Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.105887
Revised: March 25, 2025
Accepted: April 23, 2025
Published online: June 15, 2025
Processing time: 124 Days and 10.8 Hours
Lenvatinib and sorafenib are tyrosine kinase inhibitors that are effective in the treatment of unresectable hepatocellular carcinoma (uHCC). The efficacy of which of them is better suited to combine transarterial chemoembolization (TACE) for the treatment of uHCC is ripe.
To compare the effectiveness of TACE combined with lenvatinib (TACE-lenvatinib) and TACE combined with sorafenib (TACE-sorafenib) in the treatment of uHCC, this study was carried out.
Publicly available studies comparing the efficacy of TACE-lenvatinib and TACE-sorafenib in the treatment of uHCC were collected from PubMed, Embase and Cochrane Library, with a cut-off date of December 2024. Stata SE 15 software was used for statistical analysis.
A total of six studies involving 547 patients were included, 248 in the TACE-lenvatinib group and 299 in the TACE-sorafenib group. Meta-analysis results showed that TACE-lenvatinib was more effective than TACE-sorafenib in complete response [relative risk (RR) = 1.81, 95% confidence interval (CI): 1.11-2.96, P = 0.02], partial response (RR = 1.38, 95%CI: 1.12-1.70, P = 0.002), objective response rate (RR = 1.47, 95%CI: 1.24-1.74, P < 0.0001) and disease control rate (RR = 1.22, 95%CI: 1.00-1.49, P = 0.05). TACE-lenvatinib was significantly lower than TACE-sorafenib in progressive disease rate (RR = 0.54, 95%CI: 0.39-0.74, P = 0.002). No significant difference was found in stable disease rate (RR = 0.89, 95%CI: 0.60-1.33, P = 0.58) between the two groups. TACE-lenvatinib was significantly more effective than TACE-sorafenib in overall survival (hazard ratio = 2.00, 95%CI: 1.59-2.50, P < 0.05) and progression free survival (hazard ratio = 2.04, 95%CI: 1.49-2.86, P < 0.05). As regards adverse events, TACE-lenvatinib was better in reducing the incidence of hypertension than TACE-sorafenib, while no significant difference was found in overall adverse events, abdominal pain, fever, fatigue, nausea and vomiting, decreased appetite, liver dysfunction, hand-foot skin reaction, diarrhea, thrombocytopenia, and rash between the two groups.
In patients with uHCC, TACE-lenvatinib induced a better tumor response rate and survival outcome than TACE-sorafenib, while TACE-lenvatinib resulted in a higher incidence of hypertension than TACE-sorafenib. However, these conclusions are derived from currently available medical evidence, and further confirmation by more rigorously designed randomized controlled studies is still needed.
Core Tip: In this study, we compared the efficacy of transarterial chemoembolization (TACE)-lenvatinib and TACE-sorafenib against unresectable hepatocellular carcinoma. The TACE-lenvatinib group had higher tumor response rate than the TACE-sorafenib group. We also found that both overall survival and progression-free-survival were higher in the TACE-lenvatinib group than in the TACE-sorafenib group. The TACE-lenvatinib group had a higher incidence of hypertension. Therefore, the combination of TACE and lenvatinib resulted in better tumor response rates and survival outcomes in unresectable hepatocellular carcinoma patients compared to TACE-sorafenib, although the former was associated with a higher incidence of hypertension.
- Citation: Zhang W, Fu H, Liu ZR, Xu L, Che X, Ning YT, Zhan ZY, Zhou GC. Transarterial chemoembolization combined with lenvatinib vs transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: A systematic review and meta-analysis. World J Gastrointest Oncol 2025; 17(6): 105887
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/105887.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.105887
Liver cancer is the most common and third deadliest cancer worldwide, and hepatocellular carcinoma (HCC) is the predominant subtype that accounts for 75%-85% of all liver cancer-related deaths[1]. Surgical resection is still considered a curative approach for HCC, although two-thirds of the patients are diagnosed in the middle to advanced stages of the disease, which precludes the possibility of surgery[2-4]. Transarterial chemoembolization (TACE) has been established as the standard local therapy for unresectable HCC (uHCC), whereas targeted drugs is the mainstay of treating advanced HCC, such as sorafenib and lenvatinib[5,6]. Sorafenib was the first drug approved for the first-line treatment of uHCC, and has been effective when used in combination with TACE[7,8]. While TACE contributes to local tumor control through ischemia and necrosis, sorafenib inhibits TACE-induced upregulation of vascular endothelial growth factor (VEGF), thereby inhibiting tumor re-vascularization and progression.
Lenvatinib is a novel tyrosine kinase inhibitor (TKI) that targets VEGF receptors (VEGFRs) 1-3, fibroblast growth factor receptors (FGFRs) 1-4, and platelet-derived growth factor receptor alpha[8,9] The REFLECT trial[8] demonstrated a significant improvement in the objective response rate (ORR) and progression-free survival (PFS) of liver cancer patients treated with lenvatinib compared to that of the sorafenib arm. Currently, lenvatinib and sorafenib are recommended as first-line therapeutic agents for patients with uHCC. However, monotherapy regimens based on TKIs have shown limited efficacy with response rates below 20%[7,10,11]. A growing number of studies have suggested that the combination of TACE with sorafenib or lenvatinib may increase the tumor response rate and provide a survival benefit for patients with uHCC[12,13]. The aim of this study was to compare the efficacy and safety of TACE combined with lenvatinib (TACE-lenvatinib) with that of TACE combined with sorafenib (TACE-sorafenib) in patients with uHCC.
The systematic review was reported in accordance with PRISMA guidelines[14]. This study was exempt from the requirement of institutional review-board approval or informed consent from patients on account of the public availability of the data.
The Embase, PubMed, and Cochrane Library databases were searched for relevant articles published till December 2024. The search strategy is described in Supplementary Table 1. The original authors were contacted for extra information if necessary. In case of multiple studies from the same authors or medical centers with duplicate samples, only the most recent, highest quality studies were selected. The inclusion criteria were as follows: (1) The target population of the study is patients diagnosed with histologically or clinically confirmed uHCC, including patients with TACE-refractory or non-refractory HCC; (2) Comparative analysis of the efficacy of TACE-lenvatinib and TACE-sorafenib; (3) No restriction on study sample size; (4) No restriction on follow-up time; (5) No restriction on language type; (6) Human subjects; and (7) Studies assessing treatment response based on Response Evaluation Criteria for Solid Tumors and adverse events (AEs) according to Common Terminology Criteria for AEs v5.0. The exclusion criteria were as follows: (1) Incomplete information, inability to extract valid data and/or contact authors, duplicate publications, unpublished studies; (2) Single-arm studies reporting only the efficacy of TACE-lenvatinib or TACE-sorafenib; and (3) Reviews, case reports, and animal experiments. A risk assessment was conducted for all randomized control trials (RCTs) according to the “risk assessment tool” recommended by the Cochrane Collaboration Network. The quality of the cohort studies was assessed with the Newcastle-Ottawa Scale as detailed in Supplementary Table 2.
Stata SE 15 software was used for statistical analysis. The relative risk (RR) was calculated to compare effect sizes for dichotomous variables by the Mantel-Haenszel method, and hazard ratios (HRs) were used to compare effect sizes for survival data by the inverse variance method. Heterogeneity among studies was qualitatively evaluated using a χ2-based Q test with I2 statistics; I2 < 30%, 30% ≤ I2 ≤ 50%, I2 > 50% were defined as low, moderate, and high heterogeneity respectively. A random model which based on the Wald-type framework was used in this study. Sensitivity analysis was performed by removing one study at a time to assess whether the results were markedly affected by a single study. Publication bias was quantified through Begg’s test and Egger’s test, with P < 0.05 as the threshold for significance. Funnel plots were used for the qualitative assessment of publication bias.
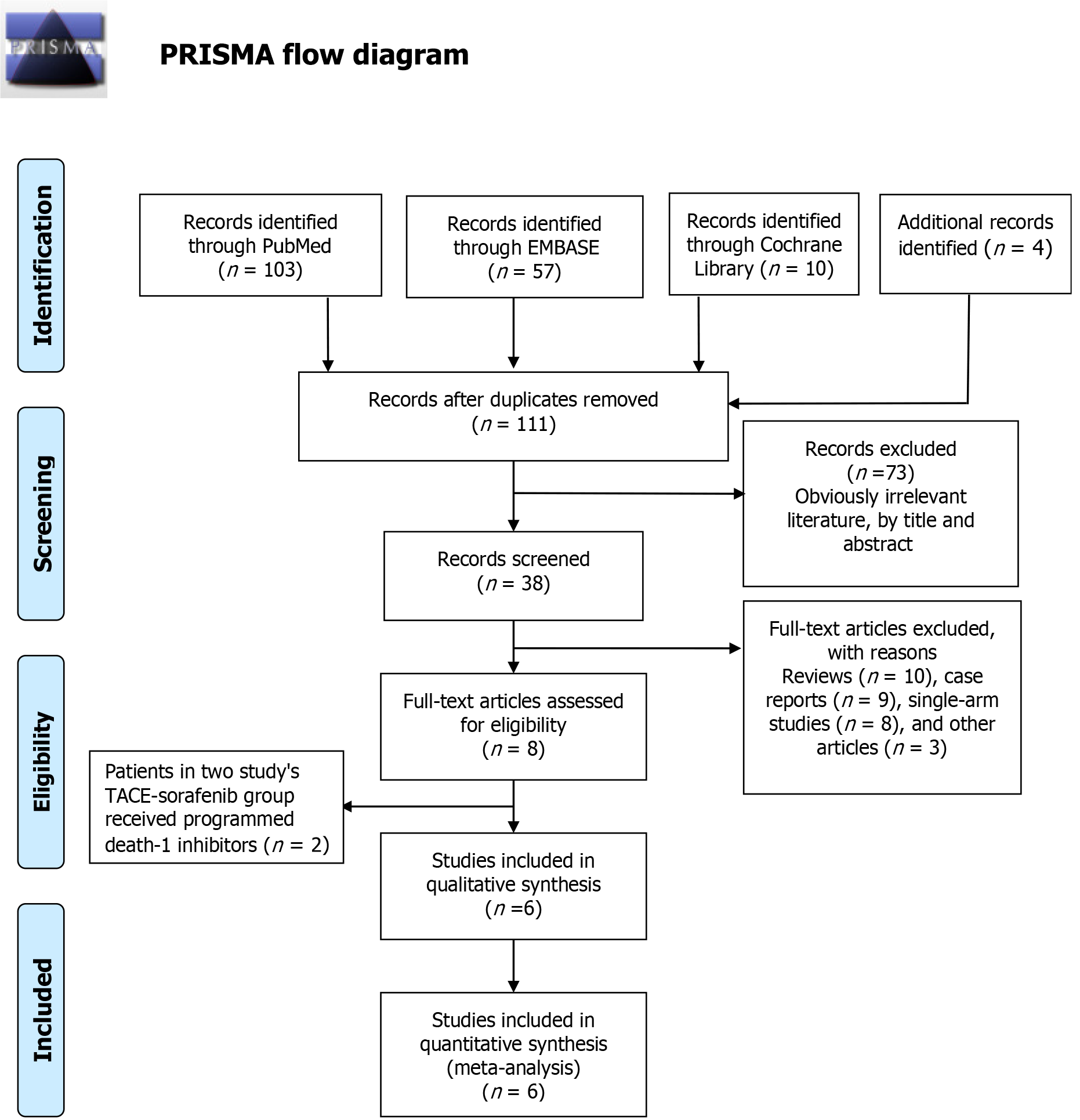
A total of 63 articles were retrieved, of which 38 remained after excluding the duplicates. We then excluded reviews, case reports and other types of literature, and incorporated the remaining 6 articles[15-20] in the meta-analysis. The detailed steps of literature search are outlined in Figure 1. The 6 studies[15-20] included a total of 547 patients, of which 248 (45.34%) received TACE-sorafenib and 299 (54.66%) received TACE-sorafenib. The characteristics of these studies are summarized in Tables 1 and 2.
| Ref. | Country | Period | Medical center | Study type | TACE + LEN vs TACE + SOR, n | Gender (F/M), TACE + LEN | Gender (F/M), TACE + SOR | Age, TACE + LEN | Age, TACE + SOR | Quality |
| Ding et al[15], 2021 | China | 2018-2020 | Beijing Ditan Hospital | R | 32 vs 32 | 7/25 | 5/27 | 57 ± 11 | 56 ± 11 | 5 |
| Xue et al[16], 2021 | China | 2017-2020 | First Affiliated Hospital of Sun Yat-Sen University | RCT | 50 vs 100 | 4/46 | 3/97 | 54 (49-61) | 54 (49-63) | 6 |
| Xu et al[17], 2023 | China | 2019-2021 | First Affiliated Hospital of Anhui Medical University | R | 43 vs 41 | 18/25 | 17/24 | 55 ± 12.26 | 52.5 ± 11.47 | 6 |
| Yang et al[18], 2021 | China | 2017-2020 | West China Medical School | R | 38 vs 38 | 4/34 | 4/34 | 55.18 ± 10.94 | 54.39 ± 12.17 | NA |
| Zhang et al[19], 2022 | China | 2018-2021 | The First Affiliated Hospital with Nanjing Medical University | R | 53 vs 59 | 9/44 | 7/52 | 57.7 ± 11.8 | 58.8 ± 11.1 | 7 |
| Zhao et al[20], 2022 | China | 2018-2020 | Chinese People’s Liberation Army General Hospital, Beijing | R | 32 vs 29 | 1/31 | 4/25 | 57.38 ± 9.44 | 55.90 ± 8.18 | 7 |
| Ref. | Prior HBV infection, TACE + LEN1 | Prior HBV infection, TACE + SOR1 | ECOG-PS (0-1/2), TACE + LEN | ECOG-PS (0-1/2), TACE + SOR | Child-Pugh (A/B), TACE + LEN | Child-Pugh (A/B), TACE + SOR | Invading blood vessels, TACE + LEN1 | Invading blood vessels, TACE + SOR1 | Distant metastasis, TACE + LEN1 | Distant metastasis, TACE + SOR1 | AFP (< 400/≥ 400, ng/mL), TACE + LEN | AFP (< 400/≥ 400, ng/mL), TACE + SOR |
| Ding et al[15], 2021 | 30/2 | 29/3 | 24/7 | 22/10 | 22/10 | 28/4 | 32/0 | 32/0 | 19/13 | 23/9 | 16/16 | 14/18 |
| Xue et al[16], 2021 | 46/4 | 97/3 | 37/13 | 84/16 | 41/9 | 84/16 | 36/14 | 81/19 | 27/23 | 45/55 | NA | NA |
| Xu et al[17], 2023 | 38/5 | 36/5 | 20/23 | 23/18 | 32/11 | 28/13 | 17/26 | 15/26 | 11/32 | 12/29 | 23/20 | 26/15 |
| Yang et al[18], 2021 | 36/2 | 34/4 | 27/11 | 27/11 | 37/1 | 37/1 | 14/24 | 15/23 | ||||
| Zhang et al[19], 2022 | 50/3 | 55/4 | 43/10 | 45/14 | 52/1 | 57/2 | 36/17 | 41/18 | 36/17 | 39/20 | 20/33 | 25/34 |
| Zhao et al[20], 2022 | 22/1 | 21/11 | 12/11 | 18/14 | 19/4 | 27/5 | 10/13 | 18/14 | 1/22 | 22/10 | NA | NA |
The efficacy and safety of TACE-lenvatinib and TACE-sorafenib were compared in terms of tumor response rates, long-term outcomes, and AEs.
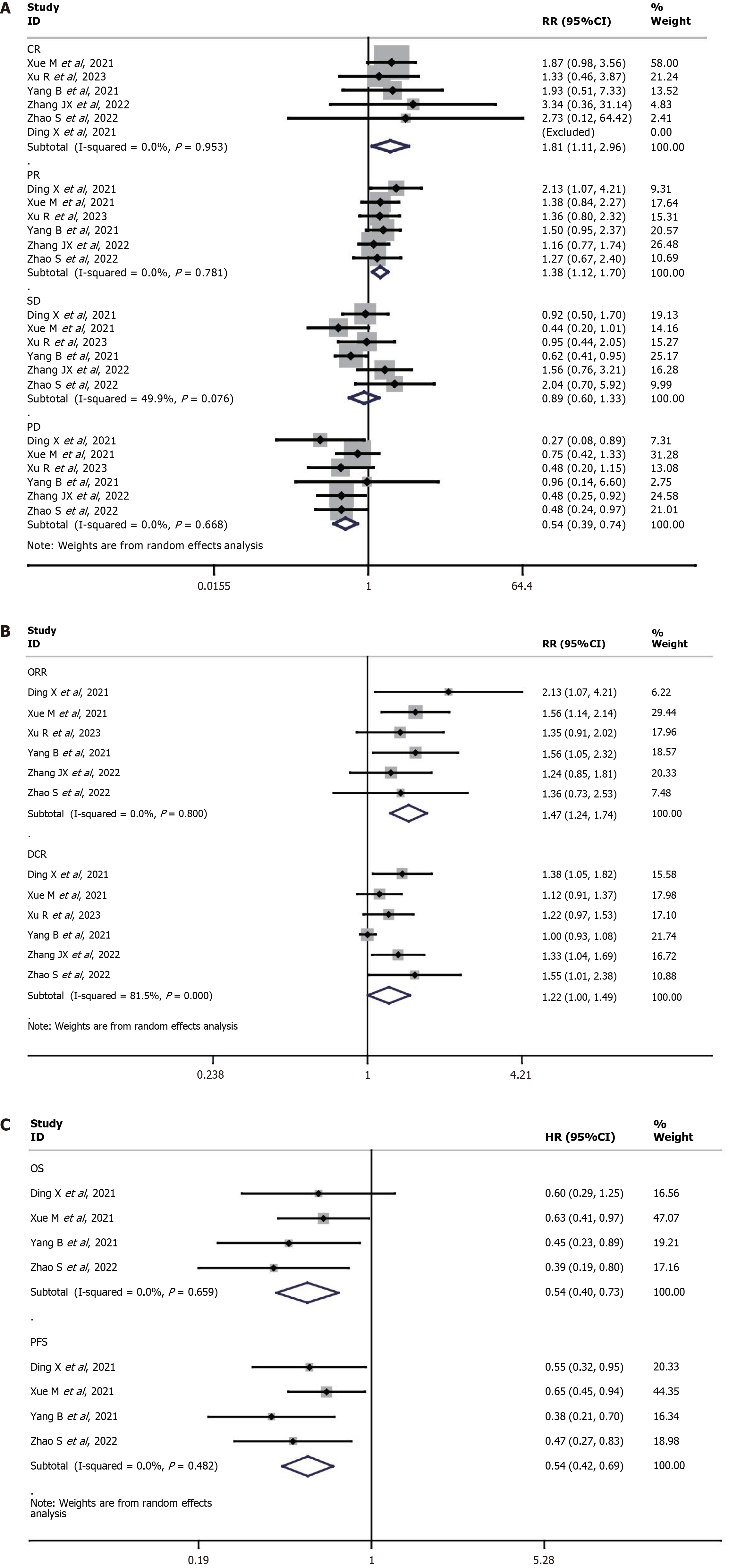
Six studies[15-20] reported complete response (CR), partial response (PR), stable disease, progressive disease (PD), ORR, and disease control rate (DCR). The CR (RR = 1.81, 95%CI: 1.11-2.96, P = 0.02), PR (RR = 1.38, 95%CI: 1.12-1.70, P = 0.002), ORR (RR = 1.47, 95%CI: 1.24-1.74, P < 0.0001) and DCR (RR = 1.22, 95%CI: 1.00-1.49, P = 0.05) were significantly higher in the TACE-lenvatinib group compared to the TACE-sorafenib group. On the other hand, the TACE-lenvatinib group had lower PD rate than the TACE-sorafenib group (RR = 0.54, 95%CI: 0.39-0.74, P = 0.0002). The rate of stable disease was similar in both groups (RR = 0.89, 95%CI: 0.55-1.44, P > 0.05). The results are shown in Table 3 and Figure 2A.
| Measured outcomes | Studies, n | Heterogeneity test, I2 (%) | Heterogeneity test, P value | Model | RR/HR | 95%CI | P value |
| Complete response | 6 | 0 | 0.95 | Random | 1,81 | 1.11-2.96 | 0.02 |
| Partial response | 6 | 0 | 0.78 | Random | 1.38 | 1.12-1.70 | 0.002 |
| Stable disease | 6 | 50 | 0.08 | Random | 0.89 | 0.60-1.33 | 0.58 |
| Progressive disease | 6 | 0 | 0.67 | Random | 0.54 | 0.39-0.74 | 0.0002 |
| Objective response rate | 6 | 0 | 0.80 | Random | 1.47 | 1.24-1.74 | < 0.0001 |
| Disease control rate | 6 | 82 | < 0.0001 | Random | 1.22 | 1.00-1.49 | 0.05 |
| Overall survival | 4 | 39.8 | 0.173 | Random | 0.28 | 0.19-0.42 | < 0.05 |
| Disease free survival | 4 | 0 | 0.482 | Random | 0.54 | 0.42-0.69 | < 0.05 |
Four studies[16,18-20] reported overall survival (OS) and disease-free survival. The TACE-lenvatinib group showed significantly higher OS (HR = 2.00, 95%CI: 1.59-2.50, P < 0.05) and PFS (HR = 2.04, 95%CI: 1.49-2.86, P < 0.05) than the TACE-sorafenib group. The results are shown in Table 3 and Figure 2B and C.
The included studies[15-20] reported all AEs, in addition to abdominal pain, fever, fatigue, nausea, vomiting, decreased appetite, liver dysfunction, hypertension, hand- foot skin reaction, diarrhea, thrombocytopenia, and rashes. The incidence of all AEs (RR = 0.97, 95%CI: 0.79-1.20, P = 0.80), abdominal pain (RR = 0.95, 95%CI: 0.65-1.41, P = 0.81), fever (RR = 0.95, 95%CI: 0.72-1.25, P = 0.69), fatigue (RR = 1.28, 95%CI: 0.79-2.05, P = 0.32), nausea and vomiting (RR = 1.25, 95%CI: 0.85-1.83, P = 0.25), decreased appetite (RR = 1.18, 95%CI: 0.81-1.71, P = 0.40), liver dysfunction (RR = 1.04, 95%CI: 0.62-1.75, P = 0.87), hand-foot skin reaction (RR = 0.74, 95%CI: 0.18-3.04, P = 0.68), diarrhea (RR = 1.38, 95%CI: 0.55-3.44, P = 0.49), thrombocytopenia (RR = 1.18, 95%CI: 0.71-1.98, P = 0.52), and rashes (RR = 0.88, 95%CI: 0.19-4.16, P = 0.88) were similar in both treatment groups. However, the TACE-lenvatinib group had a significantly higher incidence of hypertension (RR = 2.53, 95%CI: 1.19-5.39, P = 0.02) compared to the TACE-sorafenib group. The results are shown in Table 4 and Supplementary Table 3.
| Adverse events | Studies, n | Heterogeneity test, I2(%) | Heterogeneity test, P value | Model | RR | 95%CI | P value |
| All adverse events | 3 | 0 | 0.53 | Random | 0.97 | 0.79-1.20 | 0.80 |
| Abdominal pain | 4 | 0 | 0.68 | Random | 0.95 | 0.65-1.41 | 0.81 |
| Fever | 3 | 0 | 0.66 | Random | 0.95 | 0.72-1.25 | 0.69 |
| Fatigue | 4 | 0 | 0.68 | Random | 1.28 | 0.79-2.05 | 0.32 |
| Nausea and vomiting | 5 | 0 | 0.70 | Random | 1.25 | 0.85-1.83 | 0.25 |
| Decreased appetite | 5 | 0 | 0.76 | Random | 1.18 | 0.81-1.71 | 0.40 |
| Liver dysfunction | 4 | 0 | 0.46 | Random | 1.04 | 0.62-1.75 | 0.87 |
| Hypertension | 5 | 0 | 0.44 | Random | 2.53 | 1.19-5.39 | 0.02 |
| Hand-foot skin reaction | 5 | 75 | 0.003 | Random | 0.74 | 0.18-3.04 | 0.68 |
| Diarrhea | 6 | 39 | 0.16 | Random | 1.38 | 0.55-3.44 | 0.49 |
| Thrombocytopenia | 3 | 0 | 0.71 | Random | 1.18 | 0.71-1.98 | 0.52 |
| Rash | 6 | 65 | 0.04 | Random | 0.88 | 0.19-4.16 | 0.88 |
According to the sensitivity analyses, the results of the meta-analysis were stable. We did not detect publication bias with Begg’s test and Egger’s test which are shown in Supplementary Table 4. The funnel plots with pseudo 95% confidence limits are shown in Supplementary Figure 1.
A growing body of research suggests that TACE in combination with sorafenib may increase tumor response rates and provide a survival benefit in patients with uHCC[12,13]. However, TACE can upregulate VEGF, which is a key factor driving neoangiogenesis and tumor progression[21]. While sorafenib inhibits VEGF[22], sorafenib also activates the FGF family of proteins which may weaken VEGF inhibition[23]. In contrast, lenvatinib exerts inhibitory effects on the FGF/FGFR as well as the VEGF/VEGFR pathways. In addition, lenvatinib may also inhibit other signaling pathways with pro-angiogenic effects, such as platelet-derived growth factor, which is not affected by sorafenib[24,25]. It stands to reason that the differences in the mechanisms of both TKIs may influence tumor control in the TACE-lenvatinib and TACE-sorafenib groups. Given its broad-spectrum inhibitory action against VEGFRs 1-3, platelet-derived growth factor receptor alpha, FGFRs 1-4, and RET, lenvatinib in combination with TACE could theoretically result in stronger inhibition of tumor neovascularization, and therefore a greater reduction in tumor growth.
In this study, we compared the efficacy of TACE-lenvatinib and TACE-sorafenib against uHCC. The TACE-lenvatinib group had higher CR, PR, ORR, and DCR, and significantly lower PD rates than the TACE-sorafenib group. Although TACE-lenvatinib group exhibited superior tumor response as per the Response Evaluation Criteria for Solid Tumors criteria, the tumor response often does not reflect the direct clinical benefits of anti-neoplastic agents, such as improved survival, improved quality of life for patients, or reduction of tumor-related symptoms. Therefore, we also analyzed the long-term survival outcomes in both groups, and found that both OS and PFS were higher in the TACE-lenvatinib group than in the TACE-sorafenib group. The combination of TACE and sorafenib is considered clinically safe[7,8]. Consistent with this, there were no difference in the incidence of AEs between the two treatment groups in our study, although the TACE-lenvatinib group had a higher incidence of hypertension. Furthermore, the most common side effects of lenvatinib are hand-foot skin reactions, diarrhea and hypertension, whereas fever, pain and transient transaminase elevation primarily result from TACE. These treatment-related AEs are predominantly grade 1 or 2, which can be resolved or eliminated with appropriate and timely management.
A previous meta-analysis[26] showed that although TACE-lenvatinib was significantly more effective than TACE-sorafenib in patients with uHCC, the former was associated with serious AEs. The authors concluded that TACE-sorafenib should be used as an alternative to TACE-lenvatinib in case of serious AEs. However, two studies in that meta-analysis[27,28] included patients with uHCC refractory to TACE. In fact, some uHCC patients are unresponsive to TACE due to considerable tumor heterogeneity, and repeated ineffective TACE can even aggravate liver injury and worsen prognosis. The Global Liver Cancer Society proposed the concept of “TACE failure refractoriness” as a criterion for timely termination of TACE in order to avoid repeated ineffective treatments[29-31]. In the event that TACE is ineffective, the response of patients depends on the efficacy of the TKI, making the combination treatment redundant. According to the principle of intention to treat, a patient with uHCC cannot know before TACE whether the treatment will be effective. Therefore, inclusion of patients who are unresponsive to TACE[27,28] likely introduced bias in the meta-analysis. Therefore, we excluded these studies in our meta-analysis, and our conclusions were close to real-world unresectable intermediate and advanced HCC who are about to receive their initial treatment, including those refractory and non-refractory to TACE.
While retrospective studies can reflect real-world scenarios, their inherent bias cannot be avoided. Meta-analyses of well-designed non-randomized comparative studies are likely to be as accurate as RCTs[32]. We performed comparative meta-analysis, which is better accepted in the medical literature as a complementary tool to qualitative reviews[33]. We believe that our findings are significant and provide a comprehensive picture of the real-world clinical efficacy of combining TACE and lenvatinib in the treatment of uHCC. Taken together, TACE-based combination therapy warrants further investigation in future RCTs.
The limitations of the study were as followed: First, the included studies were retrospective, which may have led to selection bias. Second, most of the studies on uHCC patients did not perform further subgroup analysis, which limited the further subdivision of the conclusions. Finally, most of the studies were conducted on limited number of cases from single medical centers, which reduced the interpretive power for the conclusions.
The combination of TACE and lenvatinib resulted in better tumor response rates and survival outcomes in uHCC patients compared to TACE-sorafenib, although the former was associated with a higher incidence of hypertension. However, further validation with more rigorously designed RCTs and longer follow-up are still needed in the future.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64583] [Article Influence: 16145.8] [Reference Citation Analysis (176)] |
| 2. | Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang W, Wang X, Wang Z, Xiang B, Xing B, Xu J, Yang J, Yang J, Yang Y, Yang Y, Ye S, Yin Z, Zhang B, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Liu R, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Wang W, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 574] [Article Influence: 114.8] [Reference Citation Analysis (1)] |
| 3. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 574] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6052] [Article Influence: 864.6] [Reference Citation Analysis (3)] |
| 5. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4102] [Article Influence: 586.0] [Reference Citation Analysis (6)] |
| 6. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1106] [Article Influence: 100.5] [Reference Citation Analysis (1)] |
| 7. | Goh MJ, Oh JH, Park Y, Kim J, Kang W, Sinn DH, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Efficacy and Safety of Lenvatinib Therapy for Unresectable Hepatocellular Carcinoma in a Real-World Practice in Korea. Liver Cancer. 2021;10:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3822] [Article Influence: 546.0] [Reference Citation Analysis (1)] |
| 9. | Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 10. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4649] [Article Influence: 273.5] [Reference Citation Analysis (0)] |
| 11. | Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, Ying J, Lu Y, Meng Z, Pan H, Yang P, Zhang H, Chen X, Xu A, Cui C, Zhu B, Wu J, Xin X, Wang J, Shan J, Chen J, Zheng Z, Xu L, Wen X, You Z, Ren Z, Liu X, Qiu M, Wu L, Chen F. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol. 2021;39:3002-3011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 257] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 12. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 502] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 13. | Qiu Z, Shen L, Jiang Y, Qiu J, Xu Z, Shi M, Yu Z, Ma Y, He W, Zheng Y, Li B, Wang G, Yuan Y. Transarterial chemoembolization (TACE) combined with apatinib versus TACE combined with sorafenib in advanced hepatocellular carcinoma patients: a multicenter retrospective study. Ann Transl Med. 2021;9:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 7636] [Article Influence: 477.3] [Reference Citation Analysis (1)] |
| 15. | Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, Liu X, Zheng L, Li W, Chen J. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer. 2021;127:3782-3793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 16. | Xue M, Wu Y, Zhu B, Zou X, Fan W, Li J. Advanced hepatocellular carcinoma treated by transcatheter arterial chemoembolization with drug-eluting beads plus lenvatinib versus sorafenib, a propensity score matching retrospective study. Am J Cancer Res. 2021;11:6107-6118. [PubMed] |
| 17. | Xu R, Ji X, Pei X, Yu Y. Comparison of efficacy and safety between transarterial chemoembolization (TACE) combined with lenvatinib versus TACE combined with sorafenib in the treatment of intermediate and advanced hepatocellular carcinoma. Am J Transl Res. 2023;15:1117-1128. [PubMed] |
| 18. | Yang B, Jie L, Yang T, Chen M, Gao Y, Zhang T, Zhang Y, Wu H, Liao Z. TACE Plus Lenvatinib Versus TACE Plus Sorafenib for Unresectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Prospective Cohort Study. Front Oncol. 2021;11:821599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Zhang JX, Chen YX, Zhou CG, Liu J, Liu S, Shi HB, Zu QQ. Transarterial chemoembolization combined with lenvatinib versus transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: A comparative retrospective study. Hepatol Res. 2022;52:794-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Zhao S, Zhou M, Wang P, Yang J, Zhang D, Yin F, Song P. Sorafenib, Lenvatinib, or Lenvatinib Combining PD-1 Inhibitors Plus TACE in Unresectable Hepatocellular Carcinoma: A Retrospective Analysis. Technol Cancer Res Treat. 2022;21:15330338221133640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 21. | Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 22. | Scartozzi M, Faloppi L, Svegliati Baroni G, Loretelli C, Piscaglia F, Iavarone M, Toniutto P, Fava G, De Minicis S, Mandolesi A, Bianconi M, Giampieri R, Granito A, Facchetti F, Bitetto D, Marinelli S, Venerandi L, Vavassori S, Gemini S, D'Errico A, Colombo M, Bolondi L, Bearzi I, Benedetti A, Cascinu S. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: the ALICE-1 study. Int J Cancer. 2014;135:1247-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1215] [Cited by in RCA: 1266] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 24. | Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3471] [Cited by in RCA: 4027] [Article Influence: 287.6] [Reference Citation Analysis (0)] |
| 25. | Finn RS, Kudo M, Cheng AL, Wyrwicz L, Ngan RKC, Blanc JF, Baron AD, Vogel A, Ikeda M, Piscaglia F, Han KH, Qin S, Minoshima Y, Kanekiyo M, Ren M, Dairiki R, Tamai T, Dutcus CE, Ikezawa H, Funahashi Y, Evans TRJ. Pharmacodynamic Biomarkers Predictive of Survival Benefit with Lenvatinib in Unresectable Hepatocellular Carcinoma: From the Phase III REFLECT Study. Clin Cancer Res. 2021;27:4848-4858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Liu JN, Li JJ, Yan S, Zhang GN, Yi PS. Transarterial chemoembolization combined with lenvatinib versus transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: A systematic review and meta-analysis. Front Oncol. 2023;13:1074793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 27. | Shimose S, Kawaguchi T, Tanaka M, Iwamoto H, Miyazaki K, Moriyama E, Suzuki H, Niizeki T, Shirono T, Nakano M, Suga H, Yamaguchi T, Yokokura Y, Noguchi K, Koga H, Torimura T. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: A multicenter cohort study using data mining analysis. Oncol Lett. 2020;20:2257-2265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Lee J, Sung PS, Yang H, Lee SK, Nam HC, Yoo SH, Lee HL, Kim HY, Lee SW, Kwon JH, Jang JW, Kim CW, Nam SW, Bae SH, Choi JY, Yoon SK. A Real-World Comparative Analysis of Lenvatinib and Sorafenib as a Salvage Therapy for Transarterial Treatments in Unresectable HCC. J Clin Med. 2020;9:4121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Arizumi T, Ueshima K, Chishina H, Kono M, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, Minami Y, Sakurai T, Nishida N, Kudo M. Validation of the criteria of transcatheter arterial chemoembolization failure or refractoriness in patients with advanced hepatocellular carcinoma proposed by the LCSGJ. Oncology. 2014;87 Suppl 1:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Cheng AL, Amarapurkar D, Chao Y, Chen PJ, Geschwind JF, Goh KL, Han KH, Kudo M, Lee HC, Lee RC, Lesmana LA, Lim HY, Paik SW, Poon RT, Tan CK, Tanwandee T, Teng G, Park JW. Re-evaluating transarterial chemoembolization for the treatment of hepatocellular carcinoma: Consensus recommendations and review by an International Expert Panel. Liver Int. 2014;34:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Raoul JL, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Cancer. 2014;3:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Abraham NS, Byrne CJ, Young JM, Solomon MJ. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol. 2010;63:238-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Shapiro S. Meta-analysis/Shmeta-analysis. Am J Epidemiol. 1994;140:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 202] [Article Influence: 6.5] [Reference Citation Analysis (0)] |