Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.106393
Revised: April 3, 2025
Accepted: April 25, 2025
Published online: June 15, 2025
Processing time: 93 Days and 5.8 Hours
Colorectal cancer (CRC) ranks among the most prevalent malignancies in elderly populations, and chemotherapy resistance remains a critical clinical challenge. Emerging evidence highlights the interplay between chronic inflammation, gut microbiome dysbiosis, and CRC progression. Proinflammatory cytokines [e.g., interleukin (IL)-6, tumor necrosis factor-alpha (TNF-α)] and mediators like S100 calcium-binding protein A12 (S100A12)/soluble receptor for advanced glycation end products (sRAGE) are implicated in tumorigenesis, while gut microbial imbalances may exacerbate inflammatory microenvironments conducive to che
To analyze the correlation between serum levels of S100A12, sRAGE, gut microbiome dysbiosis, and systemic inflammation in elderly patients with CRC and to assess their predictive value for chemotherapy efficacy.
A retrospective analysis was conducted on the clinical data of 120 elderly patients with advanced-stage CRC who visited our hospital from August 2023 to May 2024. These patients were enrolled in the study group. Additionally, 120 healthy individuals undergoing routine health check-ups during the same period were selected as the control group. Serum S100A12, sRAGE, IL-6, and TNF-α levels were measured by ELISA, and fresh stool samples were collected before chemotherapy to analyze gut microbiome composition in the study group. Follow-up observations were conducted after chemotherapy. Pearson correlation analysis was used to explore the relationship between serum S100A12, sRAGE levels, and gut microbiome dysbiosis in patients with CRC. The predictive diagnostic value of pre-chemotherapy serum S100A12 and sRAGE levels for chemotherapy efficacy was assessed using receiver operating characteristic curves.
Pre-chemotherapy serum S100A12, sRAGE, IL-6, and TNF-α levels were significantly elevated in patients with CRC vs controls (all P < 0.05). These biomarkers progressively increased with microbiota dysbiosis severity (severe vs mild dysbiosis: S100A12: 340.26 ± 52.39 μg/L vs 302.53 ± 56.97 μg/L; sRAGE: 525.64 ± 37.32 ng/L vs 441.38 ± 48.73 ng/L, P < 0.05) and correlated strongly with IL-6 (r = 0.712) and TNF-α (r = 0.698). Post-chemotherapy, biomarker levels decreased (P < 0.05), coinciding with beneficial microbiota recovery (Bifidobacterium 176%, Lactobacillus 153%) and pathogenic taxa reduction (Escherichia coli 62%). The combined S100A12/sRAGE model predicted chemotherapy resistance with an area under the curve of 0.914 (sensitivity = 86.07%, specificity = 88.89%), outperforming individual biomarkers.
Elevated serum S100A12 and sRAGE in elderly patients with CRC reflected gut microbiome dysbiosis and systemic inflammation, driven by IL-6/TNF-α signaling. Their post-chemotherapy decline parallels microbiota restoration, supporting a microbiome-inflammation-biomarker axis. The combined biomarker model offers robust clinical utility for chemotherapy efficacy prediction and personalized therapeutic strategies.
Core Tip: Elevated serum S100 calcium-binding protein A12 and soluble receptor for advanced glycation end products in elderly patients with colorectal cancer reflected gut microbiome dysbiosis and systemic inflammation that was driven by interleukin-6/tumor necrosis factor-α signaling. Their post-chemotherapy decline parallels microbiota restoration, supporting a microbiome-inflammation-biomarker axis. The combined biomarker model offers robust clinical utility for chemotherapy efficacy prediction and personalized therapeutic strategies.
- Citation: Qiao SB, Niu ML, Liang WT, Zhang LJ, Chen X, Zhu YK. Analysis of serum S100A12, soluble advanced glycation end products receptor, and gut microbiome in elderly patients with colorectal cancer. World J Gastrointest Oncol 2025; 17(6): 106393
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/106393.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.106393
In recent years, the incidence and mortality rates of colorectal cancer (CRC) have been steadily rising worldwide, particularly among the elderly, with the incidence significantly higher than that in younger patients[1]. This phenomenon is closely related to the decline in immune function and metabolic changes in elderly individuals as well as the potential long-term effects of gut microbiome dysbiosis[2]. The gut microbiome, as an essential microecosystem in the human body, is closely involved in immune regulation, inflammatory responses, and cancer progression[3]. A study[4] found that gut microbiome imbalance can promote the occurrence and progression of CRC and may affect the efficacy of chemotherapy.
In molecular mechanism studies, serum S100 calcium-binding protein A12 (S100A12) and soluble receptor for advanced glycation end products (sRAGE) have gained increasing attention as important inflammatory markers and immune-modulating factors in recent years. S100A12 is mainly secreted by neutrophils and is involved in cytokine release and inflammation, with significantly elevated levels observed in various inflammatory diseases[5]. sRAGE, a soluble form of the advanced glycation end product receptor (RAGE), antagonizes RAGE signaling, alleviating inflammation and oxidative stress[6]. However, the expression levels of these two biomarkers in the serum of patients with CRC and their specific relationship with gut microbiome dysbiosis remain unclear.
Furthermore, chemotherapy, as a key treatment for CRC, is influenced by various factors, including the patient’s gut microbiome status and inflammatory response levels[7]. Therefore, it is crucial to investigate the correlation between serum S100A12 and sRAGE levels and gut microbiome dysbiosis as well as their predictive value for chemotherapy efficacy. Based on the above considerations, this study aimed to assess the potential value of serum S100A12 and sRAGE levels in chemotherapy efficacy prediction and disease management by analyzing gut microbiome status and chemotherapy outcomes in elderly patients with CRC. This research may provide a theoretical basis and reference for individualized treatment strategies.
A retrospective analysis was conducted on the clinical data of 120 elderly patients with advanced-stage CRC who visited our hospital from August 2023 to May 2024, and they were included in the study group. All participants and their families provided informed consent and signed related documents. Among them, 67 were male and 53 were female; the age range was 66-79 years, with an average age of 70.51 ± 4.12. According to the tumor node metastasis staging, 79 patients were in stage IIIb, and 41 patients were in stage IV. Additionally, 120 healthy individuals undergoing routine health check-ups at our hospital during the same period were selected as the control group, which included 72 males and 48 females, with an age range of 63-80 years and an average age of 69.37 ± 4.35. There were no significant differences in basic information such as age and gender between the two groups (P > 0.05), indicating comparability.
Inclusion criteria: (1) Participants aged ≥ 60 years, with no gender restrictions; (2) The study group patients were diagnosed with CRC by clinical testing and confirmed through relevant examinations, with no surgical indication; (3) The study group patients were in tumor node metastasis stages IIIb to IV, including those with lymph node metastasis and/or distant metastasis, and expected survival time > 3 months; (4) The study group patients had not received chemotherapy or any other anticancer treatments, and had no contraindications such as severe infections or immunosuppressive conditions; and (5) Clinical data of the participants were complete and authentic for analysis.
Exclusion criteria: (1) Patients with other malignancies; (2) Severe heart, kidney, or liver dysfunction (such as heart failure, end-stage kidney disease, liver failure, etc.), or poorly controlled chronic diseases such as diabetes and hypertension; (3) Patients with acute infectious diseases (e.g., acute viral infections, bacterial infections); (4) Patients with congenital or acquired immune deficiencies (e.g., HIV-infected individuals, those undergoing immunosuppressive therapy after organ transplantation); (5) Use of antibiotics, probiotics, or similar medications for extended periods within 3 months before enrollment; (6) Allergic reactions or contraindications to the chemotherapy or procedures in this study; (7) Cognitive, communication disorders, and/or mental illness; (8) Failure to sign informed consent or incomplete clinical data; and (9) Participants who died during the study or were unable to complete follow-up due to any reason.
Detection of serum S100A12 and sRAGE levels: Venous blood samples were collected from both groups of participants. Blood samples from the control group were collected from health check-up participants on the day of the check-up, while blood samples from the study group were collected before and after chemotherapy. The collection process was done in the morning on an empty stomach, and 5 mL of blood was drawn from the elbow vein. Immediately after collection, the blood samples were centrifuged using a high-speed centrifuge at 3000 rpm for 15 min (centrifugal radius: 5 cm) to ensure complete separation of the blood components. After centrifugation, the supernatant was carefully separated, and serum was collected. The samples were then stored at -70 °C to ensure stability and accuracy for subsequent analysis. The serum levels of S100A12 and sRAGE were measured using an ELISA. The S100A12 kit (ZY-S100A12-Hu) was provided by Shanghai Zeye Biotechnology Co., Ltd., and the sRAGE kit (ml038107) was provided by Shanghai Enzyme-Linked Biotechnology Co., Ltd.
Inflammatory pathway analysis: To preliminarily explore potential mechanisms linking S100A12/sRAGE with microbiome dysbiosis, serum levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) were concurrently measured using ELISA kits (IL-6 kit: Ml002859, TNF-α kit: Ml002301, Shanghai Enzyme-Linked Biotechnology Co., Ltd.) as adjunct inflammatory markers.
Fresh stool samples (0.1 g) from patients in the study group were collected before chemotherapy and diluted with 0.9 mL of saline. After dilution, a series of standardized dilutions were made, and the diluted samples were inoculated onto appropriate culture media for incubation for 72 h. During the incubation period, the researchers observed and recorded colony formation, specifically counting the numbers of Escherichia coli, Enterococcus faecalis, Bifidobacterium, and Lactobacillus. To assess the degree of dysbiosis, the samples were categorized based on bacterial count and the ratio of gram-negative to gram-positive bacteria (adapted from Gagnière et al[8], 2016 with modifications for CRC populations). Specifically, degree I dysbiosis was defined as a bacterial count of 300-500 bacteria per field of view (40 × magnification) with a taxonomic composition of gram-positive rods (40%-50%), gram-negative rods (30%), and gram-positive cocci (< 10%). Degree II dysbiosis was characterized by a bacterial count of fewer than 300 bacteria per field of view, with gram-positive rods comprising less than 30%, gram-negative rods less than 30%, and gram-positive cocci exceeding 10%.
All patients received the CapeOX regimen (oxaliplatin + capecitabine), which was selected based on its established efficacy in elderly patients with CRC[9] and reduced neurotoxicity risk compared to FOLFOX[10]. On the first day of treatment, patients were given intravenous oxaliplatin (130 mg/m², infused over 2 h), followed by oral capecitabine (1000 mg/m², twice daily) starting on the same day for 2 weeks. Chemotherapy cycles were repeated every 3 weeks for a total of four cycles. After chemotherapy, treatment efficacy was assessed during follow-up visits.
The efficacy evaluation was based on the Response Evaluation Criteria in Solid Tumors[11] with patients categorized as follows: Complete response (CR). Complete disappearance of all tumors with no new lesions; partial response (PR). At least 30% reduction in tumor size but not complete disappearance; stable disease (SD). Tumor size change within 20%; and progressive disease (PD). Tumor growth of more than 20% or appearance of new lesions. Patients in the study group were further divided into the chemotherapy response group (including patients with CR and PR) and the chemotherapy non-response group (including patients with SD and PD).
To preliminarily assess the dynamic relationship between chemotherapy and microbiome recovery, stool samples from the chemotherapy response group were re-collected 4 weeks post-treatment for follow-up bacterial quantification.
GraphPad Prism 8 software was used for graphing, and SPSS 25.0 was used for statistical analysis. Normally distributed continuous variables were expressed as mean ± SD. The comparison between two independent groups was conducted using the independent samples t-test. For multiple group comparisons, one-way analysis of variance was used, and pairwise comparisons were performed using the Student-Newman-Keuls method. For within-group comparisons before and after chemotherapy, the paired t-test was used. Pearson’s correlation analysis was used to analyze the correlation between serum S100A12, sRAGE levels, and intestinal dysbiosis in patients with CRC. The predictive diagnostic value of serum S100A12 and sRAGE levels before chemotherapy for chemotherapy failure was analyzed using receiver operating characteristic curves. A P value of < 0.05 was considered statistically significant.
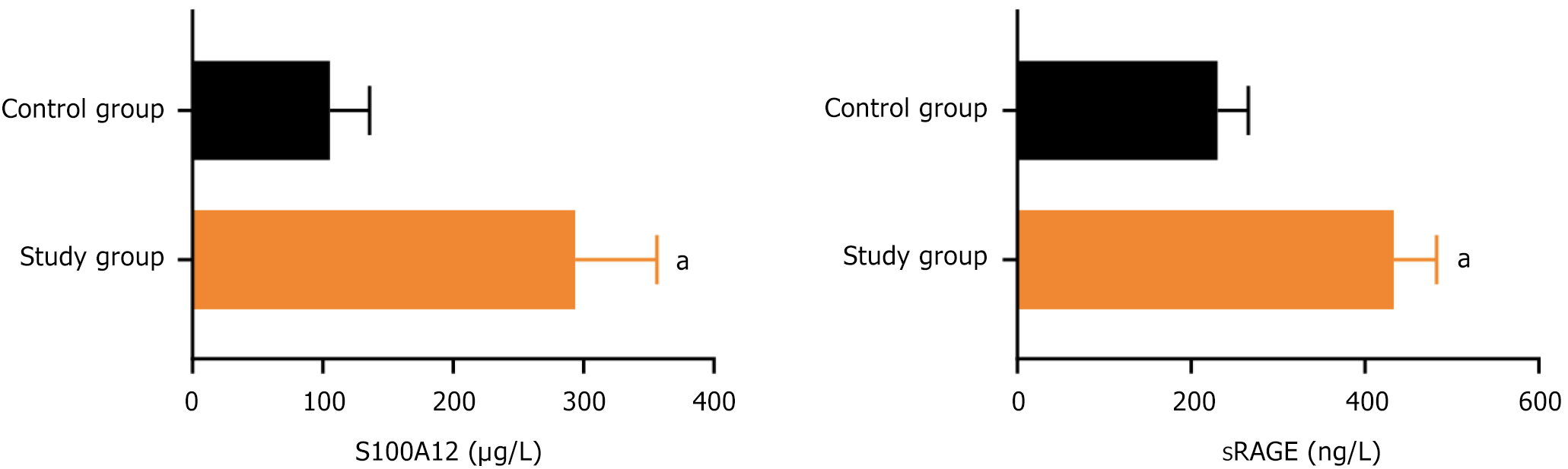
The serum S100A12 and sRAGE levels before chemotherapy in the study group were 293.76 ± 62.54 and 433.12 ± 49.27, respectively, while those in the control group were 105.41 ± 30.39 and 230.48 ± 35.25, respectively. The serum S100A12 and sRAGE levels before chemotherapy in the study group were significantly higher than those in the control group (P < 0.05), as shown in Figure 1.
A total of 120 patients with CRC were tested. Among them, 50 had normal gut microbiota, 43 had mild dysbiosis, and 27 had severe dysbiosis. The serum S100A12 and sRAGE levels in the mild dysbiosis and severe dysbiosis groups were higher than those in the normal microbiota group before chemotherapy, and the levels in the severe dysbiosis group were higher than those in the mild dysbiosis group (P < 0.05), as shown in Table 1.
Pearson correlation analysis showed that serum S100A12 and sRAGE levels in patients with CRC were positively correlated with the numbers of Escherichia coli and Enterococcus faecalis (P < 0.05) and negatively correlated with the numbers of Bifidobacterium and Lactobacillus (P < 0.05), as shown in Table 2.
| Indicator | S100A12 | sRAGE | ||
| r | P value | r | P value | |
| Escherichia coli | 0.551 | < 0.001 | 0.627 | < 0.001 |
| Enterococcus faecalis | 0.587 | < 0.001 | 0.529 | < 0.001 |
| Bifidobacterium | -0.623 | < 0.001 | -0.584 | < 0.001 |
| Lactobacillus | -0.615 | < 0.001 | -0.606 | < 0.001 |
Systemic inflammation in patients with CRC: Pre-chemotherapy serum IL-6 and TNF-α levels in the study group were significantly elevated compared to the control group (IL-6: 48.32 ± 12.47 pg/mL vs 12.15 ± 3.82 pg/mL; TNF-α: 36.75 ± 8.93 pg/mL vs 8.64 ± 2.51 pg/mL; P < 0.001) (Supplementary Table 1).
Association with gut microbiota dysbiosis: The levels of IL-6 and TNF-α progressively increased with the severity of gut microbiota dysbiosis (Supplementary Table 2). In the normal microbiota group, IL-6 was 34.21 ± 9.15 pg/mL and TNF-α was 28.43 ± 6.72 pg/mL. In the mild dysbiosis group, IL-6 increased to 52.67 ± 11.38 pg/mL and TNF-α to 39.56 ± 7.85 pg/mL (vs normal: P < 0.01). In the severe dysbiosis group, IL-6 further increased to 68.92 ± 13.24 pg/mL and TNF-α to 48.31 ± 9.12 pg/mL (vs mild: P < 0.05).
Correlation with S100A12/sRAGE: IL-6 and TNF-α levels showed strong positive correlations with both S100A12 and sRAGE (Supplementary Table 3).
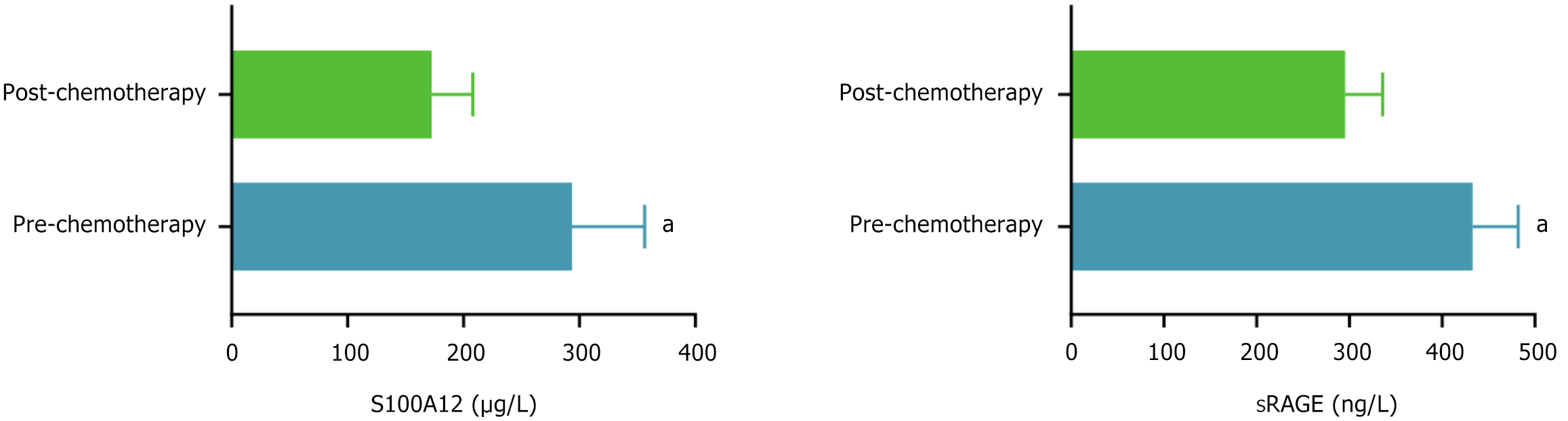
The serum S100A12 and sRAGE levels before chemotherapy in patients with CRC were 293.76 ± 62.53 and 432.78 ± 49.24, respectively, and after chemotherapy, they were 172.54 ± 35.67 and 295.46 ± 40.59, respectively. The serum S100A12 and sRAGE levels after chemotherapy were significantly lower than those before chemotherapy (P < 0.05), as shown in Figure 2.
In the chemotherapy effective group (n = 75), post-treatment Bifidobacterium and Lactobacillus counts significantly increased compared to pre-chemotherapy levels (P < 0.001), while pathogenic bacteria (Escherichia coli and Enterococcus faecalis) decreased (P < 0.01) (Supplementary Table 4). Specifically, Bifidobacterium increased from 2.13 × 106 colony forming units (CFU)/g pre-chemotherapy to 5.82 × 106 CFU/g post-chemotherapy, and Lactobacillus increased from 1.72 × 106 CFU/g to 4.35 × 106 CFU/g. Meanwhile, Escherichia coli decreased from 8.56 × 107 CFU/g to 3.21 × 107 CFU/g, and Enterococcus faecalis decreased from 6.93 × 107 CFU/g to 2.74 × 107 CFU/g.
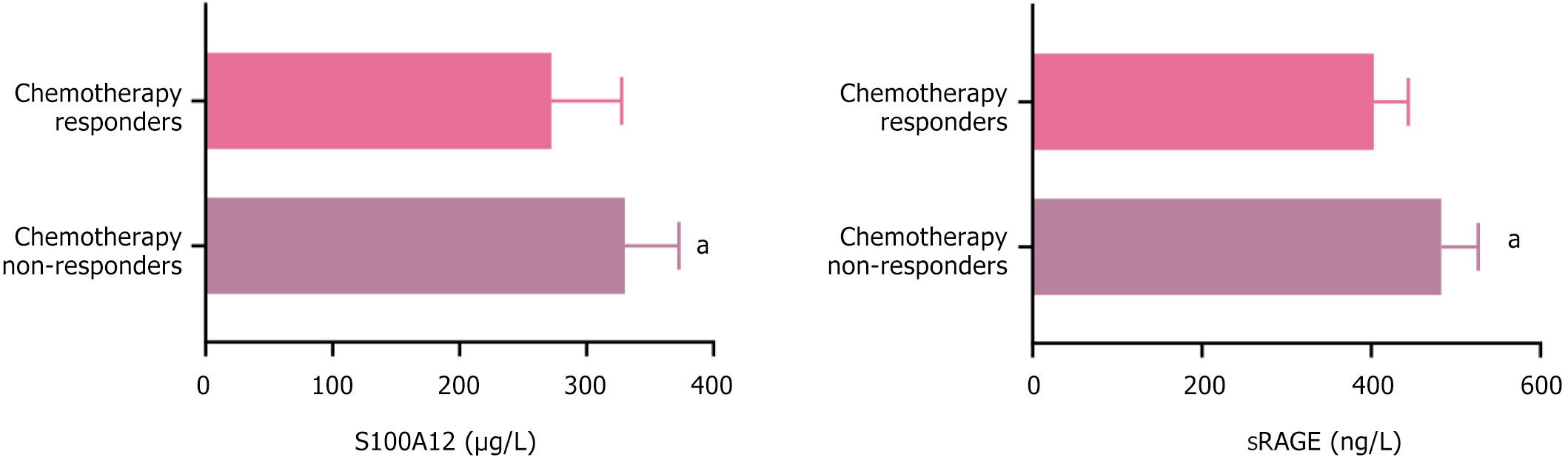
After chemotherapy, 17 patients achieved CR, 58 patients achieved PR, 30 patients had SD, and 15 patients had PD. The remission group consisted of 75 patients, and the ineffective group consisted of 45 patients. The serum S100A12 and sRAGE levels before chemotherapy in the ineffective group were 330.12 ± 42.67 and 482.89 ± 43.65, respectively, while those in the remission group were 272.36 ± 55.38 and 403.21 ± 40.55, respectively. The serum S100A12 and sRAGE levels before chemotherapy were higher in the ineffective group than in the remission group (P < 0.05), as shown in Figure 3.
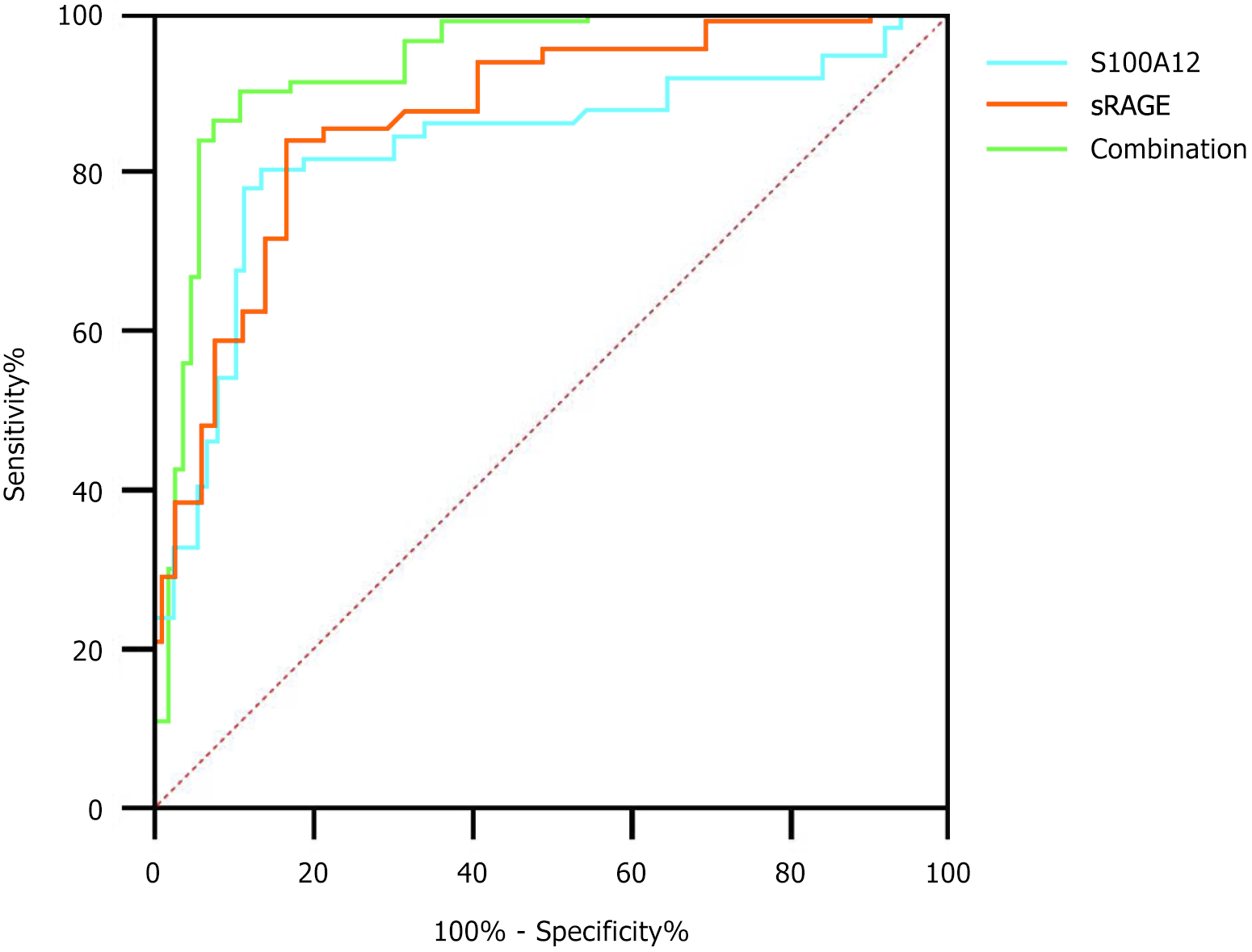
The combined diagnostic area under the curve (AUC), sensitivity, and specificity were higher than those of S100A12 and sRAGE alone, as shown in Table 3 and Figure 4. Specifically, the combined model (AUC = 0.914, 95% confidence interval: 0.843-0.962) demonstrated excellent discriminative power, surpassing the individual performance of S100A12 (AUC = 0.802) and sRAGE (AUC = 0.841). At optimal cutoffs, this model achieved 86.07% sensitivity and 88.89% specificity, indicating robust potential for identifying patients at high risk of chemotherapy failure.
| Indicator | Optimal cutoff value | AUC | 95%CI | P value | Sensitivity (%) | Specificity (%) |
| S100A12 | 324.89 μg/L | 0.802 | 0.713-0.864 | < 0.05 | 79.04 | 82.03 |
| sRAGE | 435.76 ng/L | 0.841 | 0.776-0.915 | < 0.05 | 81.43 | 75.32 |
| Combined | - | 0.914 | 0.843-0.962 | < 0.05 | 86.07 | 88.89 |
With the population in China becoming increasingly older, the incidence of CRC among the elderly continues to rise, making its early diagnosis, treatment, and prognosis evaluation increasingly important[11,12]. Treatment options for CRC include surgical resection, radiotherapy, chemotherapy, and targeted therapy, with chemotherapy being one of the key methods for treating advanced-stage CRC[13]. The CapeOX regimen (oxaliplatin + capecitabine) was selected for this study based on its established efficacy in elderly populations and reduced neurotoxicity compared to FOLFOX[9], as elderly patients are more susceptible to cumulative neurotoxicity and require regimens balancing tolerability with therapeutic benefit[10].
However, despite chemotherapy effectively controlling the disease and prolonging survival, its therapeutic effects vary significantly between individuals. Moreover, chemotherapy itself is associated with a range of adverse reactions, particularly in elderly patients in whom side effects are more pronounced, complicating the treatment response[14,15]. Therefore, there is an urgent need to identify more precise biomarkers to help evaluate chemotherapy efficacy and predict patient prognosis.
The gut microbiome, as an important microecological system in the human body, has attracted widespread attention in recent years. It plays a crucial role in regulating immune functions, food digestion, and nutrient absorption. Dysbiosis, or an imbalance in the gut microbiome, has been closely associated with the occurrence and development of various diseases, particularly those related to the digestive system[16-18]. Research[19] indicates that the development of CRC is influenced not only by genetic and environmental factors but also by changes in the gut microbiome, which is considered a significant factor in promoting CRC onset and progression. Increasing studies[20,21] have shown that dysbiosis can promote tumor development through various mechanisms (such as promoting intestinal inflammation and impairing intestinal barrier function), and this imbalance is more pronounced in elderly patients, as aging, long-term antibiotic use, and chemotherapy can all contribute to dysbiosis[22], which in turn affects immune function, treatment response, and disease prognosis. Therefore, exploring the relationship between gut microbiome dysbiosis and CRC as well as its impact on chemotherapy outcomes holds important clinical significance.
S100A12 is a member of the calcium-binding protein family and is primarily expressed by immune cells such as neutrophils and monocytes. It serves as an important inflammatory biomarker involved in regulating immune responses[23]. RAGE is the primary receptor of S100A12, and it is widely distributed on the surface of cells such as monocytes, macrophages, and endothelial cells[24]. sRAGE is the soluble form of RAGE, which can competitively bind its ligands, thus modulating its biological effects[25]. Studies[26,27] have shown that S100A12 and sRAGE play important roles in various inflammatory diseases (such as bacterial pneumonia, sepsis, severe pancreatitis), and their levels are closely related to disease severity and prognosis.
Our findings demonstrated a stepwise increase in serum IL-6 and TNF-α levels across microbiota dysbiosis severity (Supplementary Table 2). Patients with severe dysbiosis exhibited IL-6 levels nearly double those of the normal microbiota group (68.92 pg/mL vs 34.21 pg/mL), paralleling the progressive rise in S100A12/sRAGE (Table 1). The strong correlations between IL-6/TNF-α and both biomarkers (Supplementary Table 3; r = 0.68-0.71, P < 0.001) suggest that gut dysbiosis may amplify systemic inflammation, driving S100A12/sRAGE overexpression via RAGE/nuclear factor kappa-B signaling[28]. This aligns with experimental evidence that Escherichia coli-enriched microbiota promote IL-6 secretion through TLR4 activation, which in turn upregulates S100A12 in myeloid cells[29].
The results of this study indicated that in patients with CRC, the levels of S100A12 and sRAGE are significantly higher than those in healthy individuals, and their levels are positively correlated with the degree of microbiome dysbiosis (P < 0.05), consistent with previous studies[30,31]. This suggests that both may serve as biomarkers reflecting gut microbiome dysbiosis. Further analysis in this study revealed that serum levels of S100A12 and sRAGE in patients with CRC after chemotherapy were significantly lower than before chemotherapy, and the levels of S100A12 and sRAGE in the chemotherapy-resistant group were significantly higher than in the chemotherapy-response group (P < 0.05). This phenomenon is speculated to be related to the elevated expression of S100A12 and sRAGE in tumors. S100A12, through binding to RAGE and TLR4 receptors, activates multiple signaling pathways (such as extracellular regulated protein kinases 1/2, phosphatidylinositol 3-kinase/protein kinase B pathways)[32]. This promotes the release of inflammatory mediators and the expression of adhesion molecules, which contribute to the occurrence and development of intestinal cancer. Moreover, S100A12 and sRAGE are closely associated with inflammatory responses triggered by microbiome dysbiosis. Dysbiosis activates inflammatory pathways, suppresses anti-tumor immune responses, and accelerates tumor progression[33], all of which further affects the patient’s chemotherapy response.
Notably, patients who were responsive to chemotherapy showed a concurrent recovery of beneficial bacteria (Bifidobacterium and Lactobacillus) and a reduction in proinflammatory taxa (Escherichia coli, Enterococcus faecalis) (Supplementary Table 4). The post-chemotherapy decline in S100A12/sRAGE (Figure 2) coincided with decreased IL-6 (-62.1%) and TNF-α (-39.5%), supporting a “microbiome-inflammation-S100A12/sRAGE axis” wherein microbiota restoration dampens inflammatory signaling. This bidirectional relationship implies that pre-chemotherapy S100A12/sRAGE levels may reflect both tumor burden and dysbiosis-driven immune dysfunction, offering a composite biomarker for treatment resistance[34-36].
In the chemotherapy-resistant group, the higher levels of S100A12 and sRAGE may reflect immune suppression and inflammation induced by gut microbiome dysbiosis and negatively impact tumor control. It is also noteworthy that S100A12 and sRAGE are closely related to patient prognosis. Receiver operating characteristic curve analysis in this study found that the combined detection of serum S100A12 and sRAGE showed higher sensitivity and specificity in predicting the effectiveness of chemotherapy for CRC, with an AUC value of 0.914, sensitivity of 86.07%, and specificity of 88.89%, which was superior to single biomarker detection. This provides strong evidence for clinical practice, indicating that S100A12 and sRAGE can not only serve as biomarkers for assessing chemotherapy efficacy in CRC but also offer new insights and methods for developing personalized treatment plans.
Although this study revealed the potential association between S100A12 and sRAGE and chemotherapy outcomes in CRC, there are still some limitations that need further improvement and refinement: (1) While focusing on elderly patients with CRC addresses a critical demographic, our findings may not be generalizable to younger populations or early-stage disease. Future studies should include broader age ranges and disease stages; (2) Microbiome analysis limitations. Culture-based methods, while cost-effective, lack resolution for unculturable taxa. Integrating 16S rRNA sequencing will provide a more comprehensive microbial profile; (3) While this study explored the correlation between changes in serum S100A12 and sRAGE levels and chemotherapy efficacy, it did not delve into the mechanisms underlying the actions of these biomarkers. The specific roles of S100A12 and sRAGE in the CRC immune microenvironment are not yet clear. Future research could further investigate their functions in the tumor microenvironment and their relationship with microbiome dysbiosis through in vitro and in vivo experiments; and (4) This study did not explore other potential factors that may affect chemotherapy outcomes, such as patients’ nutritional status and immune function, which could interact with the changes in S100A12 and sRAGE levels and influence chemotherapy outcomes. Therefore, future research should consider multiple factors and further refine the predictive model for chemotherapy efficacy in patients with CRC.
This study highlighted the potential of serum S100A12 and sRAGE as dual biomarkers for predicting chemotherapy efficacy in elderly patients with CRC, with gut microbiome dysbiosis serving as a key modulator of inflammation and treatment resistance. Translating these findings into clinical practice requires validation in multicenter cohorts and integration with microbiome-targeted therapies to optimize personalized oncology strategies.
| 1. | Li N, Zhou YY, Lu M, Zhang YH, Lu B, Luo CY, Luo JH, Cai J, Chen HD, Dai M. [Participation rate and detection of colorectal neoplasms based on multi-round fecal immunochemical testing for CRC screening in the Chinese population]. Zhonghua Zhong Liu Za Zhi. 2023;45:1041-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Zhao X, Dou LZ, Zhang YM, Liu Y, He S, Ke Y, Liu XD, Liu YM, Wu HR, Li ZQ, Chen ZH, Wang GQ. [Risk factors for residual cancer or lymph node metastasis after endoscopic noncurable resection of early colorectal cancer]. Zhonghua Zhong Liu Za Zhi. 2023;45:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Wang HY, Liu LX, Chen XY, Zhang YD, Li WX, Li WW, Wang L, Mo XL, Wei H, Ji P, Xie P. Comprehensive analysis of the gut microbiome and post-translational modifications elucidates the route involved in microbiota-host interactions. Zool Res. 2024;45:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Tortora SC, Bodiwala VM, Quinn A, Martello LA, Vignesh S. Microbiome and colorectal carcinogenesis: Linked mechanisms and racial differences. World J Gastrointest Oncol. 2022;14:375-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (3)] |
| 5. | Bobek D, Sestan M, Mijacika L, Kovacic N, Lukic IK, Grcevic D, Jelusic M. Serum S100A12 levels in children with childhood-onset systemic lupus erythematosus, systemic juvenile arthritis, and systemic undefined recurrent fevers. Z Rheumatol. 2023;82:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Wang J, Zeng AM, Liang SF. [The Value of Serum HMGB1 and sRAGE in the Diagnosis, Efficacy Monitoring and Prognosis of Newly Diagnosed Multiple Myeloma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022;30:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Duan JJ, Ning T, Bai M, Zhang L, Li HL, Liu R, Ge SH, Wang X, Yang YC, Ji Z, Wang FX, Sun YS, Ba Y, Deng T. [The efficacy of chemotherapy re-challenge in third-line setting for metastatic colorectal cancer patients: a real-world study]. Zhonghua Zhong Liu Za Zhi. 2023;45:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Gagnière J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, Bringer MA, Pezet D, Bonnet M. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. 2016;22:501-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 613] [Cited by in RCA: 557] [Article Influence: 61.9] [Reference Citation Analysis (5)] |
| 9. | Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll HJ. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 576] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 10. | Kang L, Tian Y, Xu S, Chen H. Oxaliplatin-induced peripheral neuropathy: clinical features, mechanisms, prevention and treatment. J Neurol. 2021;268:3269-3282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 11. | Colorectal Oncology Committee of Chinese Medical Doctor Association. [Chinese expert consensus on multidisciplinary treatment of bone metastasis from colorectal cancer (2020 version)]. Zhonghua Zhong Liu Za Zhi. 2020;42:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Chen ZY, Wang X, Wu T, Liu YC, Wang PY. [Progress of molecular subtypes of colorectal cancer]. Zhonghua Zhong Liu Za Zhi. 2017;39:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Wang J, Wei WS, Jiang LJ, Zhang ZL, Guo SJ, Han H, Zhou FJ, Dong P. [Efficacy and safety evaluation of immunotherapy combined with targeted therapy as second-line treatment in patients with metastatic non-clear cell renal cell carcinoma]. Zhonghua Zhong Liu Za Zhi. 2023;45:704-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | An Q, Shi JX, Cui J, Li ZJ, Ma FH, Xiao G, Jia WW, Tang DN, Zhao G, Wu GJ. [Analysis of prognosis and related factors in oldest-old patients with left-side or right-side colon cancer after hemicolectomy]. Zhonghua Yi Xue Za Zhi. 2023;103:1666-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Pei W, Zhou SC, Liang JW, Zheng ZX, Wang Z, Liu Z, Jiang Z, Liu Q, Zhou ZX, Wang XS. [Analysis of risk factors of severe postoperative complications in elderly patients with colorectal cancer aged over 80 years]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:695-700. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Muyasha A, Liu WY, Jin J, Li S, Tang Y, Li N, Ren H, Fang H, Lu NN, Tang Y, Chen B, Wang SL, Song YW, Liu YP, Qi SN, Li YX. [Comparison of preoperative chemotherapy with concurrent chemoradiotherapy combined with TME for 305 patients with locally advanced rectal cancer]. Zhonghua Zhong Liu Za Zhi. 2021;43:1122-1131. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Lin SM, Wang XJ, Huang SH, Xu ZB, Huang Y, Lu XR, Xu DB, Chi P. [Construction of artificial neural network model for predicting the efficacy of first-line FOLFOX chemotherapy for metastatic colorectal cancer]. Zhonghua Zhong Liu Za Zhi. 2021;43:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Wang JJ, Lyu JD, Peng X. [Analysis the effect of cytokine-induced killer cells combined with mFOLFOX6 regimen in chemotherapy for advanced colorectal cancer]. Zhonghua Yi Xue Za Zhi. 2023;103:2000-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Koustas E, Trifylli EM, Sarantis P, Papadopoulos N, Aloizos G, Tsagarakis A, Damaskos C, Garmpis N, Garmpi A, Papavassiliou AG, Karamouzis MV. Implication of gut microbiome in immunotherapy for colorectal cancer. World J Gastrointest Oncol. 2022;14:1665-1674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (2)] |
| 20. | Bartolini I, Risaliti M, Ringressi MN, Melli F, Nannini G, Amedei A, Muiesan P, Taddei A. Role of gut microbiota-immunity axis in patients undergoing surgery for colorectal cancer: Focus on short and long-term outcomes. World J Gastroenterol. 2020;26:2498-2513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 21. | Baxter BA, Parker KD, Nosler MJ, Rao S, Craig R, Seiler C, Ryan EP. Metabolite profile comparisons between ascending and descending colon tissue in healthy adults. World J Gastroenterol. 2020;26:335-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Grobbee EJ, Lam SY, Fuhler GM, Blakaj B, Konstantinov SR, Bruno MJ, Peppelenbosch MP, Kuipers EJ, Spaander MC. First steps towards combining faecal immunochemical testing with the gut microbiome in colorectal cancer screening. United European Gastroenterol J. 2020;8:293-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 23. | Huang X, Chen L, Li Z, Zheng B, Liu N, Fang Q, Jiang J, Rao T, Ouyang D. The efficacy and toxicity of antineoplastic antimetabolites: Role of gut microbiota. Toxicology. 2021;460:152858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Tan B, Liu YX, Tang H, Chen D, Xu Y, Chen MJ, Li Y, Wang MZ, Qian JM. Gut microbiota shed new light on the management of immune-related adverse events. Thorac Cancer. 2022;13:2681-2691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (1)] |
| 25. | Li L, Ye J. Characterization of gut microbiota in patients with primary hepatocellular carcinoma received immune checkpoint inhibitors: A Chinese population-based study. Medicine (Baltimore). 2020;99:e21788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Mester P, Keller D, Kunst C, Räth U, Rusch S, Schmid S, Krautbauer S, Müller M, Buechler C, Pavel V. High Serum S100A12 as a Diagnostic and Prognostic Biomarker for Severity, Multidrug-Resistant Bacteria Superinfection and Herpes Simplex Virus Reactivation in COVID-19. Viruses. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Page TH, Chiappo D, Brunini F, Garnica J, Blackburn J, Dudhiya F, Prendecki M, McAdoo SP, Pusey CD. Danger-associated molecular pattern molecules and the receptor for advanced glycation end products enhance ANCA-induced responses. Rheumatology (Oxford). 2022;61:834-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 28. | Yoon T, Ahn SS, Ha JW, Ko E, Song JJ, Park YB, Lee SW. Serum Soluble Receptors for Advanced Glycation End-Products May Predict Mortality in Microscopic Polyangiitis and Granulomatosis with Polyangiitis. Yonsei Med J. 2024;65:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Korucu B, Yeter H, Gonen S, Derici MK, Ronco C, Derici U. Impact of medium cut-off membranes on S100A12 and soluble receptor for advanced glycation end products. Semin Dial. 2023;36:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Wu HP, Chuang LP, Liu PH, Chu CM, Yu CC, Lin SW, Kao KC, Li LF, Chuang DY. Decreased Monocyte HLA-DR Expression in Patients with Sepsis and Acute Kidney Injury. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 31. | Mitrović Ajtić O, Subotički T, Diklić M, Đikić D, Vukotić M, Dragojević T, Živković E, Antić D, Čokić V. Regulation of S100As Expression by Inflammatory Cytokines in Chronic Lymphocytic Leukemia. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Zhang Q, Zuo Y, Xu M. The correlation of serum Vaspin, S100A12 and PCT levels with the severity of ulcerative colitis and its clinical significance. Am J Transl Res. 2021;13:7914-7920. [PubMed] |
| 33. | Melin EO, Dereke J, Hillman M. Higher levels of the soluble receptor for advanced glycation end products and lower levels of the extracellular newly identified receptor for advanced glycation end products were associated with lipid-lowering drugs in patients with type 1 diabetes: a comparative cross-sectional study. Lipids Health Dis. 2020;19:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 34. | Tsirebolos G, Tsoporis JN, Drosatos IA, Izhar S, Gkavogiannakis N, Sakadakis E, Triantafyllis AS, Parker TG, Rallidis LS, Rizos I. Emerging markers of inflammation and oxidative stress as potential predictors of coronary artery disease. Int J Cardiol. 2023;376:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 35. | Cabrera-García AI, Suchodolski JS, Steiner JM, Heilmann RM. Association between serum soluble receptor for advanced glycation end-products (RAGE) deficiency and severity of clinicopathologic evidence of canine chronic inflammatory enteropathy. J Vet Diagn Invest. 2020;32:664-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Menzies J, Sundararaj A, Cardamone M, McHarg A, Leach S, Krishnan U. Ketogenic Diets in Children With Intractable Epilepsy and its Effects on Gastrointestinal Function, Gut Microbiome, Inflammation, and Quality of Life. J Pediatr Gastroenterol Nutr. 2023;77:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |