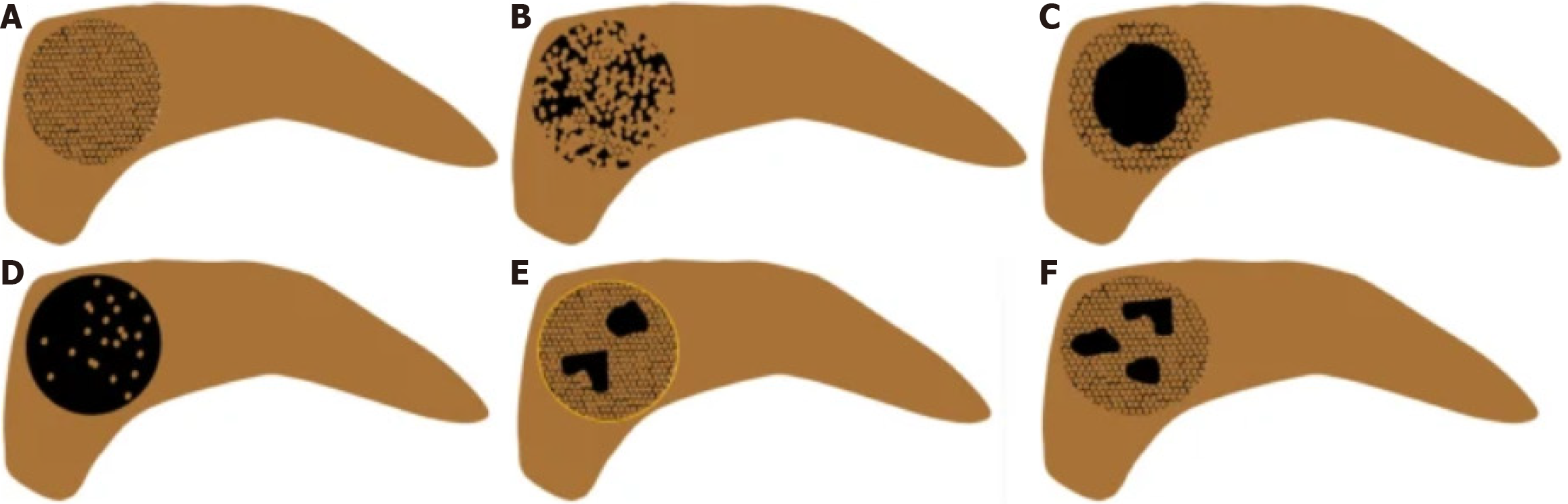
Currently, there is no standardized method for the qualitative analysis of CEUS. However, six peak enhancement patterns have been identified in previous study[19], as illustrated in Figure 4. These include: (1) Pattern I: Homogeneous hypo enhancement (hypo vascular lesions with uniform enhancement; Figure 4A); (2) Pattern II: Heterogeneous hypo enhancement without cystic components (hypo vascular lesions with non-uniform enhancement lacking cystic components; Figure 4B); (3) Pattern III: Ring-like enhancement (contrast agent primarily distributed along the lesion’s periphery, creating a donut-like appearance; Figure 4C); (4) Pattern IV: Starry sky enhancement (contrast agent sparsely distributed, resembling a starry sky; Figure 4D); (5) Pattern V: Capsular enhancement with cystic components (hypo vascular lesions featuring distinct capsules and internal cystic areas; Figure 4E); and (6) Pattern VI: Hypo enhancement with cystic components (hypo vascular lesions containing cystic regions; Figure 4F).
Puncture pathological tissues or surgical specimens were sent to our hospital’s pathology department for Ki-67 immunohistochemical staining and differentiation degree assessment. An immunohistochemical kit (containing anti-human Ki-67 monoclonal antibody) was utilized according to the manufacturer’s instructions. The Ki-67 index was calculated as follows: Pathologists identified the Ki-67 hotspot area and evaluated a 5 μm-thick section of formalin-fixed paraffin-embedded tissue. Under a 400 × magnification microscope, they counted 1000 tumor cells and determined the percentage of positive cells by assessing the proportion of Ki-67-labeled tumor cell nuclei. Based on established criteria from prior studies, patients were categorized into two groups: < 50% (low expression) and ≥ 50% (high expression)[20,21]. PDAC was pathologically graded into well-differentiated, moderately differentiated, and poorly differentiated categories based on the 2019 WHO Classification of Tumors of the Digestive System guidelines[22].
SPSS 27.0 software was used for data analysis. Quantitative data were described as mean ± SD or median (interquartile range). Statistical inference was performed using analysis of variance or the Kruskal-Wallis H test, with long slow distance or Q tests employed for pairwise group comparisons. Categorical data were presented as case numbers (constituent ratios) and compared between groups using the χ2 test. Correlations were analyzed using Spearman rank correlation analysis. The diagnostic value of each characteristic was evaluated using receiver operating characteristic (ROC) curve analysis, with the AUC, sensitivity, specificity, and Youden’s index reported. A P value < 0.05 was considered statistically significant.
Based on the inclusion and exclusion criteria, 54 pancreatic cancer patients were enrolled in the study. Among these, 29 patients exhibited low Ki-67 expression, while 25 demonstrated high Ki-67 expression. The cohort comprised 29 males and 25 females. CA19-9 levels were normal in 14 cases and elevated in 40 cases. The distribution of lesions was as follows: pancreatic head (22 cases), neck (9 cases), body (14 cases), and tail (9 cases). For tumor node metastasis (TNM) staging, the distribution was as follows: Stage I (7 cases), stage II (5 cases), stage III (28 cases), and stage IV (13 cases). Statistical analysis revealed no significant differences between the two groups in terms of age, gender, CA19-9 level, lesion location, differentiation degree, and clinical stage (P > 0.05). However, a significant difference was observed in the maximum lesion diameter (P < 0.05), with the high-expression group showing a larger average maximum diameter (4.84 cm ± 1.68 cm) compared to the low-expression group (3.87 cm ± 1.39 cm). Details are presented in Table 1.
| Characteristics | Ki-67 < 50% | Ki-67 ≥ 50% | P value |
| Age, year | 59.93 ± 10.83 | 61.48 ± 7.90 | 0.642 |
| Gender | 0.816 | ||
| Male | 16 (29.6) | 13 (24.1) | |
| Female | 13 (24.1) | 12 (22.2) | |
| CA19-9 | 0.746 | ||
| Normal | 8 (14.81) | 6 (11.11) | |
| Elevated | 21 (38.89) | 19 (35.19) | |
| Diameter, cm | 3.87 ± 1.39 | 4.84 ± 1.68 | 0.022a |
| Location | 0.259 | ||
| Head | 14 (25.93) | 8 (14.81) | |
| Neck | 4 (7.41) | 5 (9.26) | |
| Body | 5 (9.26) | 9 (16.67) | |
| Tail | 6 (11.11) | 3 (5.56) | |
| Differentiation grade | 0.301 | ||
| Well | 11 (20.37) | 5 (9.26) | |
| Moderately | 7 (12.96) | 6 (11.11) | |
| Poorly | 11 (20.37) | 14 (25.92) | |
| TNM stage | 0.140 | ||
| I | 6 | 1 | |
| II | 3 | 2 | |
| III | 15 | 13 | |
| IV | 4 | 9 |
In this study, we evaluated Ki-67 index expression in pancreatic cancer samples across varying degrees of differentiation to investigate its association with tumor differentiation. Table 2 presents the proportions of Ki-67 index < 50% and ≥ 50% in well-differentiated, moderately differentiated, and poorly differentiated samples. In well-differentiated samples, 9 cases (11.8%) had a Ki-67 index < 50%, and 4 (6.0%) had a Ki-67 index ≥ 50%. In moderately differentiated samples, 11 cases (10.2%) had a Ki-67 index < 50%, and 8 (8.8%) had a Ki-67 index ≥ 50%. In poorly differentiated samples, 9 cases (7.0%) had a Ki-67 index < 50%, and 13 (10.2%) had a Ki-67 index ≥ 50%. A χ² test was performed, yielding a χ² value of 2.404 and a P value of 2.404 (P > 0.05). Thus, Ki-67 index expression did not differ significantly across different differentiation degrees of pancreatic cancer samples, and no significant correlation was observed between them.
| Ki-67 < 50% | Ki-67 ≥ 50% | χ² value | P value | |
| Well differentiated | 11 (20.37) | 5 (9.26) | 2.404 | 0.301 |
| Moderately differentiated | 7 (12.96) | 6 (11.11) | ||
| Poorly differentiated | 11 (20.37) | 14 (25.92) |
By comparing the quantitative analysis parameters between the high and low differentiation groups (Figures 5 and 6), we observed significant differences in the expressions of Rs50, IMAX, RT, WoutAUC, WioAUC, WoutR, Rs1090, Fs50, AUC, and TTP (P < 0.05). However, no significant differences were found in the expressions of WinAUC, FHT, FT, WinR, Fs50, and mTT (P > 0.05).Through the comparison of the data between the two groups, we concluded that the values of Rs50, IMAX, WoutAUC, WioAUC, Rs1090, and AUC were significantly higher in the high-expression group than in the low-expression group, while the values of RT, WoutR, Fs50, and TTP were significantly lower than those in the low-expression group. Details are presented in Table 3.
| Ki-67 < 50% | Ki-67 ≥ 50% | U value | P value | |
| Rs50 | 6.84 (5.23 to 10.55) | 11.35 (9.56 to 15.10) | 144.500 | < 0.001b |
| IMAX | 46.37 (37.60 to 57.56) | 74.12 (61.80 to 89.20) | 102.000 | < 0.001b |
| RT | 14.42 (10.97 to 15.30) | 11.45 (9.35 to 12.32) | 220.000 | 0.013a |
| WinAUC | 296.42 (162.34 to 550.78) | 453.86 (178.36 to 1302.49) | 278.000 | 0.143 |
| FHT | 32.58 (26.86 to 41.07) | 30.70 (25.30 to 36.00) | 311.500 | 0.376 |
| WoutAUC | 19911.74 (12434.98 to 27114.02) | 30764.31 (22543.16 to 42157.32) | 202.000 | 0.005b |
| WioAUC | 20609.49 (13213.08 to 27290.88) | 31196.99 (22668.28 to 42665.07) | 202.000 | 0.005b |
| WoutR | 19911.74 (195.31 to 354.02) | 383.07 (331.66 to 696.14) | 132.000 | < 0.001b |
| FT | 73.82 (62.50 to 99.51) | 71.36 (57.58 to 87.80) | 305.000 | 0.319 |
| Rs1090 | 4.57 (3.79 to 6.76) | 10.47 (8.76 to 11.82) | 99.000 | < 0.001b |
| WinR | 25.33 (17.77 to 51.06) | 37.99 (18.41 to 134.44) | 275.500 | 0.131 |
| Fs50 | -0.52 (-0.68 to 0.38) | -0.92 (-1.16 to 0.76) | 117.500 | < 0.001b |
| AUC | 25841.89 (18120.31 to 37170.46) | 39426.38 (31969.68 to 59121.85) | 198.00 | 0.004b |
| TTP | 15.31 (11.28 to 17.36) | 11.74 (10.30 to 13.10) | 226.500 | 0.018a |
| mTT | 68.89 (54.54 to 91.66) | 76.38 (48.34 to 94.23) | 356.000 | 0.910 |
Through correlation analysis, we concluded that there is a significant correlation between the CEUS parameters and the expression of the Ki-67 index. Specifically, Rs50, IMAX, WoutAUC, WioAUC, WoutR, Rs1090, and AUC are positively correlated with Ki-67 expression. Among these, Rs50, IMAX, WoutAUC, WioAUC, WoutR, and Rs1090 show highly significant correlations (P < 0.001), while AUC exhibits a moderate correlation (r = 0.373). On the other hand, RT, Fs50, and TTP are negatively correlated with the expression of the Ki-67 protein. Among these, Fs50 demonstrates the most significant correlation (P < 0.001). While RT (r = -0.323) exhibits a moderate negative correlation, TTP (r = -0.280) shows a low negative correlation. However, no significant correlation was observed between WinAUC, FHT, WinR, mTT, and the expression of the Ki-67 protein (P > 0.05). Details are presented in Table 4.
We compared the medians of the quantitative analysis parameters across different degrees of differentiation. Notably, among the three differentiation groups, only mTT exhibited differential expression. Specifically, the mTT value in the well-differentiated group was significantly lower than that in the poorly-differentiated group. However, correlation analysis revealed that the significance level of the correlation between mTT and the degree of differentiation was 0.075, indicating a lack of a clear-cut association between the two variables. Details are presented in Table 5.
| Well differentiated | Moderately differentiated | Poorly differentiated | Z value | P value | r value | P’ value | |
| mTT | 57.7 (49.42 to 74.12) | 77.88 (41.81 to 88.58) | 78.40 (64.33 to 100.52) | 6.037 | 0.049a | -0.244 | 0.075 |
| Rs50 | 9.31 (7.63 to 10.73) | 10.59 (5.28 to 13.78) | 10.30 (6.84 to 12.81) | 0.367 | 0.832 | -0.095 | 0.491 |
| IMAX | 54.30 (43.41 to 72.76) | 54.37 (40.84 to 74.12) | 58.56 (49.43 to 88.76) | 0.687 | 0.709 | -0.144 | 0.295 |
| RT | 12.34 (9.80 to 14.86) | 12.67 (11.61 to 14.80) | 11.84 (10.07 to 13.74) | 0.687 | 0.709 | 0.084 | 0.084 |
| WinAUC | 408.93 (241.28 to 1082.16) | 364.44 (155.50 to 850.82) | 296.42 (158.84 to 557.33) | 0.687 | 0.709 | 0.134 | 0.330 |
| FHT | 31.50 (24.84 to 35.99) | 28.93 (26.85 to 41.07) | 33.30 (26.25 to 38.29) | 1.942 | 0.378 | -0.135 | 0.329 |
| WoutAUC | 22454.57 (12938.39 to 29406.73) | 25091.22 (14283.42 to 35206.22) | 29167.97 (18501.77 to 42157.32) | 0.687 | 0.709 | -0.200 | 0.147 |
| WioAUC | 22967.65 (13223.69 to 29874.76) | 25172.88 (15850.85 to 35361.72) | 29454.95 (18687.11 to 42335.48) | 0.687 | 0.709 | -0.185 | 0.181 |
| WoutR | 300.37 (213.11 to 395.74) | 326.56 (217.61 to 392.31) | 353.32 (265.97 to 495.21) | 0.687 | 0.709 | -0.157 | 0.258 |
| FT | 68.60 (55.90 to 79.23) | 67.97 (64.42 to 93.17) | 75.15 (62.50 to 98.85) | 2.077 | 0.353 | -0.154 | 0.266 |
| Rs1090 | 8.24 (4.33 to 8.76) | 9.12 (4.14 to 11.45) | 8.76 (5.85 to 10.83) | 0.367 | 0.832 | -0.189 | 0.172 |
| WinR | 36.73 (18.20 to 60.62) | 29.80 (21.45 to 105.91) | 25.33 (13.87 to 47.07) | 2.077 | 0.353 | 0.157 | 0.258 |
| Fs50 | -0.75 (-1.10 to -0.48) | -0.75 (-1.00 to 0.50) | -0.67 (-0.93 to 0.56) | 1.052 | 0.590 | -0.028 | 0.841 |
| AUC | 32655.20 (17724.16 to 41094.57) | 31111.52 (21612.44 to 43393.67) | 38174.83 (25534.96 to 51066.60) | 1.942 | 0.378 | -0.166 | 0.229 |
| TTP | 13.39 (10.32 to 15.83) | 13.70 (11.90 to 16.50) | 12.37 (10.30 to 14.80) | 0.709 | 0.378 | 0.125 | 0.367 |
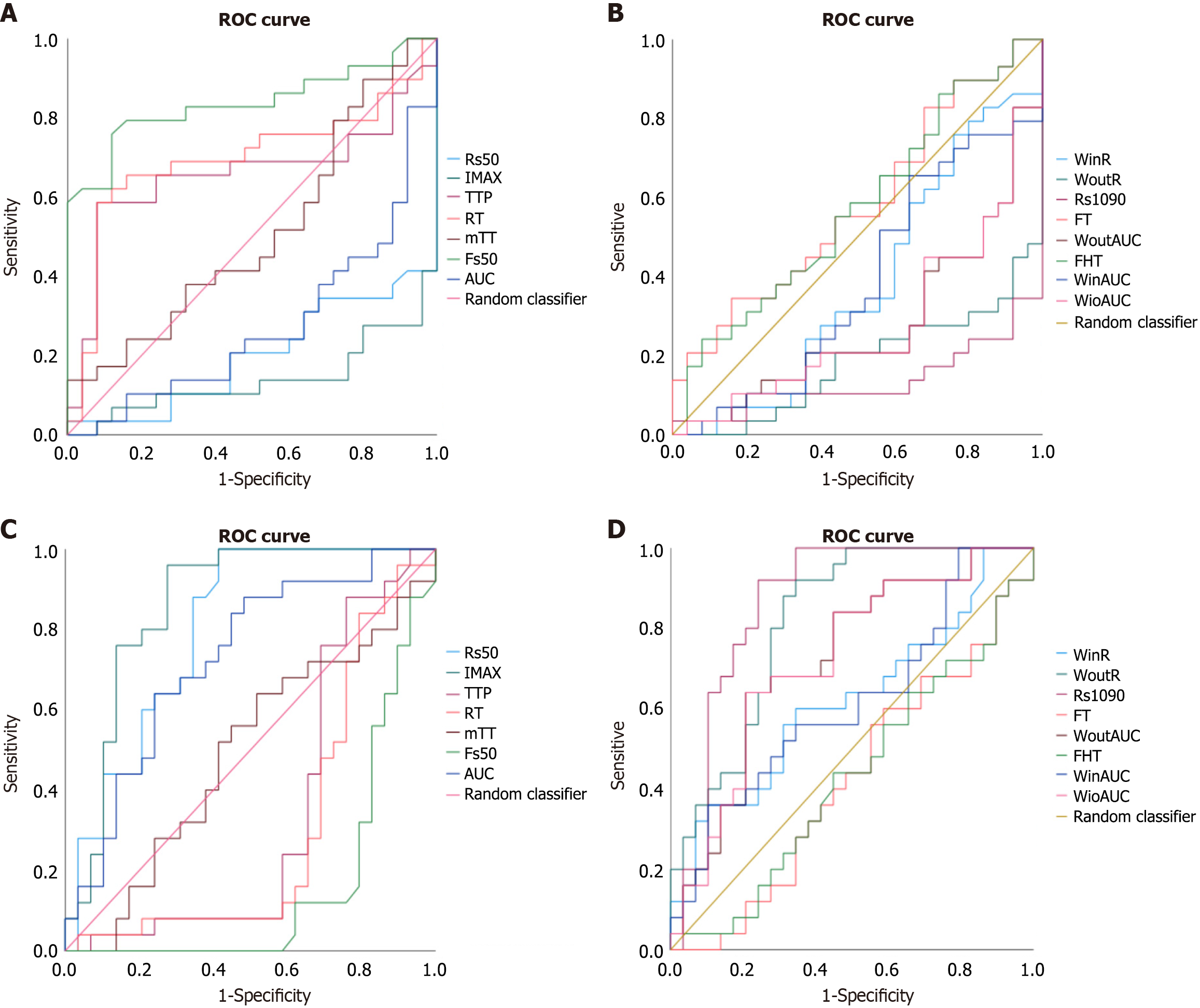
From the ROC curve analysis of Ki-67 low expression, Fs50 had the highest AUC (0.838), demonstrating high accuracy in differentiating low Ki-67 protein expression. RT (0.697) and TTP (0.657) had AUCs between 0.6 and 0.7 but were below 0.75, indicating low diagnostic efficacy. Rs50, IMAX, Rs1090, WinR, AUC and diameter exhibited near-zero AUCs, contributing minimally to differentiating Ki-67 levels. Fs50, with the highest AUC, also displayed good sensitivity (0.759), specificity (0.88), and Youden’s index (0.639), with an optimal threshold of -0.695. Refer to Figure 7A and Table 6 for details.
| AUC (95%CI) | P value | Diagnostic threshold | Specificity | Sensitive | Youden | |
| Rs50 | 0.199 (0.081-0.318) | 0.000b | 28.09 | 1 | 0.034 | 0.034 |
| IMAX | 0.141 (0.036-0.246) | 0.000b | 17.93 | 0 | 1 | 0 |
| RT | 0.697 (0.548-0.845) | 0.013b | 13.305 | 0.92 | 0.586 | 0.506 |
| WinAUC | 0.383 (0.232-0.535) | 0.143 | 247.535 | 0.36 | 0.655 | 0.015 |
| FHT | 0.570 (0.416-0.724) | 0.376 | 44.05 | 0.92 | 0.241 | 0.161 |
| WoutAUC | 0.279 (0.141-0.416) | 0.005b | 122023.5 | 1 | 0 | 0 |
| WioAUC | 0.279 (0.141-0.416) | 0.005b | 128423.37 | 1 | 0 | 0 |
| WoutR | 0.380 (0.141-0.294) | 0.000b | 1233.34 | 1 | 0 | 0 |
| FT | 0.579 (0.426-0.732) | 0.319 | 90.095 | 0.84 | 0.345 | 0.185 |
| Rs1090 | 0.137 (0.032-0.241) | 0.000b | 23.195 | 1 | 0.034 | 0.034 |
| WinR | 0.380 (0.227-0.533) | 0.131 | 75.55 | 0 | 1 | 0 |
| Fs50 | 0.838 (0.726-0.949) | 0.000b | -0.695 | 0.88 | 0.759 | 0.639 |
| AUC | 0.273 (0.137-0.409) | 0.004b | 164597.03 | 1 | 0 | 0 |
| TTP | 0.657 (0.502-0.812) | 0.048a | 14.33 | 0.92 | 0.586 | 0.506 |
| mTT | 0.509 (0.352-0.666) | 0.910 | 128.52 | 1 | 0.138 | 0.138 |
| Size | 0.138 (0.172-0.464) | 0.022a | 0.70 | 1 | 0 | 0 |
Based on the AUC analysis of high-expression values, for differentiating high Ki-67 expression, Rs50 (0.801), IMAX (0.859), WoutR (0.818) and Rs1090 (0.863) had AUCs > 0.75, showing good diagnostic efficacy. Rs1090 (AUC = 0.863) had sensitivity 0.92, specificity 0.759, and an optimal threshold of 6.958. IMAX also performed well with sensitivity 0.96, specificity 0.724, and a threshold of 53.825. The diagnostic performance of tumor diameter was suboptimal, as reflected by an AUC of 0.682, falling below the threshold of 0.75 commonly associated with clinically meaningful discrimination. In contrast, RT (0.303), FHT (0.430), FT (0.303) and Fs50 (0.162) had non-significant AUCs. See Figure 7B and Table 7.
| AUC (95%CI) | P value | Diagnostic threshold | Specificity | Sensitive | Youden | |
| Rs50 | 0.801 (0.682-0.919) | 0.000b | 7.105 | 0.586 | 1 | 0.586 |
| IMAX | 0.859 (0.754-0.964) | 0.000b | 53.825 | 0.724 | 0.96 | 0.684 |
| RT | 0.303 (0.155-0.452) | 0.013b | 7.31 | 0.103 | 0.96 | 0.063 |
| WinAUC | 0.617 (0.465-0.768) | 0.143 | 1034.04 | 0.897 | 0.36 | 0.257 |
| FHT | 0.430 (0.276-0.584) | 0.376 | 236.155 | 1 | 0.04 | 0.04 |
| WoutAUC | 0.721 (0.584-0.859) | 0.005b | 29092.74 | 0.793 | 0.64 | 0.443 |
| WioAUC | 0.721 (0.584-0.859) | 0.005b | 29327.415 | 0.793 | 0.64 | 0.433 |
| WoutR | 0.818 (0.706-0.930) | 0.000b | 283.9 | 0.655 | 0.92 | 0.575 |
| FT | 0.421 (0.268-0.574) | 0.319 | 67.805 | 0.414 | 0.6 | 0.014 |
| Rs1090 | 0.863 (0.759-0.968) | 0.000b | 6.958 | 0.759 | 0.92 | 0.679 |
| WinR | 0.620 (0.497-0.773) | 0.131 | 72.18 | 0.897 | 0.36 | 0.257 |
| Fs50 | 0.162 (0.051-0.274) | 0.000b | 0.82 | 1 | 0 | 0 |
| AUC | 0.727 (0.591-0.863) | 0.004b | 37672.645 | 0.759 | 0.65 | 0.399 |
| TTP | 0.343 (0.188-0.498) | 0.048a | 8.84 | 0.241 | 0.88 | 0.121 |
| mTT | 0.491 (0.334-0.648) | 0.910 | 66.745 | 0.483 | 0.64 | 0.123 |
| Size | 0.682 (0.536-0.828) | 0.022a | 3.45 | 0.552 | 0.84 | 0.392 |
Qualitative analysis of imaging data from all patients revealed distinct patterns in enhancement modes based on Ki-67 expression levels. Enhancement mode II was the most common in cases with Ki-67 < 50%, accounting for 62.07% of samples. In contrast, enhancement mode III predominated in cases with Ki-67 ≥ 50%, representing 40% of samples. Notably, enhancement mode VI was exclusively observed in Ki-67 ≥ 50% samples, constituting 16% of this subgroup. Additionally, in well-differentiated tumors, enhancement mode II was the most prevalent, observed in 37.5% of cases. In moderately differentiated tumors, enhancement mode II remained the most common, observed in 69.23% of cases. Conversely, in poorly differentiated tumors, enhancement mode III was predominant, accounting for 40% of samples. Notably, enhancement mode V was not observed in any of the analyzed cases. Fisher’s exact test revealed no significant association between enhancement modes and Ki-67 expression levels (Table 8) or between enhancement modes and tumor differentiation grade (Table 9).
| Ki-67 < 50% | Ki-67 ≥ 50% | χ² value | P value | |
| I | 1 (3.45) | 2 (8) | 5.246 | 0.245 |
| II | 18 (62.07) | 8 (32) | ||
| III | 8 (27.59) | 10 (40) | ||
| IV | 2 (6.89) | 1 (4) | ||
| V | 0 (0) | 0 (0) | ||
| VI | 0 (0) | 4 (16) |
| Well differentiated | Moderately differentiated | Poorly differentiated | χ² value | P value | |
| I | 2 (12.5) | 0 (0) | 1 (4) | 6.094 | 0.659 |
| II | 6 (37.5) | 9 (69.23) | 11 (44) | ||
| III | 5 (31.25) | 3 (23.08) | 10 (40) | ||
| IV | 0 (0) | 0 (0) | 1 (4) | ||
| V | 0 (0) | 0 (0) | 0 (0) | ||
| VI | 1 (6.25) | 1 (7.69) | 2 (8) |
Pancreatic cancer, characterized by its high malignancy and poor prognosis, necessitates precise outcome prediction to facilitate personalized treatment strategies. This study employs CEUS as a non-invasive method to evaluate tumor microcirculation, thereby circumventing the risks associated with invasive biopsy procedures[14]. This study analyzed CEUS parameters in 54 pancreatic cancer patients, revealing significant differences in Rs50, IMAX, RT, WoutAUC, WioAUC, WoutR, Rs1090, Fs50, AUC, and TTP between Ki-67 expression groups. Fs50 accurately identified low Ki-67 (AUC = 0.838), while Rs50, IMAX, WoutR, and Rs1090 were effective for high Ki-67. mTT varied by tumor differentiation, with lower values in well-differentiated tumors. These findings support non-invasive assessment of pancreatic cancer malignancy and guide personalized treatment.
This study revealed no significant correlation between Ki-67 expression and pancreatic cancer differentiation, potentially because of the small sample size. However, Table 2 reveals that 56% of patients with high Ki-67 expression presented poorly differentiated tumors, suggesting that Ki-67 expression in > 50% is frequently associated with lower tumor differentiation. This observation aligns with the findings reported by Pergolini et al[23], suggesting that elevated Ki-67 expression may serve as an indicator of aggressive tumor biology. Previous studies have consistently demonstrated that Ki-67 expression and tumor differentiation are reliable predictors of prognosis and survival. Zhu et al[20] reported that patients with Ki-67 expression in ≥ 50% exhibited shorter survival and higher recurrence rates in critical vascular regions. Notably, prior studies[21] have also employed Ki-67 expression in > 50% as the standard cutoff for evaluating prognosis. Furthermore, tumor differentiation may play a pivotal role in treatment decisions, with evidence suggesting that poorly differentiated tumors are associated with longer survival following neoadjuvant therapy[10]. The incorporation of differentiation degree into TNM staging may improve prognostic accuracy[24].
In this study, we employed quantitative analysis of CEUS parameters to indirectly assess neovascularization in pancreatic cancer lesions. Ki-67 protein expression was utilized as an indicator of tumor proliferative activity and a predictor of prognosis. The analysis revealed significant differences in multiple CEUS parameters including Rs50, IMAX, RT, WoutAUC, WioAUC, WoutR, Rs1090, Fs50, AUC, and TTP between high (Ki-67 ≥ 50%) and low (Ki-67 < 50%) expression groups. Specifically, Rs50, IMAX, WoutAUC, WioAUC, WoutR, and Rs1090 demonstrated positive correlations with Ki-67 expression, with Rs50, IMAX, WoutR, and Rs1090 exhibiting particularly strong associations (r values > 0.5). Conversely, RT, Fs50, and TTP showed negative correlations with Ki-67 protein expression, with Fs50 displaying the most significant inverse relationship. These findings underscore the utility of CEUS parameters in evaluating tumor vascularization and proliferative activity in pancreatic cancer. On the basis of on previous biological mechanism analyses[25], when Ki-67 expression increases, tumor proliferative activity increases, which is accompanied by an increase in MVD. This accelerates tissue blood perfusion, raising Rs50, Rs1090, and WoutR while reducing TTP and RT. Additionally, IMAX and the distribution breadth of microbubbles increase, which, along with the elevated MVD, accelerates microbubble clearance. This results in a reduction of clearance-related parameters such as Fs50 and WoutR. Further ROC analysis validated the clinical discriminatory performance of these parameters. In the low Ki-67 expression group, Fs50 (AUC = 0.838, cutoff value ≥ -0.695) demonstrated the best balance between specificity (0.88) and sensitivity (0.759). In the high Ki-67 group, Rs50 (AUC = 0.801), IMAX (AUC = 0.859), WoutR (AUC = 0.818), and Rs1090 (AUC = 0.863) all exhibited excellent diagnostic performance. For instance, Rs50 achieved a sensitivity of 1.0, and IMAX demonstrated a specificity of 0.724. These results underscore the utility of CEUS parameters in distinguishing between high and low Ki-67 expression groups in pancreatic cancer.
Through this study, we found that CEUS parameters effectively reveal blood perfusion characteristics, and quantitative analysis demonstrated high accuraccy in predicting Ki-67 expression levels in pancreatic cancer. These findings suggest that CEUS parameters serve as a powerful, noninvasive tool for analyzing tumor proliferative activity, assessing prognosis, and guiding the selection of chemotherapy, radiotherapy, or antiangiogenic treatment strategies. Previous studies have already employed CEUS to evaluate blood perfusion in liver tumors, particularly in assessing the efficacy of antiangiogenic therapies, further supporting its clinical utility[26]. The formation of microvessels and tumor blood perfusion are regarded as critical rate-determining steps in tumor growth and metastasis, making them a central focus in the treatment of pancreatic cancer. Numerous therapeutic agents target key signaling pathways involved in pancreatic cancer angiogenesis, such as the vascular endothelial growth factor and platelet-derived growth factor receptor pathways, underscoring their importance the treatment of this disease[27]. These approaches emphasize the critical role of vascular mechanisms in pancreatic cancer progression and highlight the potential of antiangiogenic therapies to improve patient outcomes. Moreover, there is a complex interplay between microvascular formation and the tumor immune microenvironment in pancreatic cancer. A growing body of research has demonstrated that enhanced vascular perfusion and the remodeling of the tumor vasculature can synergistically increase the efficacy of chemotherapy, radiotherapy, and immunotherapy[28]. In this study, certain quantitative parameters, such as Fs50, Rs50, and IMAX, were found to effectively evaluate the prognosis and malignancy level of pancreatic cancer. These findings provide a novel non-invasive assessment tool and prognostic stratification basis for clinical decision-making, which has the potential to optimize individualized treatment strategies for pancreatic cancer patients. Additionally, these parameters may offer clues for the development of new therapeutic targets in the future, further advancing the field of pancreatic cancer treatment.
In addition to assessing Ki-67 expression levels, we performed a systematic analysis of the relationships between the degree of tumor differentiation degree and quantitative parameters. Among the parameters analyzed, the mTT significantly differed across tumors with different degrees of differentiation. Notably, the mTT value in the well-differentiated group was significantly lower than that in the moderately and poorly differentiated groups. The mTT, which reflects the average time required for a contrast agent to enter and exit the tissue, serves as a valuable indicator of the tissue microcirculation status. A shorter mTT typically indicates faster blood flow, which may suggest an abundant blood supply or greater vascular permeability within the tissue. This finding aligns with previous studies showing that patients with well-differentiated PDAC exhibits greater intratumoral vascular density than those with moderately and poorly differentiated PDAC[29]. These results further validate the utility of mTT as a valuable parameter for assessing tumor differentiation and microcirculation characteristics in pancreatic cancer. The ability to non-invasively evaluate such features could enhance clinical decision-making and contribute to the development of more precise and individualized treatment strategies.
We also conducted correlation analyses on qualitative features (i.e., pancreatic cancer CEUS enhancement patterns. Ki-67 expression levels and pathological differentiation degree). The results revealed no significant correlation between enhancement patterns and either Ki-67 expression or differentiation, which aligns with previous findings[30]. This suggests that qualitative CEUS features, such as enhancement patterns, may not directly reflect tumor proliferation activity or differentiation degree. This discrepancy may arise because enhancement patterns primarily reflect overall blood supply distribution and vascular morphology, while Ki-67 expression focuses on cell proliferation activity, highlighting distinct biological mechanisms.
This study has certain limitations. Firstly, it is a single-center, retrospective study, which may result in a small sample size, selection bias, and significant differences in data volume among subgroups. Secondly, as a single-center study, it lacks external validation, meaning the results may only be applicable to the specific center or equipment used, and there is no multi-center, multi-platform external validation cohort. Thirdly, this study focused solely on imaging features under CEUS and did not include other imaging examinations such as computed tomography or magnetic resonance imaging. Therefore, the findings may have limitations in comprehensively assessing lesion characteristics, and their applicability in scenarios requiring integrated diagnosis based on multi-modal imaging information may be restricted. Fourthly, the uneven distribution of samples across differentiation levels in this study introduced data bias, which could affect the statistical results.
In future research, we should address the potential bias caused by uneven sample distribution, further optimize sample selection criteria, and ensure balance and representativeness of data across groups to improve the reliability and generalizability of the conclusions. Additionally, integrating data from multiple imaging modalities, expanding the sample size through multi-center collaboration to collect larger datasets, or utilizing artificial intelligence algorithms for annotation could further enhance the consistency and reproducibility of the results. Through these improvements, we can provide more accurate and comprehensive diagnostic information, advance the field of pancreatic cancer imaging assessment, and ultimately offer more personalized medical services and better clinical outcomes for patients.
Quantitative analysis parameters of CEUS demonstrate objective correlations with the expression of Ki-67 in pancreatic cancer, with certain parameters such as Rs50, IMAX, WoutAUC, WioAUC, WoutR, and Rs1090 showing particularly significant correlations. Fs50 exhibits high accuracy in predicting low Ki-67 expression, while Rs50, IMAX, WoutR, and Rs1090 are highly accurate in predicting high Ki-67 expression. This indicates that CEUS, as a non-invasive examination, can be utilized to predict the proliferative activity of pancreatic cancer and the formation of tumor neovascularization. This approach holds certain value in assessing tumor malignancy and prognostic evaluation.
The authors thank the patients who agreed to participate in this study and all researchers, pathologists, and clinical staff who supported this study.
| 1. | Yi LX, Fang HL, Li JY, Liu YW, Mo M, Xie J. [Incidence and mortality of pancreatic cancer in the world and China in 2022]. Haijun Junyi Daxue Xuebao. 2024;45:1470-1477. [DOI] [Full Text] |
| 2. | Zeng H, Zheng R, Sun K, Zhou M, Wang S, Li L, Chen R, Han B, Liu M, Zhou J, Xu M, Wang L, Yin P, Wang B, You J, Wu J, Wei W, He J. Cancer survival statistics in China 2019-2021: a multicenter, population-based study. J Natl Cancer Cent. 2024;4:203-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 3. | Fahrmann JF, Schmidt CM, Mao X, Irajizad E, Loftus M, Zhang J, Patel N, Vykoukal J, Dennison JB, Long JP, Do KA, Zhang J, Chabot JA, Kluger MD, Kastrinos F, Brais L, Babic A, Jajoo K, Lee LS, Clancy TE, Ng K, Bullock A, Genkinger J, Yip-Schneider MT, Maitra A, Wolpin BM, Hanash S. Lead-Time Trajectory of CA19-9 as an Anchor Marker for Pancreatic Cancer Early Detection. Gastroenterology. 2021;160:1373-1383.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (1)] |
| 4. | Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep. 2015;11:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 540] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 5. | Van der Kwast TH. Proliferative cribriform prostate cancer: a new opportunity for 'promising' marker KI-67? Histopathology. 2023;83:850-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Luo Y, Zhang X, Mo M, Tan Z, Huang L, Zhou H, Wang C, Wei F, Qiu X, He R, Chen G. High Ki-67 Immunohistochemical Reactivity Correlates With Poor Prognosis in Bladder Carcinoma: A Comprehensive Meta-Analysis with 13,053 Patients Involved. Medicine (Baltimore). 2016;95:e3337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153:477-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 194] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 8. | Klöppel G, La Rosa S. Ki67 labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018;472:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 9. | Liu L, Xu HX, He M, Wang W, Wang WQ, Wu CT, Wei RQ, Liang Y, Gao HL, Liu C, Xu J, Long J, Ni QX, Shao CH, Wang J, Yu XJ. A novel scoring system predicts postsurgical survival and adjuvant chemotherapeutic benefits in patients with pancreatic adenocarcinoma: Implications for AJCC-TNM staging. Surgery. 2018;163:1280-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Nurmi A, Mustonen H, Parviainen H, Peltola K, Haglund C, Seppänen H. Neoadjuvant therapy offers longer survival than upfront surgery for poorly differentiated and higher stage pancreatic cancer. Acta Oncol. 2018;57:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Chai WL, Kuang XF, Yu L, Cheng C, Jin XY, Zhao QY, Jiang TA. Percutaneous ultrasound and endoscopic ultrasound-guided biopsy of solid pancreatic lesions: An analysis of 1074 lesions. Hepatobiliary Pancreat Dis Int. 2023;22:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Yang J, Huang J, Zhang Y, Zeng K, Liao M, Jiang Z, Bao W, Lu Q. Contrast-enhanced ultrasound and contrast-enhanced computed tomography for differentiating mass-forming pancreatitis from pancreatic ductal adenocarcinoma: a meta-analysis. Chin Med J (Engl). 2023;136:2028-2036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 13. | Buxbaum J, Ko C, Varghese N, Lee A, Sahakian A, King K, Serna J, Lee H, Tchelepi H, Van Dam J, Duddalwar V. Qualitative and Quantitative Contrast-enhanced Endoscopic Ultrasound Improves Evaluation of Focal Pancreatic Lesions. Clin Gastroenterol Hepatol. 2020;18:917-925.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Wang L, Nie F, Dong T, Li M, Li Y, Yin C. Role of contrast-enhanced ultrasound with time-intensity curve analysis for differentiating hypovascular solid pancreatic lesions. Eur Radiol. 2023;33:4885-4894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 15. | Zhang R, Li D, Chen Y, Xu W, Zhou W, Lin M, Xie X, Xu M. Development and Comparison of Prediction Models Based on Sonovue- and Sonazoid-Enhanced Ultrasound for Pathologic Grade and Microvascular Invasion in Hepatocellular Carcinoma. Ultrasound Med Biol. 2024;50:414-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Huang Z, Zhou P, Li S, Li K. Prediction of the Ki-67 marker index in hepatocellular carcinoma based on Dynamic Contrast-Enhanced Ultrasonography with Sonazoid. Insights Imaging. 2022;13:199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Yan K, Fan Z, Sun L, Wu W, Yang W. Contrast-Enhanced Ultrasonography of Pancreatic Carcinoma: Correlation with Pathologic Findings. Ultrasound Med Biol. 2016;42:891-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Ping WM, Junfan L, Wensheng YM, Wenyan LM, Yuqun LM. The Correlation between Traditional Ultrasound Features and the Expression of Estrogen Receptor, Progesterone Receptor, Human Epidermal Growth Factor Receptor-2, and Ki-67 in Breast Carcinoma. AUDT. 2018;2:173. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Wang L, Wang G, Wang P, Nie F. Pancreatic ductal adenocarcinoma: CEUS characteristics are correlated with pathological findings and help predict early recurrence after resection. J Clin Ultrasound. 2024;52:230-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Zhu X, Cao Y, Ju X, Zhao X, Jiang L, Ye Y, Shen Y, Cao F, Qing S, Zhang H. Personalized designs of adjuvant radiotherapy for pancreatic cancer based on molecular profiles. Cancer Sci. 2021;112:287-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Li Q, Song Z, Li X, Zhang D, Yu J, Li Z, Huang J, Su K, Liu Q, Zhang X, Tang Z. Development of a CT radiomics nomogram for preoperative prediction of Ki-67 index in pancreatic ductal adenocarcinoma: a two-center retrospective study. Eur Radiol. 2024;34:2934-2943. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2389] [Article Influence: 477.8] [Reference Citation Analysis (3)] |
| 23. | Pergolini I, Crippa S, Pagnanelli M, Belfiori G, Pucci A, Partelli S, Rubini C, Castelli P, Zamboni G, Falconi M. Prognostic impact of Ki-67 proliferative index in resectable pancreatic ductal adenocarcinoma. BJS Open. 2019;3:646-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Liang Y, Sheng G, Guo Y, Zou Y, Guo H, Li Z, Chang S, Man Q, Gao S, Hao J. Prognostic significance of grade of malignancy based on histopathological differentiation and Ki-67 in pancreatic ductal adenocarcinoma. Cancer Biol Med. 2024;21:416-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 25. | Saman H, Raza SS, Uddin S, Rasul K. Inducing Angiogenesis, a Key Step in Cancer Vascularization, and Treatment Approaches. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 26. | Zhang Y, Zheng R, Liu M, Zhang X, Sun Y, Shen H, Chen S, Cai H, Guo W, Xie X, Liu B, Huang G. Quantitative Parameters of Contrast-Enhanced Ultrasound Predicting the Response to Combined Immune Checkpoint Inhibitor and Anti-angiogenesis Therapies for Unresectable Hepatocellular Carcinoma. Ultrasound Med Biol. 2024;50:352-357. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | An YF, Pu N, Jia JB, Wang WQ, Liu L. Therapeutic advances targeting tumor angiogenesis in pancreatic cancer: Current dilemmas and future directions. Biochim Biophys Acta Rev Cancer. 2023;1878:188958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Chen H, Tu W, Lu Y, Zhang Y, Xu Y, Chen X, Zhu M, Liu Y. Low-dose X-ray irradiation combined with FAK inhibitors improves the immune microenvironment and confers sensitivity to radiotherapy in pancreatic cancer. Biomed Pharmacother. 2022;151:113114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Barău A, Ruiz-Sauri A, Valencia G, Gómez-Mateo Mdel C, Sabater L, Ferrandez A, Llombart-Bosch A. High microvessel density in pancreatic ductal adenocarcinoma is associated with high grade. Virchows Arch. 2013;462:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Zhou T, Tan L, Gui Y, Zhang J, Chen X, Dai M, Xiao M, Zhang Q, Chang X, Xu Q, Bai C, Cheng Y, Xu Q, Wang X, Meng H, Jia W, Lv K, Jiang Y. Correlation Between Enhancement Patterns on Transabdominal Ultrasound and Survival for Pancreatic Ductal Adenocarcinoma. Cancer Manag Res. 2021;13:6823-6832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |