Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.107568
Revised: April 16, 2025
Accepted: May 21, 2025
Published online: June 15, 2025
Processing time: 78 Days and 23.1 Hours
Descending duodenal adenocarcinoma (DDA) is a rare malignancy of the digestive system, typically characterized by microsatellite instability-high (MSI-H). Pembrolizumab is a monoclonal antibody that has been approved for the treatment of MSI-H solid tumors in China.
We present the case of a 55-year-old female patient diagnosed with DDA. Biopsy findings indicated MSI-H status with high expression of programmed cell death-ligand 1 (PD-L1). The patient was unable to undergo immediate surgery due to multiple metastatic lymph nodes in the retroperitoneum. After one cycle of the SOX (S-1 + oxaliplatin) chemotherapy regimen, the patient’s performance status significantly declined, and she experienced active gastrointestinal bleeding. Following active communication with the patient's family, pembrolizumab treatment was initiated. After two cycles of treatment, the disease was assessed as a partial response. A positron emission tomography/computed tomography scan performed after two years of treatment indicated a clinical complete response (CCR). The patient maintained this CCR for four years. She has now discontinued pembrolizumab for over one year, and no disease recurrence has been observed during re-examination.
Patients with MSI-H DDA exhibiting high PD-L1 expression who are treated with pembrolizumab can achieve sustained CCR.
Core Tip: Descending duodenal adenocarcinoma (DDA) is a rare malignancy of the digestive system. We treated a patient with DDA characterized by microsatellite instability-high (MSI-H) and high programmed cell death-ligand 1 expression, customizing the treatment regimen for individualized pembrolizumab therapy based on our clinical experience and disease assessment. The patient achieved long-term clinical remission with satisfactory results. This case underscores the advantages of individualized therapy and confirms that patients with mismatch-repair deficiency/MSI-H can derive significant benefits from pembrolizumab.
- Citation: Kong XL, Lu XW, Dong SQ, Liu J, Zheng L, Chen LL, An ZH, Gao LM, Cao JL. Descending duodenal adenocarcinoma treated with pembrolizumab resulting in complete clinical response: A case report and literature review. World J Gastrointest Oncol 2025; 17(6): 107568
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/107568.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.107568
Primary duodenal malignant tumors (PDMTs) are a rare digestive system malignancy, comprising approximately 0.3%-1% of all gastrointestinal malignancies[1]. Notably, although the duodenum constitutes less than 10% of the small intestine’s length, PDMTs account for about 45% of small intestinal malignancies[2]. Adenocarcinoma is the most prevalent histological type among PDMTs, representing approximately 42% of cases. Other common tumors include lymphoma and gastrointestinal stromal tumors, which account for about 17% and 8%, respectively[3].
Microsatellite instability-high (MSI-H) results from germline or defects in mismatch repair (MMR) genes, leading to replication errors or instability in repetitive DNA sequences[4]. Approximately 8.6% of small bowel adenocarcinoma cases exhibit MSI-H, with 12% of these cases attributed to Lynch syndrome (LS)[5,6]. Programmed cell death receptor 1 (PD-1) and its ligand (PD-L1) function as immune checkpoints, allowing cancer cells to evade local immunity by upregulating their expression. PD-1/PD-L1 immune checkpoint inhibitors (ICIs) can activate the immune system against tumor cells by blocking the interaction between PD-1 and PD-L1. Research has demonstrated that PD-1/PD-L1 inhibitors are effective in treating patients with high PD-L1 expression, leading to prolonged survival and a reduced risk of disease progression[7].
Pembrolizumab, a humanized IgG4 monoclonal antibody, binds to PD-1, an immune checkpoint inhibitory receptor expressed on lymphocytes. This binding results in the reactivation of T cell-mediated destruction of tumors[8].
We describe a patient diagnosed with descending duodenal adenocarcinoma (DDA) who achieved complete remission after four years of pembrolizumab treatment. The patient has remained recurrence-free for over one year following the discontinuation of therapy.
The patient had abdominal discomfort for 3 months. She was diagnosed with a malignant duodenal tumor for over 2 months.
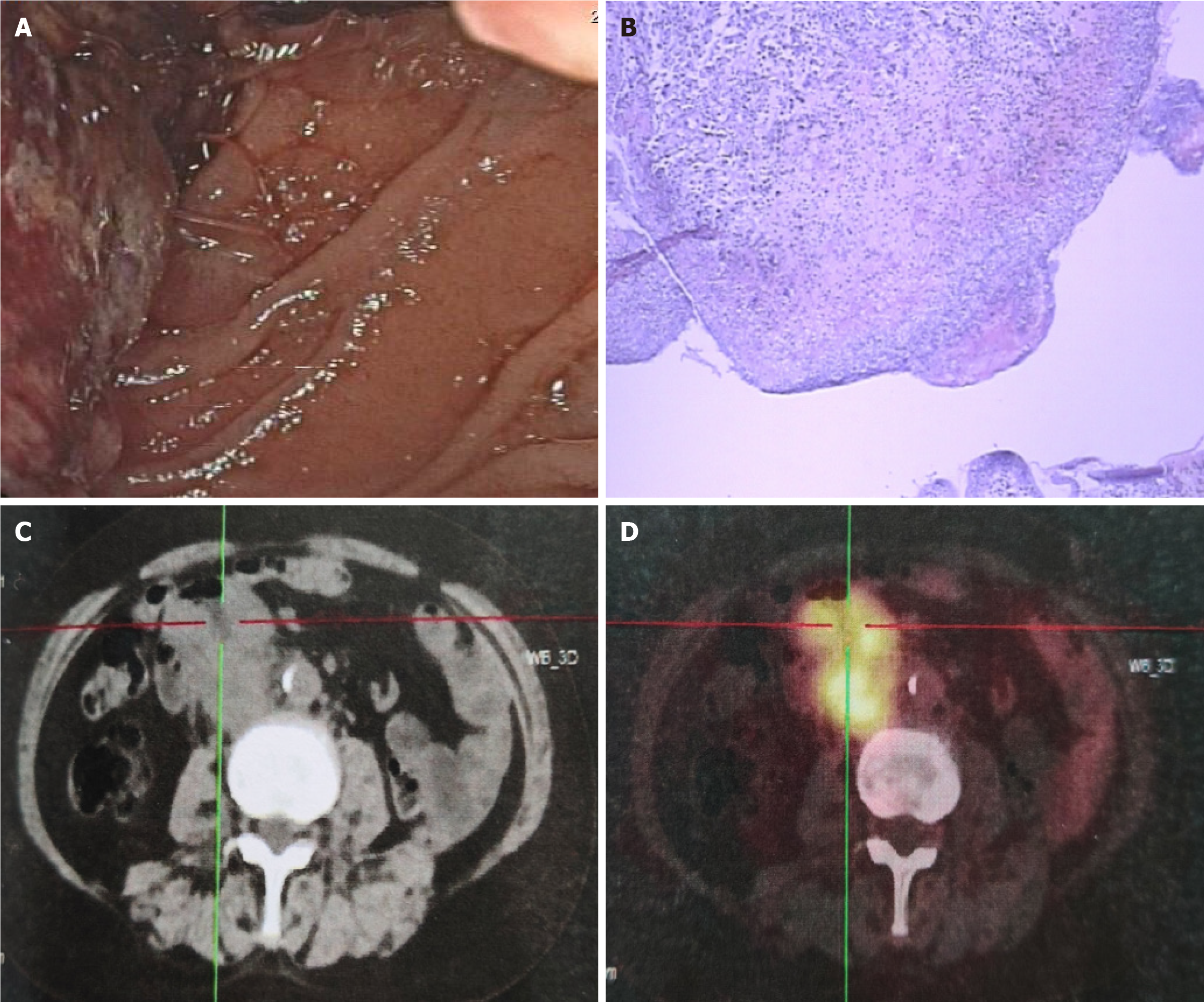
In December 2018, the patient sought medical attention at another institution due to abdominal discomfort, where gastroscopy revealed a mass in the descending duodenum (Figure 1A). Histopathological examination confirmed the presence of poorly differentiated adenocarcinoma of the duodenum (Figure 1B). Immunohistochemical analysis indicated the following characteristics of the cancer cells: Cytokeratin (CK): Positive (+); CK8 and CK18: Positive (+); human epidermal growth factor receptor 2: Negative (-); MutL Homolog (MLH1): Weakly positive (25%-50%); postmeiotic segregation increased 2: Positive (80%); MutS Homolog 2 (MSH2): Negative (-); MSH6: Negative (-). Genetic testing revealed PD-L1 protein expression, demonstrating PD-L1 positivity in tumor cells (> 90%) and stromal cells (approximately 1%).
Subsequent positron emission tomography (PET)/computed tomography (CT) imaging (Figure 1C and D) indicated 18F-fluorodeoxyglucose uptake in the intestinal wall, surrounding the intestinal wall, and retroperitoneum at the junction of the descending and horizontal segments of the duodenum, consistent with poorly differentiated adenocarcinoma of the duodenum with multiple lymph node metastases (T3N1M0, stage IIIa). On January 18, 2019, the patient commenced the SOX (S-1 + oxaliplatin) chemotherapy regimen.
There were no significant findings in her past medical history.
There were no significant findings in her past or family medical history.
On physical examination, her vital signs were as follows: Body temperature, 36.3°C; blood pressure, 112/79 mmHg; heart rate, 88 beats per min; respiratory rate, 16 breaths per minute.
Carcinoembryonic antigen level was 1.68 ng/mL (reference range: 0-4.7 ng/mL, carbohydrate antigen 19-9 level was 37.51 U/mL (reference range: 0-39 U/mL), and hemoglobin (HGB) was 67 g/L.
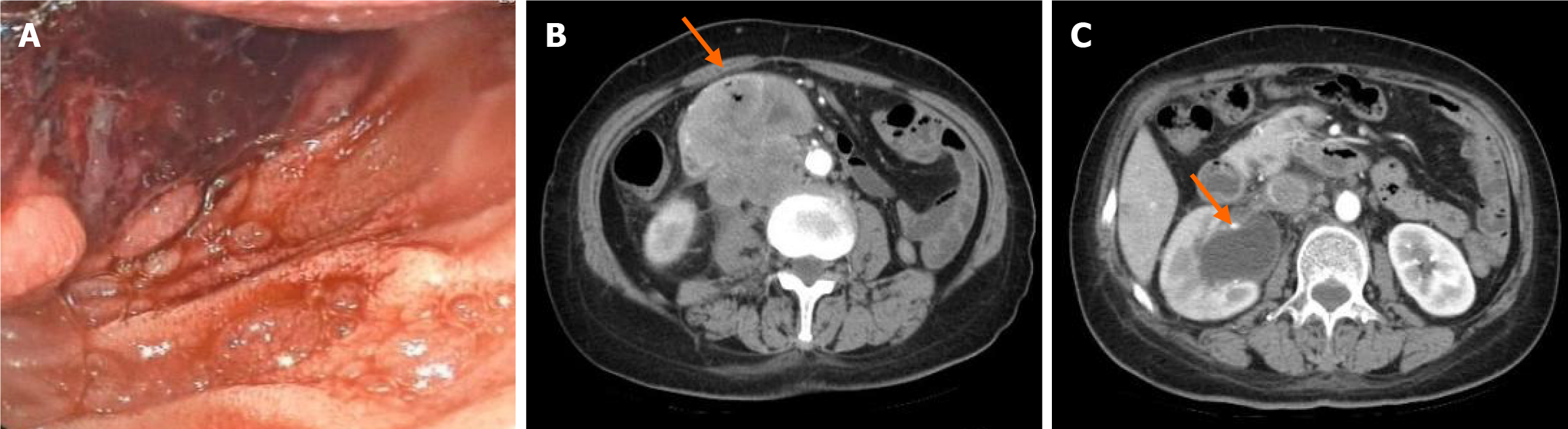
Subsequent gastroscopy revealed a protruding mass in the descending part of the duodenum with indistinct boundaries, accompanied by a substantial amount of violaceous necrotic tissue and blood clots on the surface (Figure 2A). Repeat CT scans indicated a duodenal mass, suggestive of a malignant lesion and retroperitoneal lymph node metastasis, along with dilation of the right renal pelvis and abdominal ureter (Figure 2B), and exudative changes in the right perirenal area and upper ureter (Figure 2C).
Following a multidisciplinary team consultation, it was determined that, due to the large tumor burden and the patient’s extremely poor performance status, radical resection was temporarily intolerable. Given on ongoing tumor bleeding, interventional palliative hemostasis treatment was deemed feasible.
Combined with the patient’s medical history, the final diagnosis was a PDMT.
Considering the patient's Eastern Cooperative Oncology Group PS score of 4, which rendered her unfit for surgery and aggressive anti-tumor regimens, and noting her MSI-H status with high PD-L1 expression, pembrolizumab treatment was initiated after thorough discussions with the family.
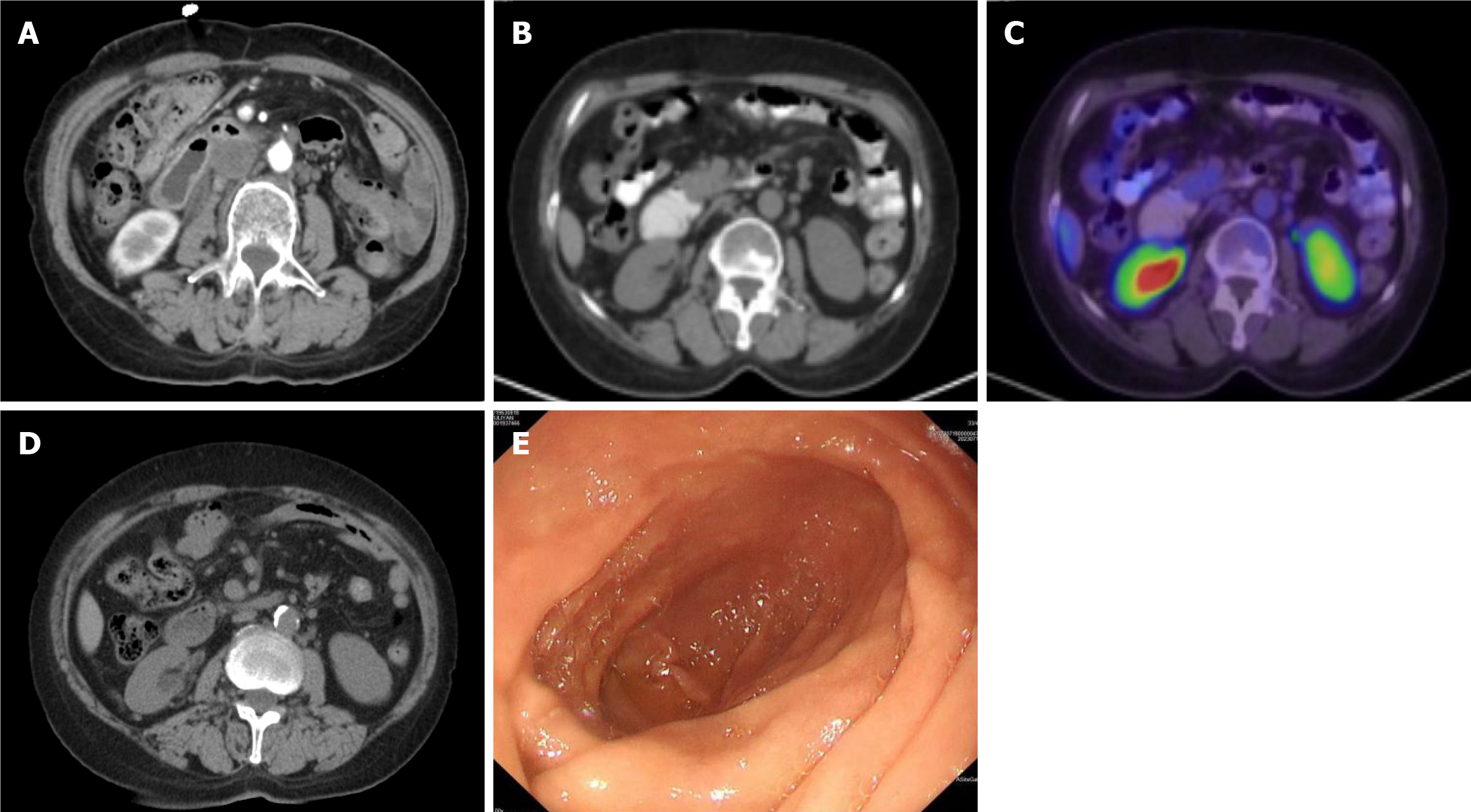
Subsequent disease assessment after 2 cycles of pembrolizumab therapy revealed a partial response on CT scans (Figure 3A), accompanied by stabilization of HGB levels, and the patient continued to receive regular immunotherapy.
Following two years of pembrolizumab maintenance therapy, a repeat PET/CT scan (Figure 3B and C) demonstrated low density at the lateral pancreatic head adjacent to the duodenum, with no discernible increase in metabolic activity, indicating that the lesion was in a hypometabolic or inactive state. Continuing the immunotherapy regimen until March 2023, subsequent CT imaging (Figure 3D) revealed no thickening of the duodenal wall, and gastroscopy (Figure 3E) indicated the absence of a mass in the duodenal bulb or descending part.
Following the discontinuation of treatment, the patient remained stable during the subsequent year, as indicated by consistent findings on regular reexaminations.
PDMTs are defined as malignant tumors originating in the duodenum, excluding the lower common bile duct and the head of the pancreas[9]. Most cases occur in the second segment of the duodenum (D2), with some in the third and fourth segments (D3/D4), and rarely in the first segment, particularly in the ampulla of Vater[10]. The typical age of onset is between 40 and 60 years. Although this type of malignancy is considered rare in clinical practice, the current incidence is reportedly increasing and affecting younger individuals[11].
The clinical presentation of a PDMT is nonspecific, often presenting with symptoms such as persistent dull pain, nausea, and gastrointestinal bleeding. Approximately 65% of cases of DDA manifest with abdominal pain and nausea. The duodenum, as the initial segment of the small bowel, accounts for only 4% of the total length of the small intestine; however, it is the most common site for small bowel malignancies, representing approximately 30% to 50% of cases, particularly in the ampullary region, which may be associated with irritation due to bile or pancreatic juice[3,12,13]. Intestinal adenocarcinoma primarily refers to colorectal cancer (CRC), which constitutes more than 80% of gastrointestinal tumors. Current studies indicate that the median survival times for small intestine cancer at stages I, II, III, and IV are 136.5, 45.6, 24.9, and 11.15 months, respectively. Additionally, the five-year survival rates for these stages are 77.7%, 43.7%, 24.9%, and 2.1%, respectively. In the case of the patient described, the main symptoms were abdominal discomfort and recurrent gastrointestinal bleeding, and the disease was localized to the junction of the second and third segments of the duodenum, and the survival period extending beyond five years.
The risk factors for primary duodenal malignancies are not fully elucidated due to the condition's low incidence. However, a systematic review of lifestyle risk factors and small bowel adenocarcinoma (SBA) has indicated some associations. The review suggested that alcohol consumption and smoking were linked to a higher risk of small bowel adenocarcinoma[14].
It is important to note that individual cases may vary, and not all patients with a PDMT have a history of these risk factors. In the case of the described patient, she denied any history of smoking or alcohol consumption.
In recent years, immunotherapy has revolutionized the treatment paradigm for many cancers, including cancers of gastrointestinal origin, which has also stimulated research interest in the immune microenvironment of DDA. In a series by Schrock et al[15], 3.9% and 4.0% of patients presented with MSI-H in gastric and CRC, respectively, compared with 7.6% in SBAs. Alterations in predominantly MSH2, MSH6, and MLH1 mismatch repair genes were observed in patients with small intestinal adenocarcinoma, with alteration rates of 1.9%, 3.8%, and 2.2%, respectively. One or more inactivating alterations in MSH2, MSH6, or MLH1 were present in 76.9% of all SBA patients with MSI-H. In another study, PD-L1 was expressed up to 50% in SBA tumors or stromal cells, a frequency similar to gastroesophageal cancer[16,17].
A retrospective study conducted by ESMO examining the relationship between MSI-H, tumor mutational burden, and PD-L1 expression indicates that among endometrioid adenocarcinoma, CRC, esophagogastric junction tumors, non-small cell lung cancer (NSCLC), and myeloid sarcoma, 0.59% of cancers exhibit both MSI-H and PD-L1 expression. In the case of CRC, this proportion is reported to be 0.6%[18]. Latham et al[5] conducted an immunohistochemical analysis of 35 DDAs and found that high PD-L1 expression (CPS ≥ 10) was observed in only 14% of the cases. Furthermore, high PD-L1 expression was not significantly associated with MSI-H status, as only one MSI-H tumor exhibited high PD-L1 expression simultaneously[19]. Our patient had loss of MSH2 and MSH6 expression, with PD-L1 positivity in more than 90% of tumor cells.
LS is an autosomal dominant disorder that significantly increases an individual's lifetime risk of developing various cancers, including colorectal, endometrial, ovarian, gastric, urothelial, small bowel, pancreatic, cholangiocarcinoma, and skin cancer[20]. Specifically, patients with LS have an estimated lifetime risk of small bowel cancer of approximately 4%, which is more than 100-fold higher than that of the general population[21]. Previous reports indicate that the most common genetic alterations associated with LS are pathogenic variants of MSH2 or MLH1, accounting for 90% of cases. Notably, pathogenic mutations in MSH2 are frequently observed in DDA[22]. Among patients with SBA, MSI-H is present in 8.6% of cases, with 12% of these patients having LS[5,6]. In this patient, deletions of MSH2 and MSH6 were identified; however, the patient did not undergo germline genetic testing, which hindered the ability of family members to receive genetic risk assessment and early screening.
Adenocarcinoma of the small intestine is considered a rare cancer, traditionally treated as CRC. Relevant clinical trials have demonstrated that the following chemotherapy regimens are effective and safe for treating certain cancers: FOLFOX (fluorouracil + oxaliplatin + leucovorin calcium)[23-25]; CAPOX (oxaliplatin + capecitabine)[26] and CAPIRINOX (oxaliplatin + capecitabine + irinotecan)[27]. These regimens can be administered with or without the addition of bevacizumab, a monoclonal antibody that inhibits angiogenesis. The above regimens were recommended as the first-line treatment by the National Comprehensive Cancer Network (NCCN) in 2019[28]. As PDMTs are rare in clinical practice, there are few clinical trials of ICIs specifically targeting this disease, and most studies have focused on a wide range of subtypes of small intestinal adenocarcinoma or on the overall study of gastrointestinal tumors. ICIs have shown some efficacy in advanced gastric, intestinal, and esophageal cancer, particularly in those patients with MSI-H or DNA repair defects[8,29]. Pembrolizumab is a monoclonal antibody that selectively binds to the PD-1 immune checkpoint on the surface of cancer cells or immune cells. By blocking the interaction between PD-1 and its ligands, PD-L1 and PD-L2, pembrolizumab relieves inhibition of the PD-1 pathway-mediated immune response. This action restores the immune function that was suppressed by PD-1, thereby enhancing the body's anti-tumor immune response and playing a significant role in cancer immunotherapy. In February 2020, the KEYNOTE-158 study published results from the mismatch-repair deficiency (dMMR)/MSI-H subgroup, which demonstrated that 15.8% of patients with unresectable or recurrent MSI-H small intestinal adenocarcinoma (including DDA) achieved clinical complete remission after receiving up to 35 cycles of pembrolizumab[30]. Based on these findings, the 2020 NCCN guidelines recommended pembrolizumab as first-line therapy for patients with advanced dMMR/MSI-H small intestinal adenocarcinoma. It is also based on the above data that pembrolizumab has been approved by the United States Food and Drug Administration and China National Medical Products Administration for patients with advanced first-line dMMR/MSI-H solid tumors in 2020 and 2023, respectively.
Before the guideline update, we developed an individualized treatment plan for this patient based on our extensive clinical experience and precise disease assessment. It is gratifying to report that the patient successfully achieved long-term clinical remission, resulting in exceptionally favorable therapeutic outcomes. This success story not only underscores the substantial benefits of personalized treatment regimens but also strongly affirms that patients with dMMR/MSI-H status can derive significant clinical benefits from ICIs. Currently, there are no separate studies specifically reporting the benefits of immunotherapy compared to standard chemotherapy in patients with MSI-H small intestinal adenocarcinoma. However, we can refer to studies on CRC, such as the KEYNOTE-177 and CHECKMATE-8HW studies, which demonstrated significant benefits of immunotherapy in MSI-H CRC patients compared to traditional chemotherapy[31,32].
It is important to note that small intestinal adenocarcinoma is often microsatellite stable (MSS), and the PD-L1 expression rate in these tumors is significantly lower than in other cancers, such as gastric or esophageal cancer. This lower expression makes it challenging for the immune system to recognize tumor cells, thereby limiting the efficacy of ICIs. Consequently, fluorouracil/oxaliplatin-based chemotherapy regimens, such as FOLFOX, remain the standard first-line treatment for most patients with MSS SBA. In this context, our patient received pembrolizumab as a second-line treatment, with the family being well-informed about the treatment options and the KEYNOTE-158 trial. Remarkably, the patient achieved a therapeutic effect characterized by clinical remission.
With regard to the duration of immunotherapy, most studies currently span 2 years. Studies such as KEYNOTE-006, KEYNOTE-024, and KEYNOTE-048 have shown that some patients can achieve long-term remission or even clinical cure after discontinuation of ICIs. This phenomenon is believed to be related to the sustained activation of the immune system and long-term regulation of the tumor microenvironment. The specific mechanisms underlying this effect include: (1) Memory T cell formation: After ICIs relieve T cell suppression, activated tumor-specific CD8+ T cells can differentiate into memory T cells. These memory T cells can persist in the body for extended periods and respond rapidly to any tumor recurrence; (2) Epigenetic modifications: ICIs may help maintain the effector function of T cells through epigenetic modifications, such as DNA methylation and histone modification, which can prevent T cell depletion; (3) Reduction of inhibitory cell infiltration: ICIs can decrease the infiltration of inhibitory cells, such as regulatory T cells and myeloid-derived suppressor cells, thereby creating an "immune licensing" microenvironment that supports effective anti-tumor responses; and (4) Improvement of tumor vascular structure: By inhibiting the vascular endothelial growth factor pathway, ICIs can enhance tumor vascular structure and promote the infiltration of effector T cells into the tumor, further facilitating an effective immune response against the cancer[33,34]. These mechanisms collectively contribute to the potential for long-term remission in patients treated with ICIs, even after therapy has been discontinued.
Sun et al[35] conducted a retrospective analysis indicating that for patients with advanced NSCLC who remained in response after 2 years, there was no statistically significant difference in overall survival rates between a fixed duration of treatment (2 years) and indefinite immunotherapy (> 2 years), suggesting that discontinuation of treatment and continuous monitoring, rather than indefinite continuation of immunotherapy, is a clinically meaningful strategy. However, in advanced NSCLC populations with PD-L1 low or non-expression, the response rate and survival rate of ICIs combined chemotherapy were significantly improved compared with chemotherapy alone[36,37], whereas MSS SBAs have limited benefit[11]. These results suggest that there are differences in the immune microenvironment between the two diseases, and further experimental data are still needed for the immune maintenance period of small intestinal malignancies or even CRC.
It is noteworthy that our patient achieved long-term survival after treatment with pembrolizumab. However, it is important to highlight that despite the clinical complete remission, the patient declined surgical treatment, leading to an absence of definitive pathology and no germline genetic testing. Given this circumstance and the potential for recurrence, it is crucial to continue close monitoring of the patient with regular and thorough follow-up visits. This ongoing supervision is essential for timely detection, intervention, and management in the event of recurrence or any other developments in the patient's condition.
Patients with MSI-H DDA exhibiting high PD-L1 expression who are treated with pembrolizumab can achieve sustained CCR.
The authors sincerely thank Ms. Yu-Jing Yao from MSD China Medical Affairs for her scientific support in literature organization and proofreading during the writing of this paper.
| 1. | Solej M, D'Amico S, Brondino G, Ferronato M, Nano M. Primary duodenal adenocarcinoma. Tumori. 2008;94:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Jiang S, Zhao R, Li Y, Han X, Liu Z, Ge W, Dong Y, Han W. Prognosis and nomogram for predicting postoperative survival of duodenal adenocarcinoma: A retrospective study in China and the SEER database. Sci Rep. 2018;8:7940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 466] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 4. | Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, Vilar E, Tie J, Broaddus R, Kopetz S, Desai J, Overman MJ. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol. 2014;25:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 5. | Latham A, Srinivasan P, Kemel Y, Shia J, Bandlamudi C, Mandelker D, Middha S, Hechtman J, Zehir A, Dubard-Gault M, Tran C, Stewart C, Sheehan M, Penson A, DeLair D, Yaeger R, Vijai J, Mukherjee S, Galle J, Dickson MA, Janjigian Y, O'Reilly EM, Segal N, Saltz LB, Reidy-Lagunes D, Varghese AM, Bajorin D, Carlo MI, Cadoo K, Walsh MF, Weiser M, Aguilar JG, Klimstra DS, Diaz LA Jr, Baselga J, Zhang L, Ladanyi M, Hyman DM, Solit DB, Robson ME, Taylor BS, Offit K, Berger MF, Stadler ZK. Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer. J Clin Oncol. 2019;37:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 448] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 6. | Akagi K, Oki E, Taniguchi H, Nakatani K, Aoki D, Kuwata T, Yoshino T. Real-world data on microsatellite instability status in various unresectable or metastatic solid tumors. Cancer Sci. 2021;112:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 7. | Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, Sahebkar A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234:16824-16837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 8. | Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, Bariani GM, De Jesus Acosta A, Doi T, Longo F, Miller WH, Oh DY, Gottfried M, Xu L, Jin F, Norwood K, Marabelle A. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022;33:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 236] [Article Influence: 78.7] [Reference Citation Analysis (1)] |
| 9. | Shao DD, Wang HX, Min JX, Wang FQ. [Study on the relationship between dietary factors and digestive tract tumors]. Jiyinzuxue Yu Yingyong Shengwuxue. 2016;35:1101-1107. [DOI] [Full Text] |
| 10. | Ross RK, Hartnett NM, Bernstein L, Henderson BE. Epidemiology of adenocarcinomas of the small intestine: is bile a small bowel carcinogen? Br J Cancer. 1991;63:143-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 11. | Jiang D, Liang X, Liang ZH, Huang ZN. [Analysis on clinical features, diagnosis and treatment of 143 cases with primary malignant tumor of the duodenum]. Zhongguo Linchuang Xinyixue. 2022;15:738-743. [DOI] [Full Text] |
| 12. | Parikh N, Howden CW. The safety of drugs used in acid-related disorders and functional gastrointestinal disorders. Gastroenterol Clin North Am. 2010;39:529-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Graziano F, De Stefanis C, Giorgio V, Pensabene L, Nobili V, Alisi A. Genic correlation between the expression of EZH2 and the degree of liver damage in NAFLD. Digest Liver Dis. 2013;45:e272. [DOI] [Full Text] |
| 14. | Bennett CM, Coleman HG, Veal PG, Cantwell MM, Lau CC, Murray LJ. Lifestyle factors and small intestine adenocarcinoma risk: A systematic review and meta-analysis. Cancer Epidemiol. 2015;39:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Schrock AB, Devoe CE, McWilliams R, Sun J, Aparicio T, Stephens PJ, Ross JS, Wilson R, Miller VA, Ali SM, Overman MJ. Genomic Profiling of Small-Bowel Adenocarcinoma. JAMA Oncol. 2017;3:1546-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 16. | Pedersen K, Smyrk TC, Harrington S, Mcwilliams RR. Programmed death-ligand 1 (PD-L1) expression in small bowel adenocarcinomas (SBA). J Clin Oncol. 2015;33:3619-3619. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Thota R, Gonzalez RS, Berlin J, Cardin DB, Shi C. Could the PD-1 Pathway Be a Potential Target for Treating Small Intestinal Adenocarcinoma? Am J Clin Pathol. 2017;148:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, Miller R, Riaz N, Douillard JY, Andre F, Scarpa A. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019;30:1232-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 684] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 19. | Watari J, Mitani S, Ito C, Tozawa K, Tomita T, Oshima T, Fukui H, Kadowaki S, Natsume S, Senda Y, Tajika M, Hara K, Yatabe Y, Shimizu Y, Muro K, Morimoto T, Hirota S, Das KM, Miwa H. Molecular alterations and PD-L1 expression in non-ampullary duodenal adenocarcinoma: Associations among clinicopathological, immunophenotypic and molecular features. Sci Rep. 2019;9:10526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Therkildsen C, Jensen LH, Rasmussen M, Bernstein I. An Update on Immune Checkpoint Therapy for the Treatment of Lynch Syndrome. Clin Exp Gastroenterol. 2021;14:181-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Koornstra JJ, Kleibeuker JH, Vasen HF. Small-bowel cancer in Lynch syndrome: is it time for surveillance? Lancet Oncol. 2008;9:901-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Hammoudi N, Dhooge M, Coriat R, Leblanc S, Barret M, Bordacahar B, Beuvon F, Prat F, Maksimovic F, Chaussade S. Duodenal tumor risk in Lynch syndrome. Dig Liver Dis. 2019;51:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Horimatsu T, Nakayama N, Moriwaki T, Hirashima Y, Fujita M, Asayama M, Moriyama I, Nakashima K, Baba E, Kitamura H, Tamura T, Hosokawa A, Yoshimura K, Muto M. A phase II study of 5-fluorouracil/L-leucovorin/oxaliplatin (mFOLFOX6) in Japanese patients with metastatic or unresectable small bowel adenocarcinoma. Int J Clin Oncol. 2017;22:905-912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Xiang XJ, Liu YW, Zhang L, Qiu F, Yu F, Zhan ZY, Feng M, Yan J, Zhao JG, Xiong JP. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs. 2012;23:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Zhang L, Wang LY, Deng YM, Wang FH, Feng F, Chen YC, An X, Chen C, Xu RH, Li YH. Efficacy of the FOLFOX/CAPOX regimen for advanced small bowel adenocarcinoma: a three-center study from China. J BUON. 2011;16:689-696. [PubMed] |
| 26. | Gulhati P, Raghav K, Shroff RT, Varadhachary GR, Kopetz S, Javle M, Qiao W, Wang H, Morris J, Wolff RA, Overman MJ. Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of vater: A single-center, open-label, phase 2 study. Cancer. 2017;123:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | McWilliams RR, Foster NR, Mahoney MR, Smyrk TC, Murray JA, Ames MM, Horvath LE, Schneider DJ, Hobday TJ, Jatoi A, Meyers JP, Goetz MP. North Central Cancer Treatment Group N0543 (Alliance): A phase 2 trial of pharmacogenetic-based dosing of irinotecan, oxaliplatin, and capecitabine as first-line therapy for patients with advanced small bowel adenocarcinoma. Cancer. 2017;123:3494-3501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming DA, Garrido-Laguna I, Grem JL, Hoffe SE, Hubbard J, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen KS, Saltz LB, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gregory KM, Gurski LA. Small Bowel Adenocarcinoma, Version 1.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:1109-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 29. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020;383:2207-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1804] [Article Influence: 360.8] [Reference Citation Analysis (0)] |
| 30. | Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2120] [Cited by in RCA: 2012] [Article Influence: 402.4] [Reference Citation Analysis (0)] |
| 31. | André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt CJA, Smith D, Garcia-Carbonero R, Alcaide-Garcia J, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zuo Y, Fogelman D, Adelberg D, Diaz LA. Pembrolizumab versus chemotherapy in microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer: 5-year follow-up from the randomized phase III KEYNOTE-177 study. Ann Oncol. 2025;36:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Andre T, Elez E, Van Cutsem E, Jensen LH, Bennouna J, Mendez G, Schenker M, de la Fouchardiere C, Limon ML, Yoshino T, Li J, Lenz HJ, Manzano Mozo JL, Tortora G, Garcia-Carbonero R, Dahan L, Chalabi M, Joshi R, Goekkurt E, Braghiroli MI, Cil T, Cela E, Chen T, Lei M, Dixon M, Abdullaev S, Lonardi S; CheckMate 8HW Investigators. Nivolumab plus Ipilimumab in Microsatellite-Instability-High Metastatic Colorectal Cancer. N Engl J Med. 2024;391:2014-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 33. | Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 4615] [Article Influence: 659.3] [Reference Citation Analysis (0)] |
| 34. | Sadasivam S, Decaprio JA. Erratum: The DREAM complex: master coordinator of cell cycle-dependent gene expression. Nat Rev Cancer. 2013;13:752-752. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Sun L, Bleiberg B, Hwang WT, Marmarelis ME, Langer CJ, Singh A, Cohen RB, Mamtani R, Aggarwal C. Association Between Duration of Immunotherapy and Overall Survival in Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2023;9:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 75] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 36. | Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, Hochmair MJ, Powell SF, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Kurata T, Gray JE, Schwarzenberger P, Jensen E, Pietanza MC, Rodríguez-Abreu D. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol. 2023;41:1992-1998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 315] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 37. | Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, Rodríguez-Cid J, Tafreshi A, Cheng Y, Lee KH, Golf A, Sugawara S, Robinson AG, Halmos B, Jensen E, Schwarzenberger P, Pietanza MC, Paz-Ares L. Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J Clin Oncol. 2023;41:1999-2006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 243] [Article Influence: 121.5] [Reference Citation Analysis (0)] |