Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.106848
Revised: April 9, 2025
Accepted: May 20, 2025
Published online: June 15, 2025
Processing time: 93 Days and 0.7 Hours
This report describes a rare case of a small gastric cancer lesion with widespread bone metastases and markedly elevated alkaline phosphatase levels that was initially misdiagnosed as rheumatoid arthritis, as the patient’s sole clinical manifestation was chronic bone pain persisting for 1 year.
An 83-year-old man was admitted due to worsening generalized joint pain over 1 year. Serum alkaline phosphatase levels were markedly elevated, and technetium-99m methylene diphosphonate single-photon emission computed tomography (CT) and fluorine-18 sodium fluoride positron emission tomography (PET)/CT images showed symmetrical diffuse uptake of the radiotracers throughout the skeleton. Initially, Paget’s disease was suspected, but abnormal hematologic tumor markers and bone biopsy confirmed metastatic adenocarcinoma. Fluorine-18-fluorodeoxyglucose PET/CT did not reveal a primary tumor. The patient had a history of colon polypectomy and tubulovillous adenoma with atypical hy
Occult gastric carcinomas with bone metastases necessitate proactive high-risk surveillance and multidisciplinary integration to improve diagnostic accuracy and clinical outcomes.
Core Tip: This case highlights an octogenarian with occult gastric cancer manifesting as extensive osteoblastic metastases initially misdiagnosed as rheumatoid arthritis. While fluorine-18 fluorodeoxyglucose positron emission tomogra
- Citation: Jin CB, Li YS, Zhang J, Wu J, Tao WJ. Extensive bone metastases from an occult gastric primary: A case report. World J Gastrointest Oncol 2025; 17(6): 106848
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/106848.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.106848
Bone metastases are more common in patients with prostate, lung, breast, and renal cancers, whereas those originating from gastric cancer are rare[1,2]. Differentiating between extensive bone metastases and metabolic bone disease in super bone imaging is challenging, particularly without primary lesion evidence. When the primary lesion is not obvious on whole-body fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT), the possibility that the lesion originated in the gastrointestinal tract should be considered because gastric tumors often do not actively take up 18F-FDG[3]. Furthermore, the diagnosis is more difficult when the lesion is small.
An 83-year-old man with a 1-year history of generalized joint pain was diagnosed with rheumatoid arthritis at a local hospital.
The patient was treated with tofacitinib and methotrexate. However, his joint pain worsened and was accompanied by severe back pain at night, which limited his ability to turn over and stand. He was referred to our hospital for further evaluation and treatment.
Ten years ago, the patient underwent endoscopic resection of a descending colon polyp in the Gastroenterology Department of our hospital due to a 1-year history of hematochezia, which had worsened with melena over the preceding month. Histopathological examination revealed a tubulovillous adenoma with moderate adenomatous dysplasia. Tumor marker carbohydrate antigen 19-9 (CA19-9) was mildly elevated at 38.72 U/mL (normal range: 0-34 U/mL). The patient did not adhere to regular follow-up evaluations post-procedure.
The patient had no family history of genetic disorders, and there was no significant history of cancer among other relatives.
The patient presented with stable vital signs. No scleral icterus, jaundice, or superficial lymphadenopathy was observed. Cardiopulmonary auscultation was unremarkable. Abdominal examination revealed no tenderness or palpable masses. The spine and extremities were without deformities, and there was no edema in the lower extremities.
Laboratory investigations revealed significantly elevated serum alkaline phosphatase (ALP) (746 U/L; normal range: 50-135 U/L), slightly increased parathyroid hormone (74.10 pg/mL; normal range: 15-65 pg/mL), and decreased blood calcium (2.01 mmol/L; normal range: 2.11-2.52 mmol/L) and total 25-hydroxyvitamin D (18.63 ng/mL; normal range: 20-100 ng/mL). There were no abnormalities in the antinuclear antibody spectrum except for a weakly positive result for anti-Scl 70 antibodies. Serum bilirubin, complement, immunoglobulin, and 24-hour urine phosphorus levels were normal. Several hematologic indicators were abnormal, including CA19-9 (60.80 U/mL; normal range: 0-34 U/mL), vascular endothelial growth factor (251.66 pg/mL; normal range: 6.25-142.20 pg/mL), progastrin-releasing peptide (129.00 pg/mL; normal range: 28.30-74.40 pg/mL), interleukin (IL)-6 (63.69 pg/mL; normal range: 0.00-5.30 pg/mL), and IL-10 (5.96 pg/mL; normal range: 0.00-4.91 pg/mL).
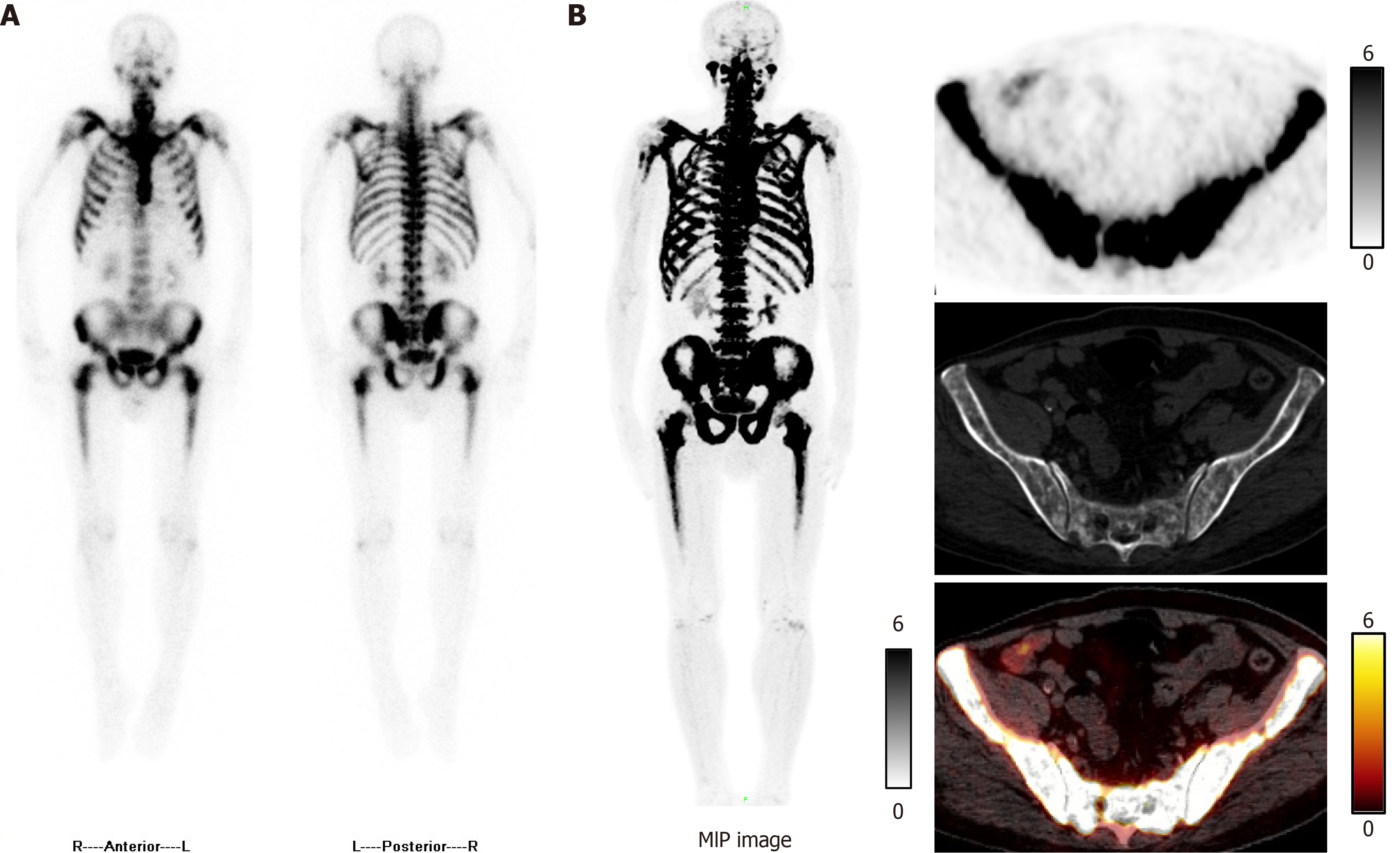
Technetium-99m methylene diphosphonate single-photon emission CT images showed diffuse increased uptake of the radiotracer throughout the skeleton (Figure 1A). Studies demonstrated that fluorine-18 sodium fluoride PET/CT was superior to technetium-99m methylene diphosphonate single-photon emission CT for detection of bone lesions[4,5]. Therefore, we performed fluorine-18 sodium fluoride PET/CT, which revealed symmetrical diffuse uptake in the ribs, vertebrae, pelvis, and both femora (Figure 1B).
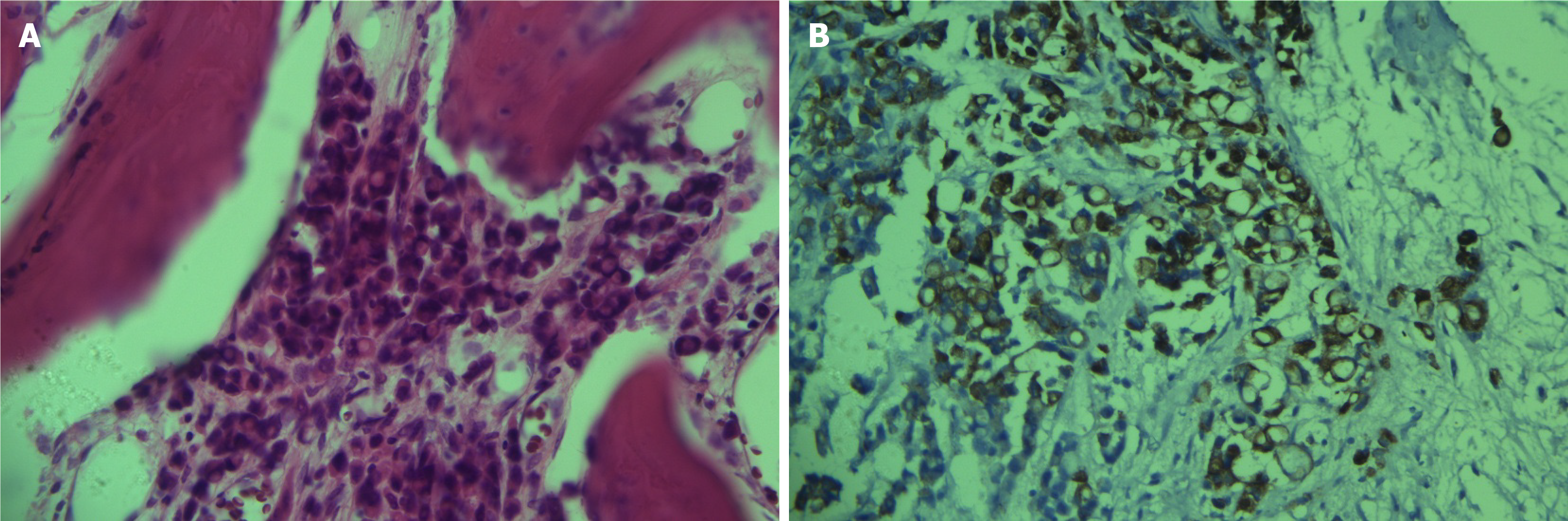
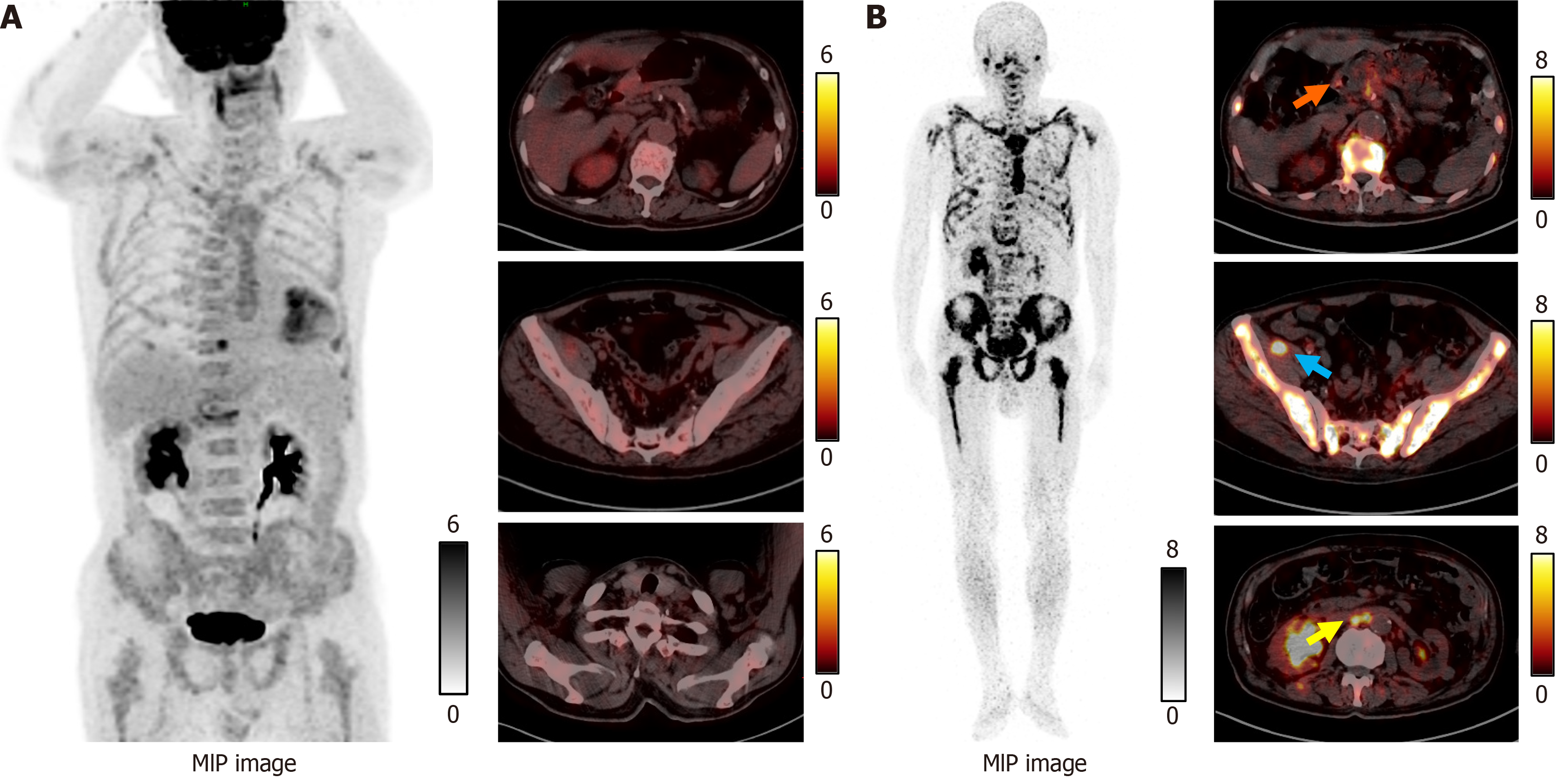
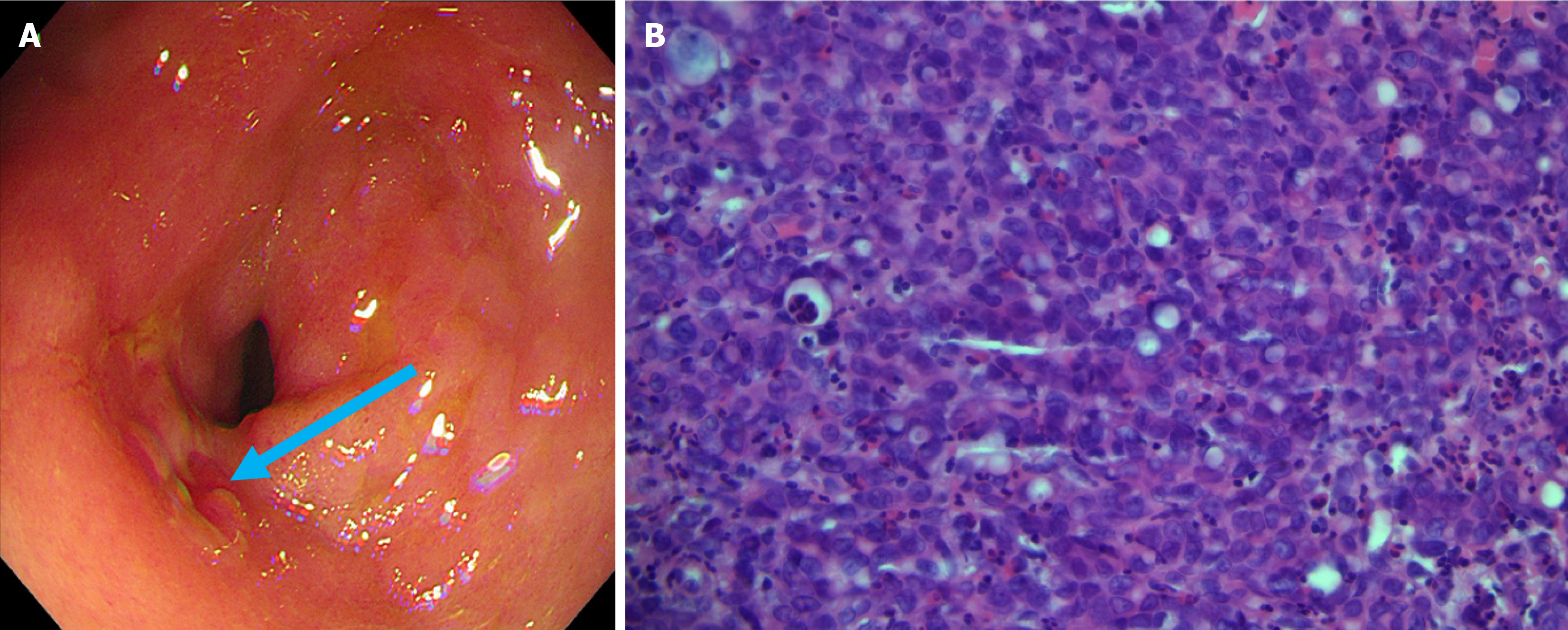
The elevation of tumor markers meant that it was impossible to rule out bone metastasis. With the patient’s consent, a bone marrow biopsy was performed on the right ilium. Hematoxylin and eosin and immunohistochemical cytokeratin (CK) 7 positivity results revealed that bone tissue was metastatic adenocarcinoma (Figure 2). However, whole-body 18F-FDG PET/CT did not display any potential primary lesion (Figure 3A). Next, gallium-68 (68Ga)-labeled fibroblast-activation protein inhibitor (FAPI) PET/CT was performed to identify the primary lesion and showed abnormal uptake in the gastric antrum, abdominal lymph nodes, right pelvic wall, and the skeleton (Figure 3B). The maximum standardized uptake value in the gastric antrum was 5.52. Gastroscopy revealed a prepyloric lesion with a diameter of approximately 1.0 cm (Figure 4A). Pathological examination of a biopsy specimen discovered poorly differentiated adenocarcinoma (Figure 4B).
Metastatic poorly differentiated adenocarcinoma of the gastric antrum with extensive osteoblastic bone metastases.
The patient and his family declined genetic testing and further treatment and opted for discharge.
The patient succumbed to his cancer 1 year later.
Inflammatory or immune metabolic disease and bone metastases are often considered in elderly patients who present with widespread joint pain. Our patient’s ALP level was several times higher than normal upon admission, which raised concern. ALP is an enzyme that is present in multiple tissues including the liver, bones, intestines, and kidneys. An elevated ALP level is typically associated with hepatic and biliary system disorders and diseases of bone. Our patient’s hematologic indicators of liver and kidney function were normal, and imaging did not reveal signs of biliary obstruction. These findings, combined with a decreased serum calcium level, suggested a high likelihood of bone disease. Primary bone tumors, especially osteosarcomas, typically present with elevated ALP levels[6]. The primary lesion shows significant tenderness and a palpable mass, with imaging revealing bone destruction and possible periosteal reaction. This differs from the patient’s presentation of symmetric, multifocal lesions and widespread pain. Paget’s disease, a chronic bone disorder characterized by abnormally active bone metabolism, leads to significantly elevated ALP levels and is more common than bone metastases from malignant tumors. Several studies have demonstrated that distinguishing between metabolic bone disease and multiple bone metastases can be challenging and often requires confirmation by bone biopsy[7-9]. Moreover, an elevated serum ALP level is not only an important diagnostic indicator in patients with bone metastases from gastric cancer but also an adverse prognostic factor in gastric cancer[2,8,10].
Our patient had a slightly increased parathyroid hormone level and slightly decreased blood calcium and total 25-hydroxyvitamin D levels but no enlargement of the parathyroid glands in the neck on imaging, which were not consistent with primary hyperparathyroidism. In this patient, the invasion of tumor cells into bone caused the release of large amounts of phosphate, which combined with calcium in the blood to form insoluble calcium phosphate, thereby reducing the calcium level in the blood. Therefore, he was diagnosed as having secondary hyperparathyroidism caused by a decrease in blood calcium.
Bone metastases were widespread in this case, indicating extensive metastatic involvement. Bone metastasis occurs mainly via the hematogenous route. Bones, especially the red bone marrow, provide a good environment for cancer cells to grow, and some parts of the skeleton, such as the spine and pelvis, are more likely to be targets for bone metastases because of their structural and functional characteristics. Mimori et al[11] showed that even in the early stages of gastric cancer, isolated tumor cells can be detected in the peripheral blood and bone marrow, and when they circulate in the presence of high levels of vascular endothelial growth factor receptor 1, they can promote hematogenous metastasis of gastric cancer. Furthermore, elevated IL-6 and IL-10 levels accelerate tumor progression by inhibiting immune regulation and promoting angiogenesis and are closely associated with deterioration of gastric cancer and an unfavorable prognosis[12-14]. However, in this case, the symptoms associated with the primary tumor were not obvious, which delayed recognition of the lesions in the stomach. Such lesions are often overlooked, and false-negative imaging results can further complicate the diagnosis.
It is important to identify the primary lesion because this can significantly affect the treatment plan. When the diagnosis is unclear, a whole-body PET/CT scan can help to localize the primary lesion. However, 18F-FDG PET/CT may occasionally miss the diagnosis, particularly when the tumor is in the gastrointestinal tract[15,16]. 68Ga-FAPI is a novel probe that has shown greater sensitivity than 18F-FDG for diagnosis of gastrointestinal tumors and serves as an important imaging tool in these cases. Of particular note, while the patient’s history of colonic tubulovillous adenoma warranted consideration of colorectal carcinoma metastasis[17], critical evidence favored gastric origin. First, the bone biopsy demonstrated CK7 positivity - a marker predominantly expressed in gastric adenocarcinomas but typically negative in colorectal carcinomas[18]. Second, 68Ga-FAPI PET/CT revealed focal tracer uptake in the gastric antrum (maximum standardized uptake value: 5.52) with no abnormal colonic accumulation. Although extended immunohistochemical profiling (e.g., CK20, caudal type homeobox 2) could further clarify tumor origin, limited biopsy material and the patient’s advanced age with poor performance status precluded additional testing. The convergence of clinical, radiological, and histopathological findings provides compelling diagnostic certainty for primary gastric malignancy.
However, early detection is more important than early diagnosis. Gastrointestinal endoscopy is an important screening tool for diseases of the digestive tract and is particularly valuable for identifying small lesions that are difficult to detect on imaging. The patient’s history of adenomatous polyps and mildly elevated CA19-9 levels increases the necessity for regular, comprehensive gastrointestinal examinations, which should not be limited to colonoscopy or gastroscopy alone. As mentioned above, it has been shown that cancer cells are present in the peripheral blood in patients with early-stage gastric cancer[11]. In this case, the rate of metastasis exceeded the growth rate of the primary lesion, with serious consequences.
This case demonstrates the importance of regular gastrointestinal endoscopy in high-risk individuals with tubulovillous adenomas in the colon and measurement of tumor marker levels to reduce the risk of gastric cancer being detected at an advanced stage. The mechanisms underlying early extensive spread of cancer cells in patients with symptoms at other body sites that manifest earlier than those associated with the primary lesion remain unclear and warrant further investigation.
| 1. | Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 2. | Turkoz FP, Solak M, Kilickap S, Ulas A, Esbah O, Oksuzoglu B, Yalcin S. Bone metastasis from gastric cancer: the incidence, clinicopathological features, and influence on survival. J Gastric Cancer. 2014;14:164-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Kaneko Y, Murray WK, Link E, Hicks RJ, Duong C. Improving patient selection for 18F-FDG PET scanning in the staging of gastric cancer. J Nucl Med. 2015;56:523-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Iagaru A, Young P, Mittra E, Dick DW, Herfkens R, Gambhir SS. Pilot prospective evaluation of 99mTc-MDP scintigraphy, 18F NaF PET/CT, 18F FDG PET/CT and whole-body MRI for detection of skeletal metastases. Clin Nucl Med. 2013;38:e290-e296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Bénard F, Harsini S, Wilson D, Zukotynski K, Abikhzer G, Turcotte E, Cossette M, Metser U, Romsa J, Martin M, Mar C, Saad F, Soucy JP, Eigl BJ, Black P, Krauze A, Burrell S, Nichol A, Tardif JC. Intra-individual comparison of (18)F-sodium fluoride PET-CT and (99m)Tc bone scintigraphy with SPECT in patients with prostate cancer or breast cancer at high risk for skeletal metastases (MITNEC-A1): a multicentre, phase 3 trial. Lancet Oncol. 2022;23:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Kim SH, Shin KH, Moon SH, Jang J, Kim HS, Suh JS, Yang WI. Reassessment of alkaline phosphatase as serum tumor marker with high specificity in osteosarcoma. Cancer Med. 2017;6:1311-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Aiba H, Nakazato T, Matsuo H, Kimura H, Saito S, Sakai T, Murakami H, Kawai J, Kawasaki S, Imamura Y. Bone Metastases from Gastric Cancer Resembling Paget's Disease: A Case Report. J Clin Med. 2022;11:7306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Kang SH, Kim JI, Moon HS, Kang HM, Kim SH, Seong JK, Lee BS, Jeong HY, Song KS, Noh SM, Shin KS, Cho JS. Overt bone marrow metastasis from early gastric cancer. Endoscopy. 2008;40 Suppl 2:E34-E35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Koyama M, Koizumi M. Two cases of diffuse osteoblastic metastases from early type gastric cancer. Clin Nucl Med. 2007;32:545-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Namikawa T, Ishida N, Tsuda S, Fujisawa K, Munekage E, Iwabu J, Munekage M, Uemura S, Tsujii S, Tamura T, Yatabe T, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K. Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric Cancer. 2019;22:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Mimori K, Fukagawa T, Kosaka Y, Kita Y, Ishikawa K, Etoh T, Iinuma H, Sasako M, Mori M. Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin Cancer Res. 2008;14:2609-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer. 2009;12:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Zhang H, Li R, Cao Y, Gu Y, Lin C, Liu X, Lv K, He X, Fang H, Jin K, Fei Y, Chen Y, Wang J, Liu H, Li H, Zhang H, He H, Zhang W. Poor Clinical Outcomes and Immunoevasive Contexture in Intratumoral IL-10-Producing Macrophages Enriched Gastric Cancer Patients. Ann Surg. 2022;275:e626-e635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 14. | Song J, Lin Z, Liu Q, Huang S, Han L, Fang Y, Zhong P, Dou R, Xiang Z, Zheng J, Zhang X, Wang S, Xiong B. MiR-192-5p/RB1/NF-κBp65 signaling axis promotes IL-10 secretion during gastric cancer EMT to induce Treg cell differentiation in the tumour microenvironment. Clin Transl Med. 2022;12:e992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Perlaza P, Ortín J, Pagès M, Buxó E, Fernández-Esparrach G, Colletti PM, Rubello D, Mayoral M, Sánchez N, Ruiz C, Ginés A, Fuster D. Should 18F-FDG PET/CT Be Routinely Performed in the Clinical Staging of Locally Advanced Gastric Adenocarcinoma? Clin Nucl Med. 2018;43:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Jayaprakasam VS, Paroder V, Schöder H. Variants and Pitfalls in PET/CT Imaging of Gastrointestinal Cancers. Semin Nucl Med. 2021;51:485-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Khsiba A, Moalla M, Mahmoudi M, Mohamed AB, Manel Y, Nechi S, Belfekih H, Zribi S, Medhioub M, Hamzaoui L, Azzouz MM. A case of gastric metastasis originating from right-sided colon cancer 4 years after colectomy. Future Sci OA. 2022;8:FSO818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Dum D, Menz A, Völkel C, De Wispelaere N, Hinsch A, Gorbokon N, Lennartz M, Luebke AM, Hube-Magg C, Kluth M, Fraune C, Möller K, Bernreuther C, Lebok P, Clauditz TS, Jacobsen F, Sauter G, Uhlig R, Wilczak W, Steurer S, Minner S, Marx AH, Simon R, Burandt E, Krech T. Cytokeratin 7 and cytokeratin 20 expression in cancer: A tissue microarray study on 15,424 cancers. Exp Mol Pathol. 2022;126:104762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |