Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.105690
Revised: March 7, 2025
Accepted: April 17, 2025
Published online: June 15, 2025
Processing time: 130 Days and 5.7 Hours
Colorectal cancer (CRC) is one of the most prevalent malignancies worldwide, ranking among the highest in both incidence and mortality rates. Traditional Chinese medicine, with a history spanning thousands of years, has demonstrated unique efficacy and advantages in the prevention and treatment of CRC, playing a pivotal role at all levels of China’s healthcare system. This article provides a comprehensive analysis and summary of traditional Chinese medicine’s contributions to CRC prevention, antitumor therapy, palliative care for advanced tumors, perioperative rehabilitation, and postoperative functional recovery.
Core Tip: Colorectal cancer (CRC) is one of the most prevalent malignancies worldwide, ranking high in both incidence and mortality rates. With a history spanning thousands of years, traditional Chinese medicine has shown unique efficacy and advantages in CRC prevention and treatment, playing a crucial role across all levels of China’s healthcare system. This article offers a comprehensive analysis and summary of traditional Chinese medicine’s contributions to CRC prevention, antitumor therapy, palliative care for advanced tumors, perioperative rehabilitation, and postoperative functional recovery.
- Citation: Mo L, Wang Y, Liang XY, Zou T, Chen Y, Tan JY, Wen J, Jian XH. Progress of traditional Chinese medicine in the prevention and treatment of colorectal cancer. World J Gastrointest Oncol 2025; 17(6): 105690
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/105690.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.105690
Colorectal cancer (CRC) is one of the most prevalent malignancies worldwide, with the highest incidence and mortality rates[1]. With a history spanning millennia, traditional Chinese medicine (TCM) has consistently played a crucial role in China’s medical systems at all levels. An increasing body of research demonstrates that various TCM components can intervene and inhibit CRC proliferation and invasion through multiple mechanisms, offering unique efficacy and advantages in CRC prevention and treatment[2,3].
The therapeutic measures of TCM encompass internal treatments such as compound herbal formulas, single herbs, herbal extracts, and patented Chinese medicines, as well as external therapies like acupuncture, massage, and qigong. Clinical studies have confirmed the efficacy of TCM in increasing survival time and improving the quality of life for CRC patients[4], while also demonstrating high safety profiles[5]. In recent years, natural plant- and mineral-based TCMs, including their extracts, multi-herb compounds and patented formulations[6], have demonstrated potent anti-tumor effects. When combined with modern treatments such as radiotherapy, chemotherapy, targeted therapy, and immunotherapy, these TCM interventions have synergistic and attenuating effects[7], garnering significant attention for TCM as alternative or complementary treatments for CRC. However, the complexity of TCM components and its unique theoretical framework, which emphasizes individualized treatment based on syndrome differentiation, pose significant challenges to high-quality clinical research and drug development. The difficulty in generating robust evidence largely continues to hinder the objective and standardized advancement of TCM.
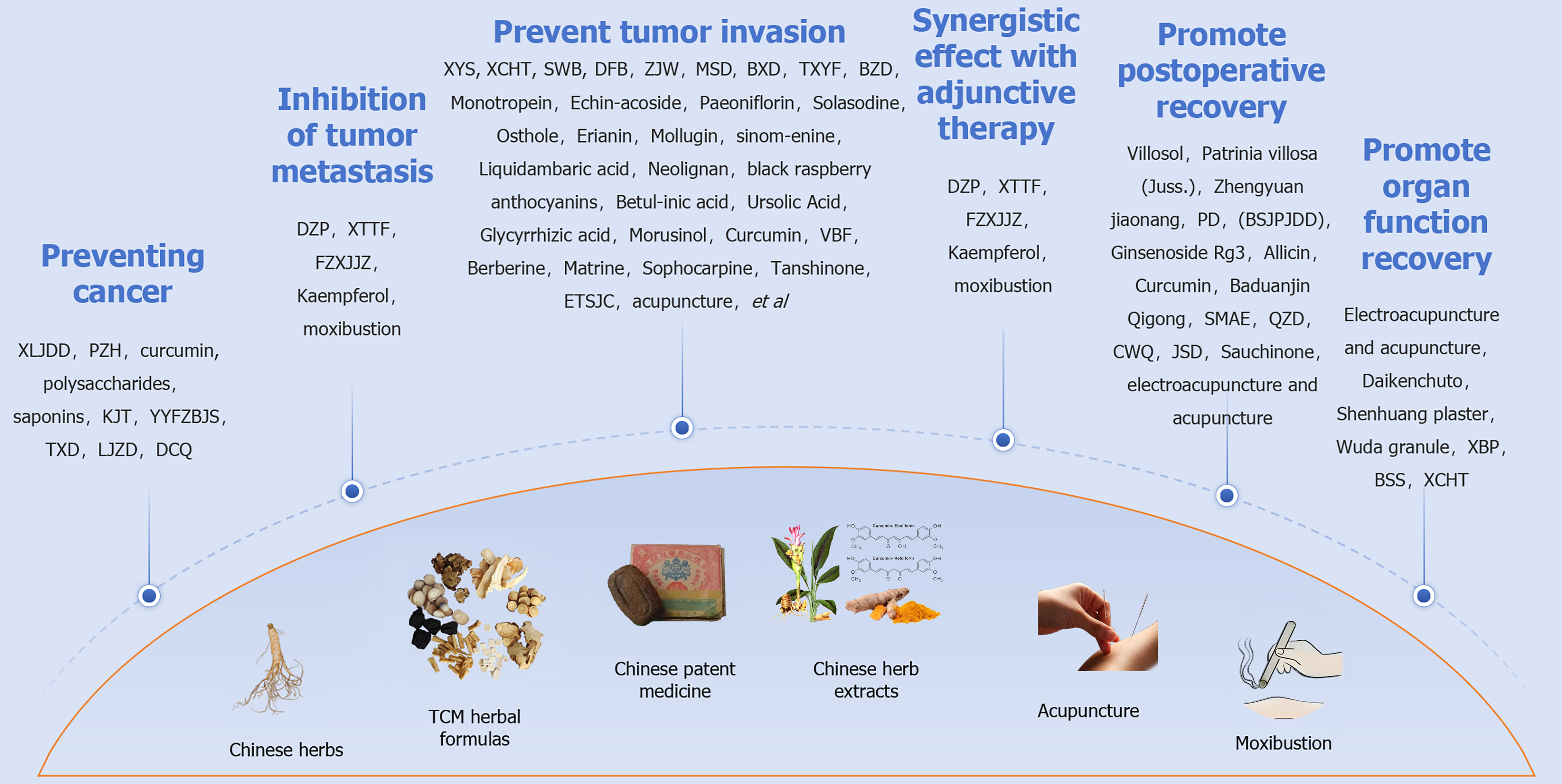
With the ongoing and increasingly integrated application of TCM and modern medical treatments, numerous meaningful therapeutic collaborations have been implemented and validated, yielding positive outcomes. Consequently, an integrated system of TCM and Western medicine with distinct Chinese characteristics has emerged, becoming one of the primary medical models in China[8]. This article aims to systematically review and analyze the application of TCM in preventing high-risk factors for cancer development, anti-tumor treatment, palliative and maintenance therapy for advanced tumors, perioperative enhanced recovery, and postoperative functional rehabilitation. The goal is to provide a comprehensive reference for understanding the role of TCM in these areas (Figure 1).
TCM possesses a comprehensive theoretical framework, placing particular emphasis on the holistic concept and syndrome differentiation, disease prevention and treatment, utilizing measures to prevent disease occurrence and their further development and progression. Previous studies have demonstrated that patients with type 2 diabetes who underwent TCM treatment for over 1 year exhibited a lower incidence of rectal cancer compared to those who did not receive such treatment[9]. Multi-center clinical studies have revealed that TCM compounds exhibit favorable efficacy in preventing the recurrence and malignant transformation of colorectal adenomatous polyps following resection[10-13].
The gut microbiota comprises a complex ecological system and plays a dual role in promoting and inhibiting tumor progression. It directly or indirectly influences CRC occurrence and development. The intestinal microbiota is closely associated with various organs in the human body, forming “gut-liver” and “lung-gut” axes that profoundly impact the immune system. TCM can regulate intestinal flora to inhibit carcinogenesis. For instance, Xianlian Jiedu decoction[14] effectively reduces inflammation, restores intestinal microbial balance , and improves metabolic disorders to inhibit colon inner wall tumor formation. Pien Tze Huang[15,16] enhances intestinal flora and metabolites while repairing intestinal barrier function. It also suppresses carcinogenic and pro-inflammatory pathways to prevent CRC by inhibiting the signal transducer and activator of transcription 3 (STAT3) pathway and regulating the transforming growth factor (TGF)-β1/zinc finger E-box-binding homeobox/miR-200 signaling network, thereby promoting cancer cell apoptosis and inhibiting proliferation and metastasis.
Specific constituents, such as curcumin[17,18], polysaccharides[19,20] (apple polysaccharide and mushroom glucan), and saponins[21,22] (Paris saponins, ginsenosides, and soy saponins) in medicinal and food homologous plants like fruits, vegetables, and spices, also exhibit preventive effects against CRC. The underlying mechanism involves modulating the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mechanistic target of rapamycin (mTOR) pathway, thereby inhibiting colon cancer cell proliferation and inducing apoptosis. Several healthy beverages, including green tea[23-25], have been consumed in China for millennia and are believed to possess preventive effects against CRC.
Certain Chinese medicine prescriptions can modulate the body’s immune system, exerting inhibitory effects on cancer. Notably, Yi-Yi-Fu-Zi-Bai-Jiang-San[26-29] significantly enhances mouse immunity and suppresses carcinogenesis and tumor progression. Additionally, TiaochangXiaoliu decoction[30] reduces serum levels of interferon-γ, interleukin (IL)-2, and tumor necrosis factor-α while increasing CD4(+), CD8(+), CD4(+)/CD8(+) ratios, as well as natural killer cell content in plasma. These findings highlight the potential of immune modulation in inhibiting ammonia methane/dextran sulfate sodium (DSS)-induced CRC.
Inflammatory bowel disease, including ulcerative colitis and Crohn’s disease, is associated with a high risk of cancer development. The role of TCM in treating inflammatory bowel disease has garnered significant attention[31]. Experimental studies have established a mouse model for colitis-associated cancer transformation, where intervention with Liujunzi decoction[32] demonstrated effective inhibition of cancer transformation in inflammatory bowel disease. Berberine (BBR) can reduce colitis-related colorectal neoplasia by modulating intestinal flora and regulating short-chain fatty acid metabolism[33,34]. Shaoyao decoction[35,36], commonly used to treat inflammatory bowel disease associated with dampness and heat accumulation, exerts its therapeutic effects by regulating the Kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response elements signaling pathway. This down-regulates pro-inflammatory cytokine release, inhibits inflammation, and prevents cell damage caused by oxidative stress. Additionally, Shaoyao decoction inhibits epithelial-mesenchymal transition (EMT) to prevent and treat inflammatory bowel diseases-related CRC. Dachengqi decoction[37] is primarily composed of flavonoids and anthraquinones, and it exhibits anti-tumor effects by potentially modulating cell proliferation and apoptosis while decreasing inflammation. Danggui Buxue Tang has been shown to possess anti-inflammatory and anti-cancer properties, potentially mitigating the inflammation-to-cancer transformation in ulcerative colitis. Additionally, Danggui Buxue Tang can enhance the efficacy of 5-fluorouracil (5-FU) by modulating the c-Jun N-terminal kinase-mediated signal transduction pathways[38,39].
Tumor invasion and metastasis constitute a highly intricate process. The initial step involves attenuating autophagy and apoptosis, thereby enhancing cancer cell proliferation. Subsequently, this facilitates angiogenesis, enabling increased nutrient absorption by the tumor from its microenvironment and subsequently stimulating cancer cell growth.
The anti-tumor mechanism of TCM is intricate, encompassing the regulation of various signaling pathways including PI3K/Akt, nuclear factor kappa B (NF-κB), mitogen-activated protein kinases (MAPK), Wnt/β-catenin, epidermal growth factor receptor, p53, TGF-β, mTOR, Hedgehog and immune regulatory signaling pathways to modulate CRC proliferation, apoptosis, cell cycle progression, migration and invasion, as well as autophagy induction and EMT[40-42]. Moreover, it primarily focuses on the following aspects[43,44]: (1) Modulating the intestinal microorganism’s internal environment; (2) Inducing apoptosis while inhibiting proliferation; (3) Suppressing tumor metastasis; and (4) Inhibiting EMT occurrence. Additionally, it can also regulate macrophage activity and intervene in the CRC microenvironment[45], thereby regulating body lipid metabolism mechanisms[46] and exhibiting antitumor activity.
Disorders in the intestinal microbiota have been implicated in promoting tumor progression. TCM can modulate cancer progression by regulating intestinal flora composition[47-50] and structure, while altering endogenous metabolite levels. These changes subsequently impact various aspects of intestinal barriers, immune system function, and systemic metabolism, thereby exerting anti-tumor effects[51,52]. Glucose, lipids, and amino acid metabolism play a crucial role in cancer metastasis by influencing cancer cell metabolism and the tumor microenvironment (TME). TCM and its bioactive compounds hold great potential for clinical applications in treating cancer metastasis[53,54].
TCM prescriptions: The prescription of TCM is the cornerstone of its treatment approach, distinguished by its extensive clinical application over thousands of years, complex composition, and synergistic effects that target multiple aspects of health (Table 1).
| Metal | Metal nanomaterials | Role of nanomaterials | Characteristics of nanomaterials | Ref. |
| Au | AuNPs spheres | Cytotoxicity: AuNPs stars were the most toxic, while AuNPs spheres were the least toxic. Induction of apoptosis. Drug delivery. Photothermal therapy | Small size, large specific surface area, good biocompatibility. The optical properties and cytotoxicity are shape and size dependent. Strong penetration | [19] |
| AuNPs rods | ||||
| AuNPs stars | ||||
| AuNPs | Cytotoxicity. Surface plasmon resonance: AuNPs have a strong surface plasmon resonance effect, which can be detected by ultraviolet-visible spectroscopy. Medium or drug carrier for photothermal therapy | Size dependence. Biocompatibility. Cell uptake efficiency is size-dependent | [20] | |
| AuNPs | Enhance the effect of hyperthermia. Enhance the effect of chemotherapy. Combined therapy: AuNPs showed synergistic effect when combined with microwave hyperthermia and chemotherapy | Size dependence. Good biocompatibility. Photothermal conversion capability. Drug delivery potential | [21] | |
| Ph-AuNPs | Antitumor activity. Cytotoxicity. Cell cycle arrest. Induction of apoptosis | Small and uniform size. Good biocompatibility. Surface modification. High stability. Environmental protection and low cost | [22] | |
| “Hedgehog ball” shaped nanoprobes (Fe3O4@Au-pep-CQDs) | Detection of inflammatory markers. Multimodal imaging. Tumor microenvironment monitoring | Multi-modal detection capability. Magnetic separation function. High sensitivity and specificity. Good biocompatibility. Targeting | [23] | |
| Glycogenic AuNPs | Anticancer activity. Good biocompatibility. Cellular uptake | Controllable size. Surface modification. Selective toxicity. Fluorescence characteristics | [24] | |
| Protein-coated AuNPs | Drug carrier. Fluorescent labeling diagnosis. Enhanced cellular uptake | Controllable size and surface charge. Good biocompatibility. High cellular uptake efficiency | [25] | |
| B-AuNPs conjugated CIS and DOX | Drug delivery. Enhance drug stability. Reduce side effects | Good biocompatibility and low toxicity. Surface chemical properties can be adjusted. Capable of loading a large number of drug molecules. It can accumulate in tumor tissues | [26] | |
| Au-GSH NPs | Inhibition of tumor cell extravasation. No toxicity. Enhanced cellular uptake and accumulation | Small size, good dispersion and stability. Negatively charged surface, which facilitates cellular uptake. No toxicity | [27] | |
| AuNR@SiO2 | Chemotherapy: DOX loading. Photothermal therapy. Anti-angiogenesis. Targeted delivery | Photothermal response. Drug carrier. Targeting. Adjustable degradation | [29] | |
| AuNPs functionalized carborane complex | BNCT treatment. Imaging and diagnosis. Drug delivery | Good biocompatibility. Targeting. Photothermal response. Adjustable water solubility | [30] | |
| PtU2-AuNPs | Drug delivery. Enhanced cytotoxicity. Targeted therapy | Biocompatibility. Drug carrier function. Enhanced cellular uptake. Enhanced cytotoxicity | [31] | |
| Au@MMSN-Ald | Bone-targeted therapy. Integrated chemotherapy and chemodynamic therapy. Dual-modality imaging. Responsive drug release | Versatility. Efficient drug release in the tumor microenvironment. Efficient targeting ability. Imaging ability | [32] | |
| Ag | AgNPs | Antibacterial effect. Cytotoxicity. Induction of oxidative stress. Cell cycle regulation | High surface area. Quantum size effect. Surface effect. Size and shape dependence. Good biocompatibility | [33] |
| AgNPs | Cytotoxicity. Antibacterial effect. Induction of apoptosis. Affect the cell cycle. Oxidative stress induction | High surface area. Quantum size effect. Surface effect. Size and shape dependence. Good biocompatibility. Cellular uptake capacity. Induction of oxidative stress | [34] | |
| AgNPs | Cytotoxicity. Antibacterial effect. Induction of apoptosis. Affect the cell cycle. Oxidative stress induction. Genotoxicity. Drug delivery vehicles | High surface area. Quantum size effect. Surface effect. Size and shape dependence. Good biocompatibility. Cellular uptake capacity. Induction of oxidative stress. Cell-specific responses | [35] | |
| AgNPs synthesized from plant extracts | Antimicrobial activity. Anti-proliferative activity. Drug delivery. Bioimaging | High surface area. Quantum size effect. Surface effect. Size and shape dependence. Good biocompatibility. Versatility. Environmentally friendly. Diversity of antimicrobial mechanisms | [36] | |
| AgNPs synthesized from leaf extracts | Antimicrobial activity. Anti-proliferative activity. Drug delivery. Bioimaging. Antioxidant activity | High surface area. Quantum size effect. Surface effect. Size and shape dependence. Good biocompatibility. Versatility. Environmentally friendly. Diversity of antimicrobial mechanisms | [37] | |
| AgNPs synthesized from grapefruit extracts | Antimicrobial activity. Anti-proliferative activity. Drug delivery. Bioimaging. Antioxidant activity | High surface area. Quantum size effect. Surface effect. Size and shape dependence. Good biocompatibility. Versatility. Environmentally friendly. Diversity of antimicrobial mechanisms | [38] | |
| AgNPs and cAgNPs synthesized by Bacillus cereus | Antimicrobial activity. Anti-proliferative activity. Drug delivery. Bioimaging. Induction of apoptosis. Reduced normal cytotoxicity | High surface area. Quantum size effect. Surface effect. Size and shape dependence. Good biocompatibility. Versatility. Environmentally friendly. Diversity of antimicrobial mechanisms | [39] | |
| AgNPs coating | Antibacterial property. Biocompatibility. Drug release. Surface modification | Size effect. Surface activity. Concentration dependence. Shape dependence | [40] | |
| f-HAp/PVP/Ag NPs | Antibacterial property. Biocompatibility. Drug release. Surface modification | Size effect. Surface can be modified. Concentration dependence. Shape dependence | [41] | |
| Cu | Cu-Fe3O4 NCs-AS-ALG | Catalytic activity. Enhanced oxidative stress. Induction of apoptosis. Targeted delivery. Good biocompatibility | High surface area. Surface activity. Surface can be modified. Concentration dependence. Shape dependence | [42] |
| Cu-Cy NPs | Photodynamic therapy. Antitumor activity. Reactive ROS generation | High surface area. Photosensitive characteristics: Microwave activation. Good biocompatibility. Versatility: Combined thermal and chemical effects | [43] | |
| Fe | “Hedgehog ball” shaped nanoprobes (Fe3O4@Au-pep-CQDs) | Detection of inflammatory markers. Multimodal imaging. Tumor microenvironment monitoring | Multi-modal detection capability. Magnetic separation function. High sensitivity and specificity. Good biocompatibility. Targeting | [23] |
| Cu-Fe3O4 NCs-AS-ALG | Catalytic activity. Enhanced oxidative stress. Induction of apoptosis. Targeted delivery. Good biocompatibility | High surface area. Surface activity. Surface can be modified. Concentration dependence. Shape dependence | [42] | |
| Zn-Fe3O4 NPsCo-Fe3O4NPs | Magnetic moment and magnetic anisotropy. Magnetic heating effect. Cell labeling and imaging. Drug delivery. Cell therapy. Good biocompatibility. Ion release. Cytotoxicity | High surface area. Surface activity. Concentration dependence. Magnetic properties. Shape dependence. Surface modification. Stability | [44] | |
| Carbon-coated iron oxide NPs | Contrast enhancement on MRI. Drug delivery. Magnetic heating therapy. Cell isolation and labeling. Cell viability and toxicity | High surface area. Surface activity. Size dependence. Concentration dependence. Good biocompatibility. Shape dependence. Surface modification. Stability | [45] | |
| Iron oxide NPs (IO-cage and IO-sphere) | Enhance drug loading. Enhance drug stability. Targeted therapy. Induction of apoptosis. Reduce tumor volume. Bioluminescence imaging. Immune escape | High surface area. Shape effect. Surface modification. Stability. Targeting. Good biocompatibility | [46] | |
| Fe3O4 NPs | Endocytosis promoted by electrical stimulation. MRI signal enhancement. Cell labeling and tracking. Magnetic heating therapy. Drug delivery | High surface area. Shape affects the endocytic mechanism. Surface modification. Targeting. Enhancing effect of electrical stimulation | [47] | |
| Superparamagnetic iron oxide NPs | Biomedical imaging. Magnetic thermotherapy. Biosensing and diagnostics. Support for tissue repair and regeneration. Fight infections | Small size effect. Surface effect. Quantum size effect. Macroscopic quantum tunneling. Good biocompatibility. Adjustability | [48] | |
| FeHA and Mag@CaP NPs | Enhanced MRI and ultrasound imaging. Drug carrier. Bone tissue repair and regeneration. Biosensors. Fight infections | Small size effect. Surface effect. Quantum size effect. Versatility. Good biocompatibility. Adjustability | [49] | |
| Fe3O4@ZIF-8 NPs | Improve drug targeting. Enhance drug stability. Ph-responsive release. Enhanced MRI and optical imaging. Chemotherapy intensification. Bone tissue repair and regeneration | Small size effect. Surface effect. Quantum size effect. Versatility. Good biocompatibility. Adjustability | [50] | |
| HA@MOF/D-Arg NPs | Improve drug targeting. Enhance drug stability. Ph-responsive release. Enhanced MRI imaging. Enhanced chemotherapy and radiotherapy. Alleviate tumor hypoxia. Bone tissue repair and regeneration | Small size effect. Surface effect. Quantum size effect. Versatility. Good biocompatibility. Adjustability | [51] | |
| D-Arg/GOX/TPZ@PDA/MOF NPs | Improve drug targeting. Enhance the efficacy of medications. Ph-responsive release. Enhanced MRI imaging. Enhanced chemotherapy and radiotherapy. Starvation therapy. Gas therapy. Bone tissue repair and regeneration | Small size effect. Surface effect. Quantum size effect. Versatility. Good biocompatibility. Adjustability | [52] | |
| FePt/MnO2@PEG NPs | Improve drug targeting. Prolong the drug circulation time. Enhanced MRI imaging. Radiotherapy enhancement. Alleviate tumor hypoxia. Induction of ferroptosis | Small size effect. Surface effect. Quantum size effect. Versatility. Good biocompatibility. Adjustability | [53] | |
| BTZ/TA/Fe3+ NPs | Improve drug stability. Control drug release. Enhance the efficacy of medications. Reduce side effects. Improve drug delivery efficiency | pH sensitivity. High drug loading efficiency and drug loading capacity. Good biocompatibility. Stability. Adjustability | [54] | |
| FeS2@CP NPs | Photothermal therapy. Chemo-dynamic therapy. Collaborative therapy. Tumor microenvironment responsiveness | Efficient Fenton catalytic activity. High photothermal conversion efficiency. Good biocompatibility. Versatility. Degradability | [55] | |
| Ca | HA-BSA-PTX NPs | Cytotoxicity. Cell cycle arrest. Inhibition of cell migration and invasion. Promote osteoblast differentiation. Regulation of osteogenic gene expression. Reduce systemic toxicity | Efficient drug loading and release. Good biocompatibility and degradability. Versatility | [56] |
| CaF2:Eu NPs | Enhance the effect of adjuvant radiotherapy. DNA damage. Inhibition of tumor growth. Reduce tumor recurrence and metastasis | Photoluminescence characteristics. Good biocompatibility. Selective toxicity. Radioenhancement characteristics | [57] | |
| Zn | ZnO NPs | Induction of apoptosis. Induction of autophagy. Oxidative stress and cell death. Interaction between autophagy and apoptosis | Stability. Good biocompatibility. Zinc ion release characteristics. Effect on cell cycle. Selective toxicity | [58] |
| ZnO NPs | Ultrasound-assisted water oxidation. ROS generation. Inhibition of tumor cell growth. Induction of apoptosis | Crystallinity. Optical properties. Piezoelectric characteristics. Catalytic activity. Surface modification and reactivity | [59] | |
| ZnO NPs | ROS production. Induction of apoptosis. Antitumor activity | Crystallinity. Optical properties. Green synthesis, environmental protection and low cost. Surface chemical properties | [60] | |
| ZnTiO3 NPs | Antibacterial. ROS generation. Cytotoxicity | Broad-spectrum antibacterial activity. Mechanical properties. Good biocompatibility | [61] | |
| Ti-ZnO-PBA-NG NPs | Antibacterial. Increased intracellular ROS levels. Induction of tumor cell apoptosis. Inhibition of tumor cell proliferation. Promote the proliferation and differentiation of osteoblasts | pH responsiveness. Antibacterial property. Anti-tumor properties. Good biocompatibility. Surface modification | [62] | |
| ZnO NPs synthesized from Rehmannia | Anticancer activity. Induction of apoptosis. ROS production. Mitochondrial membrane potential changes | Green synthesis. Good biocompatibility. Crystal structure. Bioactive ingredients. Photocatalytic activity | [63] | |
| Ti | TiO2 NPs | Acoustic catalytic activity. ROS produced. Inhibition of tumor cell growth and proliferation | Semiconductor properties. Amorphous structure. Optical inertia | [59] |
| ZnTiO3 NPs | Antibacterial. ROS generation. Cytotoxicity | Broad-spectrum antibacterial activity. Mechanical properties. Good biocompatibility | [61] | |
| TiO2 NPs | Induction of cytotoxicity. ROS production. Decrease GSH levels | Large surface area. Semiconductor properties. Good biocompatibility. Photocatalytic activity | [69] | |
| TiO2 NPs | Photocatalytic activity. Efficient generation of ROS under microwave. Selective toxicity. Microwave induced photodynamic therapy. Inhibition of osteosarcoma tumor growth | Clear lattice stripes. Surface charge. Good biocompatibility. Stability | [70] | |
| TiO2 NPs | Broad-spectrum antibacterial property. Cytotoxicity. Photocatalytic performance | Green synthesis, low environmental protection cost. Tetragonal crystal structure. Optical properties. Chemical stability | [71] | |
| TiO2 NPs | Broad-spectrum antibacterial property. Antitumor activity. ROS production. Application of bioanalog scaffolds. Biological activity. Mechanical properties. Protein adsorption capacity | Anatase phase structure of tetragonal crystal system. Low environmental cost. Photocatalytic activity. Thermal stability | [72] | |
| Fluorescent TiO2 NPs | Broad-spectrum antibacterial property. ROS produced. Drug carrier. Intracellular drug tracking and fluorescence imaging | Green synthesis, low environmental protection cost. Surface charge. Fluorescence characteristics. High drug load | [73] | |
| F-TiO2/P and F-TiO2/PC | Photothermal effect and photocatalytic effect. ROS production. Promote osteogenic differentiation. Antitumor activity | Good photothermal stability. Good photocatalytic performance. Hydrophilicity. Multifunctional integration. Surface modification | [74] | |
| TiO2 NPs | Photocatalytic degradation. Cytotoxicity. Photodynamic therapy. Antibacterial property. Oxidative stress | Photocatalytic activity. High specific surface area. Be versatile | [75] | |
| Folic acid modified TiO2 NPs | Cytotoxicity. Generation of ROS. Induction of apoptosis. Enhanced cellular uptake | Good dispersion. Tumor targeting. Surface charge | [76] | |
| Pt | PtU2-AuNPs | Drug delivery. Enhanced cytotoxicity. Targeted therapy | Biocompatibility. Drug carrier function. Enhanced cellular uptake. Enhanced cytotoxicity | [31] |
| FePt/MnO2@PEG NPs | Improve drug targeting. Prolong the drug circulation time. Enhanced MRI imaging. Radiotherapy enhancement. Alleviate tumor hypoxia. Induction of ferroptosis | Small size effect. Surface effect. Quantum size effect. Versatility. Good biocompatibility. Adjustability | [53] | |
| ALN-PtIV-Lipo | Enhance the effect of chemotherapy. Inhibits bone destruction. Targeted delivery. Drug accumulation. Tumor growth inhibition | Stability. Targeting. Good biological safety. Drug release | [77] | |
| Sr | Sr-HA NPs | Promote bone regeneration. Improve cell activity. Drug delivery | Stability. Surface charge. Good biocompatibility | [78] |
| Ir | IrO2@ZIF-8/BSA-FA | Photothermal therapy. Photodynamic therapy. Improve the efficiency of cancer treatment. Tumor targeting. Alleviate tumor hypoxia | Versatility. Photothermal conversion capability. Catalase like activity. Targeting. High drug loading capacity. Dual pH/NIR responsiveness. Good biocompatibility | [90] |
| BSA-IrO2 NPs | Chemotherapy drug carrier. Photothermal therapy. Collaborative therapy. Increase circulation time. Tumor targeting | Stability. Photothermal conversion capability. High drug loading capacity. Dual pH/NIR responsiveness. Good biocompatibility | [91] |
The primary constituent of Gegen Qinlian decoction[51,55] is Radix Pueraria[56], and its active compound is puerarin[57,58], which both exhibit significant anti-tumor effects. The underlying mechanisms involve modulation of the intestinal microbiota and alteration of the TME. Small bupleurum decoction, known as Xiao Chai Hu Tang[59,60], modulates the gut microbiota and regulates the Toll-like receptor 4/myeloid differentiation marker 88/NF-κB signaling pathways, thereby restoring intestinal flora balance by reducing the abundance of pathogenic bacteria, algae, and mucor. This intervention improves the luminal environment and exhibits antitumor and anti-anxiety properties. Xiaoyaosan[61] regulates the abundance of Bacteroides, Lactobacillus, Desulfovibrio, and Ricinidae in the intestine to ameliorate dysbiosis and effectively mitigate CRC progression in CRS-treated mice. Sanwu Baisan decoction[62] demonstrates potent antitumor efficacy in CRC mice by enhancing the secretion of anti-tumor immune cytokines, inducing cancer cell apoptosis, maintaining intestinal microbiota homeostasis, and inhibiting tumorigenesis via modulation of the Toll-like receptor 4/cyclooxygenase-2 (COX-2)/prostaglandin E2 pathway.
Recently, Dahuang Fuzi Baijiang decoction[63] was shown to inhibit the production of C-C motif ligand 2 and protect Tex+ progenitor cells in the obese microenvironment, thereby impeding CRC progression. Zuojin capsule[64,65] has been widely used to treat CRC due to its ability to target CDKN1A, Bcl2, E2F1, PRKCB, MYC, CDK2 and matrix metalloproteinase-9 (MMP9). Additionally, it inhibits the 5-HTR1D-Wnt/β-catenin signaling pathway. Shenlingbaizhu powder and its Modified Shenlingbaizhu decoction[66] effectively suppress CRC growth by inhibiting TGF-β-induced EMT. The anti-tumor mechanism of Banxia Xiexin decoction[67] involves targeting ferroautophagy mediated by the PI3K/AKT/mTOR axis. This leads to intracellular iron accumulation and generation of reactive oxygen species, ultimately resulting in ferroptosis. Tong-Xie-Yao-Fang[68] can inhibit tumor growth in CRC mice by stimulating immune responses by inhibiting hypothalamic-pituitary-adrenal axis hormone secretion and promoting mature dendritic cell activation and T cell function. Bazhen decoction[69] can reverse the abnormal expression of genes such as PI3K, AKT, MYC, epidermal growth factor receptor, hypoxia-inducible factor 1α (HIF-1α), VEGF receptor (VEGFR), JUN, STAT3, CASP3, and TP53, and regulate signaling pathways such as PI3K-AKT, P53, and VEGF to inhibit CRC progression.
TCM extract or monomer composition: Many active TCM ingredients hold potent anti-CRC activity, as demonstrated by experimental studies[3]. Notably, common compounds such as flavonoids and phenolic acids have significant anticancer effects. These bioactive components influence CRC progression through multiple mechanisms, including the regulation of molecular and signaling pathways, modulation of gut microbiota, and targeting of cancer stem cells (CSCs), all of which are critical in CRC pathogenesis.
Resveratrol, a natural compound found in grapes[70], can inhibit CRC EMT by modulating the TGF-β1/Smad signaling pathway and q/E-calcium protein. Additionally, it enhances autophagy-related cell apoptosis in CRC. Curcumin[71-73], the principal bioactive constituent of ginger with medicinal and dietary origins, possesses a rich pharmaceutical history in treating intestinal diseases. It can disrupt EMT by promoting HIF-1α degradation, inhibit CRC cell invasion and migration, and induce cell cycle arrest and apoptosis by suppressing glutaminase 1-mediated glutamine metabolism. Additionally, curcumin can enhance the anti-cancer efficacy of chemotherapeutic agents and exhibit a synergistic effect with Astragalus extract[18]. Its mechanism of action involves effectively reversing PI3K/Akt pathway activation, reducing intestinal inflammation and improving intestinal barrier function.
Additionally, mignonette and luteolin[74,75] can inhibit the MAPK pathway in CRC cells, decreasing cell proliferation, inducing cell cycle arrest, apoptosis and DNA damage progression. Moreover, they can also inhibit CRC metastasis by regulating the miR-384/PLEKHM1 axis. On the other hand, quercetin[76-81] exhibits potent anticancer activity through several signaling pathways, including MAPK/extracellular regulated kinase 1/2 (ERK1/2), p53, Janus kinase (JAK)/STAT and TNF-related apoptosis-inducing ligand, AMP-activated protein kinase α1/apoptosis signal-regulating kinase 1/p38 as well as receptor for advanced glycosylation end products/PI3K/AKT/mTOR. It also affects the high mobility group box 1 and NF-κB signaling pathways, along with Nrf2-induced signaling pathways, resulting in cell cycle arrest and subsequent death of CRC cells[81].
The plant-derived benzyl xenobiotic BBR[82] is a novel multi-target receptor tyrosine kinase inhibitor exhibiting potent activity against various malignant tumors, including CRC. It effectively suppresses cell proliferation, invasion, and metastasis while inducing apoptosis, regulating cell metabolism, blocking cell cycle progression, inhibiting angiogenesis and enhancing chemosensitivity. Studies have demonstrated that BBR can inhibit CRC cell growth, migration, invasion and colony formation without exerting toxicity on normal colon cells[83]. Moreover, in vitro experiments reveal its ability to induce apoptosis and cell cycle arrest at G(0)/G(1) in CRC cells, along with attenuated Hedgehog signaling pathway activity. Furthermore, BBR may prevent high fat diet-induced CRC by modulating intestinal microbiota-mediated lysophosphatidylcholine metabolism[84]. Additionally, it impedes CRC invasion and metastasis by inhibiting receptor tyrosine kinases/Akt axis expression[85], COX-2/prostaglandin E2-JAK2/STAT3 signaling pathway activation, and by downregulating helix-loop-helix transcription factor with YRPW motif 2 hub gene expression and metastasis-related proteins E-cadherin β-catenin[86] and cyclin D1 levels[87]. The antitumor activity of matrine and sophocarpine[88] has been investigated in various cancers, including CRC. Their mechanism of action involves interference with the miR-10b/phosphatase and tensin homolog deleted on chromosome 10 pathway[89], down-regulation of p53 and checkpoint kinase 1 expression[90], regulation of β-catenin/c-Myc signaling, and inhibition of the mitogen-activated protein kinase kinase/ERK/vascular endothelial growth factor (VEGF) pathway to disrupt metabolic reprogramming and EMT in hepatocellular carcinoma[91]. Other TCM and food extracts, including toosendanin[92], raddeanin A[93], and allicin[94], exhibit comparable anti-CRC effects.
Saponins are the primary constituents of numerous herbal medicines that exhibit inhibitory effects on CSCs. Compounds such as ginsenoside[95-97], Paris saponin VII[98], active ingredients in Rhizoma Paridis[99], notoginsenoside[100], and naringin[101] can suppress CRC cell growth and proliferation, induce apoptosis, and inhibit essential signaling pathways, including IL-6/STAT3 and Akt/mTOR/p70S6K, among others. Monotropein[102] exerts its anti-tumor effects by inhibiting the cell cycle, inducing apoptosis, and suppressing cell migration. Astragaloside[103] and its extract[104] also possess potent anti-CRC properties, which are associated with the regulation of the circ_0001615 and miR-873-5p/LIM and SH3 protein 1 axis. Additionally, these compounds can inhibit CRC metastasis by reducing extracellular vesicle release and suppressing M2-type tumor-associated macrophage activation[105]. Echinacoside[106] exhibits anti-tumor metastatic effects by promoting the growth of butyrate-producing gut bacteria, which subsequently down-regulates PI3K/Akt signaling and EMT.
The anti-tumor effect of paeoniflorin[107,108] may be attributed to its ability to inhibit the IL-6/STAT3 signaling pathway and reduce IL-17 levels. Additionally, it can regulate the miR-3194-5p/CTNNBIP1/Wnt/β-catenin axis to suppress CRC cell stemness. Moreover, paeoniflorin can impede CSC migration and invasion by downregulating histone deacetylase 2 expression, thereby reversing EMT and modulating cellular levels of E-cadherin and vimentin, ultimately leading to potent anti-tumor effects. Artesunate[109] and ziyuglycoside II[110] both suppress CRC cell proliferation by inducing reactive oxygen species-dependent cellular senescence and autophagy.
Plant-derived pentacyclic triterpenoids such as betulinic acid[111] and ursolic acid exhibit significant anti-tumor activity and hold promise in CRC prevention and treatment. The latter can induce caspase-dependent apoptosis, focal adhesion kinase/PI3K/Akt single spring cleavage, and EMT-related anoikis in human colorectal tumor cells. Inhibition of EMT[112] and Smoothened activates Akt signaling dependence, independent of the canonical Hedgehog pathway[113], regulating the miR-140-5p/TGF-β3 axis to suppress Wnt/β-catenin signaling pathway activation[114] and thereby inhibiting CRC cell proliferation and cell cycle progression while promoting cellular apoptosis. Glycyrrhizic acid can inhibit CRC progression through the AKT1/ERK1/2-glycogen synthase kinase-3β-β-catenin signaling pathway[115]. Additionally, glycyrrhizic acid can attenuate Musashi homolog 2-mediated immunopathology and immune infiltration in CRC in vitro and in vivo by inhibiting sirtuin 3 and blocking high mobility group box 1 activity[116,117]. Furthermore, it reduces the formation of neutrophil extracellular traps by inhibiting peptidylarginine deaminase 4, thereby improving colitis-associated CRC[118].
Triterpene compounds derived from Rhus chinensis extract[119,120] can effectively suppress glycolysis and glutamine pathways by targeting key enzymes including Enolase 1, aldolase A, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3, pyruvate kinase M2, and lactate dehydrogenase A to inhibit CRC. Furthermore, triterpene significantly impedes CRC cell growth and invasion by inhibiting the acid-sensing ion channel 2-induced calcineurin/nuclear factor of activated T cells pathway. Known for its spleen-strengthening and qi-replenishing properties, Jujube extract containing bioactive triterpenes[121] alleviates colorectal pixu (spleen deficiency)-associated inflammation, while also exerting an inhibitory effect on CRC via modulation of the PI3K/Akt/NF-κB signaling pathway.
Cucurbitacin B[122] can target the JAK2/STAT3 signaling pathway in CRC, thereby controlling M2 macrophage polarization and inhibiting CRC invasion and metastasis, similar to the anti-CRC effects observed with the active components of Yunnan Gargaria (YAT-17)[123,124]. Emodin, a primary constituent of TCM rhubarb, suppresses CRC proliferation and invasion by inhibiting acyl-coenzyme A synthetase long-chain family member 4 and reducing VEGF secretion and VEGFR1/VEGFR2 expression[125-127]. Additionally, emodin induces CRC cell ferroptosis via nuclear receptor coactivator 4-mediated ferritinophagy and NF-κB pathway inactivation. Shikonin, a natural quinone compound[128], possesses anti-tumor properties and can prevent acute ulcerative colitis. It upregulates 70 kDa heat shock protein to enhance immunogenicity and improve the effectiveness of programmed death receptor 1 (PD-1) blockade in CRC. Shikonin’s anti-tumor mechanism involves targeting IL6 and inhibiting the IL6R/PI3K/Akt signaling pathway, which curtails CRC cell proliferation, migration, and invasion while promoting apoptosis[129]. Total coumarins inhibit colorectal tumor growth by modulating exosome microRNA expression and CSC angiogenesis and by inducing apoptosis and autophagy[130,131].
Kras-mutant cancers exhibit heightened vulnerability to iron-dependent cell death, specifically ferroptosis, which is driven by lipid peroxidation. Osthole exerts its anti-cancer effects in KRAS-mutated CRC cells by inhibiting the AMP-activated protein kinase/Akt/mTOR signaling pathway, thereby promoting iron dislocation[132]. Similarly, erianin[133-136] suppresses CRC tumor growth and metastasis through autophagy-dependent siderosis, modulating the TMPO-AS1/miR-126-3p/PIK3R2 axis and inactivating the PI3K/Akt signaling pathway. Alizarin mollugin[137] curtails CSC proliferation and induces ferroptosis by targeting the insulin-like growth factor (IGF) 2 mRNA binding protein 3/peroxiredoxin 4 axis. Sinomenine[138], a bioactive alkaloid from TCM, inhibits cancer cell proliferation via reactive oxygen species production, ferritin phagocytosis, and iron deposition.
The Wnt/β-catenin signaling pathway is a well-established driver of colon cancer. However, targeted therapies against the pathway have not yet reached clinical application. The natural compound liquidambaric acid[139] directly targets tumor necrosis factor receptor-associated factor 2, inhibiting oncogenic Wnt/β-catenin signaling both in vitro and in vivo, and suppressing CSC growth. Neolignan[140], an extract from Rhizoma radix, also interferes with CRC development by inhibiting the Wnt/β-catenin signaling pathway. Black raspberry anthocyanins[141] upregulate miR-24-1-5p expression in human CSCs, significantly reducing β-catenin levels and curtailing tumor cell proliferation, migration, and survival. Polysaccharides[19], important functional phytochemicals found abundantly in TCM, possess anti-CRC effects by regulating intestinal flora imbalance and modulating the Wnt pathway[20].
Morusinol[142,143], a compound extracted from mulberry plants, exerts its therapeutic effects on CRC by mediating cholesterol biosynthesis through Forkhead box O-3a nuclear aggregation inhibition, thereby suppressing cell proliferation and inducing autophagy. Ethyl cinnamate[144] suppresses CRC angiogenesis and tumor growth by modulating the VEGFR2 signaling pathway.
TCM and Chinese patent medicine: CSC is closely associated with high tumorigenicity, chemotherapy/radiotherapy resistance, and metastasis and recurrence, particularly in CRC. The chemical composition of TCM is complex. Both individual TCM herbs and their isolated components[42,145] exhibit multi-target and multi-pathway effects similar to those of TCM compounds. Polysaccharides from Astragalus[103], Ginseng[146], Ganoderma lucidum[147], Dendrobium officinale[148], Puerariae Radix[149], and other TCM herbs can inhibit or kill tumor cells indirectly by enhancing immune function or directly by inducing tumor cell differentiation or apoptosis, as well as by modulating oncogene expression[19,150]. Furthermore, these polysaccharides exhibit synergistic effects with radiotherapy, chemotherapy, and immunotherapy[151]. Azalea Rhododendron molle G. Don[152] contains 17 types of anticancer bioactive compounds that induce CSC apoptosis and inhibit migration.
Salvia miltiorrhiza and its extract tanshinone exhibit potent anti-colorectal tumor activity by up-regulating 70 kDa heat shock protein to enhance immunogenicity and improve the efficacy of PD-1 blockade in CRC. Furthermore, their anti-tumor mechanism involves the regulation of the INS/SRC/IL6 pathway[153], phosphorylation of target Akt1 to modulate the PI3K/Akt signaling pathway for promoting mitochondrial fission[140], and activation of the c-Jun N-terminal kinase/mitochondrial fission factor signaling pathway[154].
Some Chinese patent medicines, such as compound Chinese medicine injections and oral preparations manufactured using modern processes, represent the integration of TCM with contemporary pharmaceutical technology. These formulations have been utilized in clinical practice for many years; however, further evidence-based research is necessary to fully establish their efficacy and safety[155,156].
Venenum Bufonis[157,158] is the primary constituent of bufonis that inhibits CRC growth and metastasis by suppressing the NF-κB, PI3K/Akt pathways and the HIF-1α/stromal cell-derived factor-1α-CXC chemokine receptor 4/PI3K/Akt signaling pathway. It also reduces VEGF synthesis and release and degrades type IV collagen[159]. Furthermore, Venenum Bufonis targets the SRC-3/MIF pathway in chemoresistant cells to enhance chemosensitivity[160,161], regulates M2 macrophage polarization in CRC[162], inhibits the C-Kit/Slug axis involved in CRC stemness, and suppresses the STAT3 signaling pathway to counteract CRC metastasis mediated by cancer-associated fibroblasts[86,163]. Additionally, compound matrine injection exhibits anti-CSC and anti-liver metastasis effects by intervening in metabolic reprogramming and EMT of CRC and hepatocellular carcinoma through regulation of the β-catenin/c-Myc signaling pathway and induction of cell cycle arrest[90,91]. Erteno-sanjie capsule[164] has demonstrated therapeutic efficacy against CRC by positively regulating cell migration, protein phosphorylation, and the PI3K/Akt signaling pathway.
Acupuncture therapy: As a complementary therapy, acupuncture or electropuncture (EA) has been extensively used to treat various inflammation-related diseases such as obesity, ulcerative colitis, tumors, and CRC. Notably, EA has demonstrated its potential in regulating the sirtuin 1-mediated miR-215/Atg14 axis by suppressing inflammation and promoting autophagy in mouse models[165]. These mechanistic insights shed light onto the anti-CRC effects of EA and highlight its promising therapeutic candidacy; however, high-quality randomized controlled trials remain scarce.
Postoperative recurrence of CRC significantly impacts treatment outcomes. A compound composed of Baizhu, Yinchen, Chenpi, and Fuling[166] can modulate tumor-infiltrating immune cells and hypoxia-related gene expression by downregulating the mRNA levels of BCL2 L1, XIAP, and TOP1, thereby reducing metastasis and recurrence in post-operative patients with stage II-III CRC. TCM has demonstrated potential in enhancing the overall condition by extending survival time and improving the quality of life for patients with stage IV CRC with liver and lung metastasis[167-170].
Dahuang Zhechong Pill[171] exerts inhibitory effects on CRC liver metastasis by suppressing the C-C motif ligand 2-mediated M2 polarization and improving the profibrotic microenvironment. Obesity disrupts IGF secretion and exacerbates CRC, whereas Xiaotan Tongfu decoction[172] hinders CRC liver metastasis by upregulating IGF-1/IGF-1R secretion and downregulating IGF binding protein 3 secretion. Fuzheng Xiaojijinzhan decoction[173] impedes CRC liver metastasis through the vitamin D receptor/TGF-β/Snail1 signaling pathway. Kaempferol[174] binds to HNRNPK and HNRNPL to regulate circ_0000345 and mediate the JMJD2C/β-catenin signaling pathway, thereby inhibiting CRC metastasis. It also exhibits favorable attenuation and synergistic effects when combined with chemotherapy drugs. Additional studies have demonstrated that as an external treatment with TCM characteristics, moxibustion can inhibit CRC liver metastasis by remodeling the disrupted intestinal microbial community structure, suggesting its potential as a complementary and alternative therapy for these patients[175].
Chemotherapy is a primary therapeutic approach for CRC; however, the cytotoxic effects of chemotherapy drugs, such as bone marrow suppression, digestive dysfunction, and hand-foot syndrome, often significantly impact treatment efficacy and sustainability while posing potential life-threatening risks to patients. Additionally, chemoresistance remains an inevitable challenge in clinical practice. TCM presents a promising alternative in addressing these concerns. Furthermore, various compounds[176], extracts[177], and Chinese patent medicines[178] including formulations[155,179] have been extensively employed in clinical settings for numerous years with validated safety and efficacy profiles.
5-FU is the most commonly used adjuvant chemotherapy for CRC. It exerts a potent cytotoxic effect on tumor cells. However, prolonged administration of 5-FU increases the risk of hematologic side effects and promotes the development of drug resistance in tumor cells. TCM containing Villosol from sauce grass and its extract Patrinia villosa Juss[180] can inhibit CDKN2A gene expression and regulate the TP53-PI3K/Akt signaling axis to reverse 5-FU resistance. Similar effects have been observed with ginsenoside Rg3[181].
Additionally, Zhengyuan jiaonang[182] inhibits CT26CRC tumor growth and enhances the efficacy of combination chemotherapy while reducing its cytotoxicity. Its mechanism involves promoting tumor fibrosis, inhibiting angiogenesis, migration and invasion, as well as modulating the tumor immune microenvironment. Pulsatilla decoction[183] exhibits similar effects by deactivating STAT3. Bioactive compounds derived from SangGui grass L[184] demonstrate strong activity against both 5-FU-sensitive and resistant CRC cells by inducing apoptosis and autophagy-mediated cell death and suppressing the Wnt signaling pathway to arrest cell cycle progression at the G0-G1 phase, thereby inhibiting CRC proliferation. Furthermore, other TCM compounds exhibit promising effects in alleviating certain chemotherapy drug-induced complications such as leukopenia[185].
The neurotoxicity of oxaliplatin can readily induce hand-foot syndrome, particularly when combined with 5-FU. The combination of Bushen Jianpi - Jiedu decoction[186] and its extract raddeanin A[93] with oxaliplatin exhibits superior therapeutic effects compared to the latter alone; however, its mechanism is complex. Bushen Jianpi - Jiedu decoction regulates 559 genes and 11 proteins. Within this compound, seven bioactive compounds interfere with 39 potential targets and modulate multiple signaling pathways, including MAPK, PI3K-Akt, and HIF-1. Identifying the optimal drug dosage is a crucial aspect of developing new Chinese medicine formulations and poses a considerable challenge in the integration of traditional Chinese and Western medicine in clinical practice.
Multiple studies have shown that acupuncture and moxibustion can effectively prevent and treat the toxic effects of chemotherapeutic agents like oxaliplatin, which often cause chemotherapy-induced peripheral neuropathy[187-193]. Commonly used acupoints include Hegu (LI 4), Neiguan (PC 6), Quchi (LI 11), Taichong (LR 3), Sanyinjiao (SP 6), and Zusanli (ST 36). The therapeutic mechanisms involve inhibiting the release of inflammatory factors and blocking the binding of related receptors. Bee venom acupuncture[194] has exhibited synergistic effects with morphine, providing potent and sustained analgesia for oxaliplatin-induced neuropathic pain. This effect is associated with the modulation of spinal opioid receptors and 5-HT3 receptors, as well as the regulation of action potential thresholds in A-fiber dorsal root ganglia neurons. For instance, the anticancer efficacy of varying concentrations of Huangqin decoction and irinotecan exhibits significant differences[195].
Radiotherapy is an important modality in CRC treatment. However, tumor cells ultimately develop resistance to radiotherapy, and there are currently no effective countermeasures. Most patients with colorectal tumors ultimately die from local recurrence after developing resistance to radiotherapy. Studies have shown that ginsenoside Rg3[96] and allicin[196] can enhance CRC radiosensitivity by inhibiting NF-κB and NF-κB-regulated gene products, thereby inhibiting tumor growth and prolonging the lifespan of CT-26 xenograft tumors in nude mice. Curcumin[71,197] can promote the sensitivity of human CRC to radiochemotherapy, and its mechanism may be related to the downregulation of DNA repair-related genes such as LIG4, PNKP, and XRCC5, as well as the upregulation of CCNH. External therapies such as acupuncture[198] have demonstrated significant efficacy in alleviating chemotherapy-induced peripheral neuropathy. In addition, certain palliative functional exercise methods, such as Baduanjin Qigong[199], have significantly alleviated the fatigue associated with adjuvant chemotherapy following CRC surgery.
PD-1/programmed death ligand 1 (PD-L1) inhibitors have demonstrated significant clinical advancements. However, monotherapy with PD-1/PD-L1 inhibitors in patients with microsatellite stability (MSS) CRC exhibits a lower objective response rate and susceptibility to tumor drug resistance, necessitating combination therapy with other immune therapies, chemotherapy, radiation therapy or alternative approaches to enhance patient response rates. Nevertheless, these combined treatments often lead to the accumulation of toxic side effects. TCM represents a potential adjunctive option due to its relatively minimal adverse effects and ability to reshape the intestinal microbiota and TME in MSS CRC patients, thereby enhancing immune potency and protecting intestinal barrier function. Consequently, TCM can augment the efficacy of PD-1 blockade in CRC patients[48,51,200].
Previous studies have demonstrated that cupping electroacupuncture can have an anti-tumor effect[201]. Furthermore, it enhances the anti-tumor immune response by elevating lymphocyte and granzyme B levels and activating the stimulator of interferon genes pathway. Combination therapy with cupping electroacupuncture and PD-1 blockade yields superior therapeutic efficacy compared to monotherapy in MSS mouse models of CRC.
Combining Salvia miltiorrhiza Bunge aqueous extract[202] with anti-PD-1 antibody has demonstrated enhanced therapeutic efficacy compared to monotherapy in controlling MC38 xenograft tumors in nude mice, suggesting its potential as an immunomodulatory strategy to augment the clinical effectiveness of anti-PD-1 treatment in MSS CRC. Ganoderma lucidum polysaccharide, an extract from Ganoderma lucidum[151], significantly inhibits CRC growth and activates anti-tumor immunity. Moreover, its combination with PD-1 inhibitors further enhance the efficacy of anti-PD-1 immunotherapy. In conjunction with ginsenoside Rg1, rosmarinic acid suppresses colon cancer metastasis by co-inhibiting the COX-2 enzyme and the PD-1/PD-L1 signaling pathway[203]. Sauchinone[204,205] exhibits inhibitory effects on various tumor cells, particularly CRC cells, by downregulating MMP2 and MMP9 expression and mediating checkpoint inhibition via the PD-1/PD-L1 axis. Additionally, combinations of certain TCM components, such as carbenoxolone-ganolol and baicalin[206], demonstrate synergistic anticancer potential and enhance the efficacy of anti-PD-1 immunotherapy in CRC by inducing GSDME-dependent pyroptosis. Individual TCM compounds, like the active ingredients in Saffron, can reduce inflammatory factor expression through the IL-17 signaling pathway, thereby improving the tumor immune microenvironment and enhancing the effectiveness of immunotherapy for CRC[207].
Several TCM compounds have demonstrated comparable efficacy. Qizhen decoction[208] exerts a similar effect. It augments the abundance of Akkermansia, facilitating dendritic cell maturation for IL-12 release, activating the JAK2/STAT4 pathway, and inducing effector T cell activation. Jianpi Jiedu Recipe[209] can inhibit colon cancer proliferation and reduce PD-L1 expression via incomplete autophagy induced by reactive oxygen species, thereby exerting a synergistic immune therapeutic effect. Chang Wei Qing decoction[210] can modulate the tumor immune microenvironment by altering the intestinal microbiota, thus enhancing the efficacy of PD-1 inhibitor therapy. When combined with PD-L1 inhibitors, Jiedu Sangen decoction[211] can reverse EMT in rectal cancer cells through the PI3K/Akt signaling pathway, significantly inhibiting CRC cell migration and invasion.
TCM in combination with targeted therapies, such as bevacizumab plus FOLFIRI regimen along with ginsenoside-modified nanostructured lipid carriers containing curcumin for treating unresectable metastatic CRC, improves long-term survival rates, tolerability, and safety[212]. However, these studies are limited by small sample sizes, lack of long-term follow-up data, unblinded methodologies, and single-center designs, which hinder their ability to provide high-quality evidence for clinical application.
Gastrointestinal dysfunction or postoperative intestinal obstruction is a prevalent complication following CRC surgery. Numerous studies have demonstrated that acupuncture holds significant potential as a therapeutic intervention for promoting gastrointestinal function recovery in patients with CRC after surgery[213-215]. Commonly employed acupuncture techniques encompass electroacupuncture and transcutaneous electrical stimulation[215-218], conventional acupuncture[219], moxibustion, scalp acupuncture, and auricular point stimulation[220]. The frequently utilized acupoints include lower He-point[216] and Zusanli[221], which exert their mechanism of action by inhibiting miR-222 up-regulation and blocking c-kit mRNA/protein and endothelial nitric oxide synthase mRNA down-regulation.
TCM prescriptions can promote postoperative intestinal obstruction recovery and synergize with modern medical rapid rehabilitation technology[222]. Among them, Daikenchuto decoction[223] is one of the most extensively studied prescriptions, and it can regulate the body’s nervous system, immune system, and inflammatory response[224]. Other prescriptions such as Shenhuang plaster[225] can significantly improve patients’ postoperative intestinal obstruction-induced inflammatory response while promoting gastrointestinal motility recovery. This may be through macrophage polarization through PI3K/Akt/NF-κB signaling pathway. Wuda granule[226] and Xiangbin prescription[227] also exhibit similar effects.
Low anterior resection syndrome is a prevalent complication in radical surgery for low rectal cancer. Defecation dysfunction, including constipation and increased defecation frequency, are the most common symptoms. However, modern medicine lacks effective treatments and mainly relies on symptomatic management. TCM treatment has shown promising results in effectively alleviating related symptoms[228]. Modified Baizhu Shaoyao San[229] can improve postoperative stool frequency without significant side effects. Acupuncture has demonstrated potential in ameliorating various symptoms associated with anterior rectal resection and holds promise as an effective intervention[228,230,231]. Nevertheless, high-quality randomized controlled trials are still lacking, and further research is needed to elucidate its mechanism of action.
Depression and anxiety can contribute to cancer development and significantly impact patients’ postoperative recovery following CRC surgery. Therefore, implementation of perioperative antidepressant interventions is imperative. TCM has been explored in this domain, with commonly employed approaches including acupuncture[232,233], Xiao-Chai-Hu-Tang, and other TCM prescriptions[60]. These interventions have demonstrated effective improvement in perioperative patients’ anxiety levels[233]; however, limited research exists.
After CRC surgery and chemotherapy, patients frequently experience spleen-stomach qi deficiency, resulting in compromised immune function and persistent fatigue. Buzhong Yiqi decoction[234] enhances immune function, thereby regulating inflammatory responses and metabolic processes to promote overall immunonutrition and restore bodily balance. Studies on acupuncture and moxibustion[235] have also confirmed the efficacy of these perioperative treatments, which are predominantly utilized for rehabilitating organ functions, including gastrointestinal, urinary, and sexual functions[236].
TCM emphasizes individualized treatment, with syndrome differentiation and treatment forming its core principle. It posits that different diseases can be treated with the same drug based on patient-specific conditions, while the same disease requires varying therapeutic approaches at different stages. This aligns with the concept of precision medicine in modern healthcare. However, TCM syndrome differentiation often lacks objective indicators, posing significant challenges for its integration with modern medicine. This remains an active area of research[237-239]. Several studies have attempted to distinguish different TCM syndromes of CRC by analyzing variations in gut microbiota, and five specific microbiotas have been identified as potential CRC markers: Dialister sp Marseille P5638, Oscillospirales, Selenomonadaceae, Dialister, and Akkermansia muciniphila. These TCM syndrome differentiation markers are anticipated to provide a biological foundation for precise syndrome differentiation in CRC[240,241]. Additionally, characteristics of the tongue microbiome[242], immunoglobulin lambda binding 3 and nuclear morphology parameters (such as nuclear texture, eccentricity, size, and pixel intensity)[243], along with metabolite differences and characteristic metabolic pathways, are included as objective indicators to facilitate accurate and objective syndrome differentiation and TCM treatment for CRC patients[244]. Therefore, in-depth research and exploration of the objective material differences between different TCM syndromes will provide new approaches for further studies on individualized treatment of CRC with TCM[245].
As one of the most significant complementary and alternative medicines worldwide, TCM has been clinically validated for its role in preventing and treating CRC and in promoting postoperative rehabilitation. TCM exhibits characteristics such as multi-target effects and relatively low side effects. However, the complexity of TCM components, particularly animal-based medicines that primarily consist of proteins, makes them susceptible to physical factors like processing and decoction. Although network pharmacology and molecular docking technology offer potential avenues for further research on TCM, there remain unresolved challenges that currently hinder the objective assessment and recognition of TCM’s efficacy. While considerable progress has been made in studying TCM extracts due to their relative ease of control, a major limitation is their inability to reflect the core principles underlying syndrome differentiation-based treatment in TCM. Additionally, identifying efficient monomeric compounds from the vast array of TCM components poses a formidable challenge.
TCM syndrome differentiation and treatment constitute an integral and pivotal component of TCM theory, serving as a guiding principle for the development of personalized therapeutic approaches. TCM syndrome differentiation and treatment is an essential component of TCM theory, guiding the design of personalized therapies. The TCM syndrome type encapsulates the comprehensive characteristics of a patient’s clinical manifestations. TCM practitioners must conduct a thorough analysis based on the patient’s overall symptoms and signs, exemplifyig the personalized nature of TCM disease management. However, due to the absence of objective measures, variability in treatment protocols may result in inconsistent clinical outcomes. Studies have demonstrated that tumor clinical stage correlates with objective indicators such as intestinal microbiota and T lymphocytes, revealing significant differences in metabolic characteristics[239,240,246]. These findings provide a foundation for establishing a predictive model for CRC progression and offer evidence supporting the scientific validity of TCM treatment principles.
In summary, TCM has unique advantages and great potential in treating CRC, particularly in its synergy with modern medicine and new drug development. While notable achievements have been made in this field, several challenges remain. Firstly, conducting high-quality multicenter prospective randomized controlled trials using blinded methods is difficult, especially for studies involving TCM compound formulations. Secondly, due to the extreme complexity of TCM components, screening active ingredients from single herbs and compound formulations, as well as understanding the synergistic effects among multiple components, remains a significant challenge in TCM development. Additionally, integrating the personalized treatment approaches characteristic of TCM, such as syndrome differentiation and treatment, with modern medicine and achieving clinical transformation poses another unresolved issue. As science and technology continue to advance and research methodologies are refined, particularly with the aid of cutting-edge technologies like artificial intelligence, these challenges are anticipated to be addressed. It is expected that in the near future, more safe and more effective TCM-based drugs and therapies will be developed, offering greater assistance and new hope to patients with CRC.
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 4681] [Article Influence: 4681.0] [Reference Citation Analysis (3)] |
| 2. | Luo Y, Zhang G, Hu C, Huang L, Wang D, Chen Z, Wang Y. The Role of Natural Products from Herbal Medicine in TLR4 Signaling for Colorectal Cancer Treatment. Molecules. 2024;29:2727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Zhang Y, Liu K, Yan C, Yin Y, He S, Qiu L, Li G. Natural Polyphenols for Treatment of Colorectal Cancer. Molecules. 2022;27:8810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 4. | Yan Y, Liu J, Pang Y, Wang Z, Peng R, Jiang D, Yang Y, Tang L, Sun L. Efficacy of Traditional Chinese Medicine Combined Online Group Psychotherapy (TCM-eRhab) on improving quality of life and relieving psychological burden for colorectal cancer survivors: a study protocol for a phase-II randomized controlled trial. BMC Complement Med Ther. 2024;24:290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Zhu Y, Yu J, Zhang K, Feng Y, Guo K, Sun L, Ruan S. Network Pharmacology Analysis to Explore the Pharmacological Mechanism of Effective Chinese Medicines in Treating Metastatic Colorectal Cancer using Meta-Analysis Approach. Am J Chin Med. 2021;49:1839-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 6. | Kong MY, Li LY, Lou YM, Chi HY, Wu JJ. Chinese herbal medicines for prevention and treatment of colorectal cancer: From molecular mechanisms to potential clinical applications. J Integr Med. 2020;18:369-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Liang Z, Xie H, Shen W, Shao L, Zeng L, Huang X, Zhu Q, Zhai X, Li K, Qiu Z, Sui X, Cheng H, Wu Q. The Synergism of Natural Compounds and Conventional Therapeutics against Colorectal Cancer Progression and Metastasis. Front Biosci (Landmark Ed). 2022;27:263. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Zou Y, Wang S, Zhang H, Gu Y, Chen H, Huang Z, Yang F, Li W, Chen C, Men L, Tian Q, Xie T. The triangular relationship between traditional Chinese medicines, intestinal flora, and colorectal cancer. Med Res Rev. 2024;44:539-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 9. | Jhang JS, Livneh H, Yang SY, Huang HJ, Chan MWY, Lu MC, Yeh CC, Tsai TY. Decreased risk of colorectal cancer among patients with type 2 diabetes receiving Chinese herbal medicine: a population-based cohort study. BMJ Open Diabetes Res Care. 2020;8:e000732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Liu F, Cao B, Zhang H, Zou Q, Liu G, Dong Y, Su D, Ren DL. Exploring the mechanism of Tengli Kangliu Decoction in the prevention and treatment of colorectal cancer precancerous based on network pharmacology. Medicine (Baltimore). 2022;101:e31690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Wu H, Huang Y, Yang L, Su K, Tian S, Chen X, Li S, Liu W. Effects of Jianpi Lishi Jiedu granules on colorectal adenoma patients after endoscopic treatment: study protocol for a randomized, double-blinded, placebo-controlled clinical trial. Trials. 2022;23:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Liu T, You J, Gao Q, Zhang Y, Xu W, Kong D, Chen L, Yuan B, Hua H. The Efficacy and Mechanism of Qinghua Jianpi Recipe in Inhibiting Canceration of Colorectal Adenoma Based on Inflammatory Cancer Transformation. J Immunol Res. 2023;2023:4319551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Ni M, Zhang Y, Sun Z, Zhou Q, Xiao J, Zhang B, Lin J, Gong B, Liu F, Meng F, Zheng G, Wang Y, Gu L, Li L, Shen W, Chen Y, Liu Y, Li L, Ling T, Cheng H. Efficacy and safety of Shenbai Granules for recurrent colorectal adenoma: A multicenter randomized controlled trial. Phytomedicine. 2024;127:155496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 14. | Cai K, Cao XY, Chen F, Zhu Y, Sun DD, Cheng HB, Duan JA, Su SL. Xianlian Jiedu Decoction alleviates colorectal cancer by regulating metabolic profiles, intestinal microbiota and metabolites. Phytomedicine. 2024;128:155385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 15. | Huang L, Zhang Y, Zhang X, Chen X, Wang Y, Lu J, Huang M. Therapeutic Potential of Pien-Tze-Huang: A Review on Its Chemical Composition, Pharmacology, and Clinical Application. Molecules. 2019;24:3274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Gou H, Su H, Liu D, Wong CC, Shang H, Fang Y, Zeng X, Chen H, Li Y, Huang Z, Fan M, Wei C, Wang X, Zhang X, Li X, Yu J. Traditional Medicine Pien Tze Huang Suppresses Colorectal Tumorigenesis Through Restoring Gut Microbiota and Metabolites. Gastroenterology. 2023;165:1404-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 17. | Xiang S, Jian Q, Chen W, Xu Q, Li J, Wang C, Wang R, Zhang D, Lin J, Zheng C. Pharmacodynamic components and mechanisms of ginger (Zingiber officinale) in the prevention and treatment of colorectal cancer. J Ethnopharmacol. 2024;324:117733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Zhang Y, Wang Y, Xin E, Zhang Z, Ma D, Liu T, Gao F, Bian T, Sun Y, Wang M, Wang Z, Yan X, Li Y. Network pharmacology and experimental verification reveal the mechanism of Hedysari Radix and Curcumae Rhizoma with the optimal compatibility ratio against colitis-associated colorectal cancer. J Ethnopharmacol. 2024;322:117555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Zhou M, Yue Y, Wang Y, Yan S. Polysaccharides from Chinese herbs as natural weapons against colorectal cancer. Biosci Rep. 2023;43:BSR20230041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Li Y, Wang S, Sun Y, Xu W, Zheng H, Wang Y, Tang Y, Gao X, Song C, Long Y, Liu J, Liu L, Mei Q. Apple polysaccharide protects ICR mice against colitis associated colorectal cancer through the regulation of microbial dysbiosis. Carbohydr Polym. 2020;230:115726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Li XM, Yuan DY, Liu YH, Zhu L, Qin HK, Yang YB, Li Y, Yan F, Wang YJ. Panax notoginseng saponins prevent colitis-associated colorectal cancer via inhibition IDO1 mediated immune regulation. Chin J Nat Med. 2022;20:258-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Zhang X, Liu Z, Chen S, Li H, Dong L, Fu X. A new discovery: Total Bupleurum saponin extracts can inhibit the proliferation and induce apoptosis of colon cancer cells by regulating the PI3K/Akt/mTOR pathway. J Ethnopharmacol. 2022;283:114742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Randisi F, Perletti G, Marras E, Gariboldi MB. Green Tea Components: In Vitro and In Vivo Evidence for Their Anticancer Potential in Colon Cancer. Cancers (Basel). 2025;17:623. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Watanabe J, Kobayashi F, Tahara M, Kitabayashi H, Shiozawa M, Kondo S, Koizumi M. Impact of Green Tea Consumption on Postoperative Ileus in Colon Cancer Patients: A Pilot Randomized Controlled Trial. Cureus. 2024;16:e72157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Khan MI, Karima G, Khan MZ, Shin JH, Kim JD. Therapeutic Effects of Saponins for the Prevention and Treatment of Cancer by Ameliorating Inflammation and Angiogenesis and Inducing Antioxidant and Apoptotic Effects in Human Cells. Int J Mol Sci. 2022;23:10665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Zhang Y, Chai N, Wei Z, Li Z, Zhang L, Zhang M, Ren J, Xu R, Pang X, Zhang B, Tang Q, Sui H. YYFZBJS inhibits colorectal tumorigenesis by enhancing Tregs-induced immunosuppression through HIF-1α mediated hypoxia in vivo and in vitro. Phytomedicine. 2022;98:153917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Chai N, Xiong Y, Zhang Y, Cheng Y, Shi W, Yao Y, Sui H, Zhu H. YYFZBJS inhibits colorectal tumorigenesis by remodeling gut microbiota and influence on M2 macrophage polarization in vivo and in vitro. Am J Cancer Res. 2021;11:5338-5357. [PubMed] |
| 28. | Li J, Zhou F, Shang L, Liu N, Liu Y, Zhang M, Wang S, Yang S. Integrated network pharmacology and experimental verification to investigate the mechanisms of YYFZBJS against colorectal cancer via CDK1/PI3K/Akt signaling. Front Oncol. 2022;12:961653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Sui H, Zhang L, Gu K, Chai N, Ji Q, Zhou L, Wang Y, Ren J, Yang L, Zhang B, Hu J, Li Q. YYFZBJS ameliorates colorectal cancer progression in Apc(Min/+) mice by remodeling gut microbiota and inhibiting regulatory T-cell generation. Cell Commun Signal. 2020;18:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 30. | Chen K, Zhang B, Li J, Pan A, Cao L, Zhao X, Huang S, Chen L. TiaochangXiaoliu decoction inhibits azomethane (AOM)/dextran sulfate sodium (DSS)-induced colorectal cancer by regulating immune response. J Gastrointest Oncol. 2021;12:2223-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 31. | Yang Y, Liu Z, Lyu H, Guo X, Jiang H, Liu L, Chen D. Traditional Chinese medicine-induced treatment in colitis-associated colorectal cancer. Chin Med J (Engl). 2023;136:1249-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 32. | Lyu D, Wang W, Xu H, Li P, Zhang W, Meng X, Liu S. Establishment of Coloproctitis Cancer Model in Mice and Evaluation of Therapeutic Effect of Chinese Medicine. J Vis Exp. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Yan S, Chang J, Hao X, Liu J, Tan X, Geng Z, Wang Z. Berberine regulates short-chain fatty acid metabolism and alleviates the colitis-associated colorectal tumorigenesis through remodeling intestinal flora. Phytomedicine. 2022;102:154217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 34. | Chen H, Zhang F, Zhang J, Zhang X, Guo Y, Yao Q. A Holistic View of Berberine Inhibiting Intestinal Carcinogenesis in Conventional Mice Based on Microbiome-Metabolomics Analysis. Front Immunol. 2020;11:588079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | Wang X, Saud SM, Wang F, He S, Zhang X, Hua B, Li W. Protective effect of ShaoYao decoction on colitis-associated colorectal cancer by inducing Nrf2 signaling pathway. J Ethnopharmacol. 2020;252:112600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Wang X, Saud SM, Zhang X, Li W, Hua B. Protective effect of Shaoyao Decoction against colorectal cancer via the Keap1-Nrf2-ARE signaling pathway. J Ethnopharmacol. 2019;241:111981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Yin FT, Zhou XH, Kang SY, Li XH, Li J, Ullah I, Zhang AH, Sun H, Wang XJ. Prediction of the mechanism of Dachengqi Decoction treating colorectal cancer based on the analysis method of " into serum components -action target-key pathway". J Ethnopharmacol. 2022;293:115286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Zhang Y, Kang Q, He L, Chan KI, Gu H, Xue W, Zhong Z, Tan W. Exploring the immunometabolic potential of Danggui Buxue Decoction for the treatment of IBD-related colorectal cancer. Chin Med. 2024;19:117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 39. | Gong G, Zheng Y, Ganesan K, Xiong Q, Tsim KWK. Danggui Buxue Tang potentiates the cytotoxicity of 5-fluorouracil on colorectal adenocarcinoma cells: A signaling mediated by c-Jun N-terminal kinase. Phytother Res. 2023;37:2864-2876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 40. | Chen JF, Wu SW, Shi ZM, Hu B. Traditional Chinese medicine for colorectal cancer treatment: potential targets and mechanisms of action. Chin Med. 2023;18:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 41. | Ziyang T, Xirong H, Chongming A, Tingxin L. The potential molecular pathways of Astragaloside-IV in colorectal cancer: A systematic review. Biomed Pharmacother. 2023;167:115625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Liao W, Zhang L, Chen X, Xiang J, Zheng Q, Chen N, Zhao M, Zhang G, Xiao X, Zhou G, Zeng J, Tang J. Targeting cancer stem cells and signalling pathways through phytochemicals: A promising approach against colorectal cancer. Phytomedicine. 2023;108:154524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 43. | Sun J, Wei Y, Wang J, Hou M, Su L. Treatment of colorectal cancer by traditional Chinese medicine: prevention and treatment mechanisms. Front Pharmacol. 2024;15:1377592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 44. | Wang K, Chen Q, Shao Y, Yin S, Liu C, Liu Y, Wang R, Wang T, Qiu Y, Yu H. Anticancer activities of TCM and their active components against tumor metastasis. Biomed Pharmacother. 2021;133:111044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 45. | Yang Y, Zhao M, Kuang Q, You F, Jiang Y. A comprehensive review of phytochemicals targeting macrophages for the regulation of colorectal cancer progression. Phytomedicine. 2024;128:155451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 46. | Liu H, Li X, Dong Y, Zhou C, Rezeng C. Lipid metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. J Cancer. 2023;14:2066-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 47. | Wei X, Wang F, Tan P, Huang H, Wang Z, Xie J, Wang L, Liu D, Hu Z. The interactions between traditional Chinese medicine and gut microbiota in cancers: Current status and future perspectives. Pharmacol Res. 2024;203:107148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 48. | Lv J, Jia Y, Li J, Kuai W, Li Y, Guo F, Xu X, Zhao Z, Lv J, Li Z. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10:415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 49. | Bu F, Tu Y, Wan Z, Tu S. Herbal medicine and its impact on the gut microbiota in colorectal cancer. Front Cell Infect Microbiol. 2023;13:1096008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 50. | Sun L, Pang Y, Wang Z, Liu J, Peng R, Yan Y, Yang Y, Tang L. Effect of traditional Chinese medicine combined group psychotherapy on psychological distress management and gut micro-biome regulation for colorectal cancer survivors: a single-arm phase I clinical trial. Support Care Cancer. 2023;31:698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 51. | Li Y, Li ZX, Xie CY, Fan J, Lv J, Xu XJ, Lv J, Kuai WT, Jia YT. Gegen Qinlian decoction enhances immunity and protects intestinal barrier function in colorectal cancer patients via gut microbiota. World J Gastroenterol. 2020;26:7633-7651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Zhao H, He M, Zhang M, Sun Q, Zeng S, Chen L, Yang H, Liu M, Ren S, Meng X, Xu H. Colorectal Cancer, Gut Microbiota and Traditional Chinese Medicine: A Systematic Review. Am J Chin Med. 2021;49:805-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Wang D, Wang F, Kong X, Li Q, Shi H, Zhao S, Li W, Li Y, Meng J. The role of metabolic reprogramming in cancer metastasis and potential mechanism of traditional Chinese medicine intervention. Biomed Pharmacother. 2022;153:113376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 54. | Wang S, Fu JL, Hao HF, Jiao YN, Li PP, Han SY. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol Res. 2021;170:105728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 55. | Wang ZJ, Wang XH, Li J, Zheng SH, Zhang FP, Hao SL, Wang XX, Liu LK. The efficacy and safety of modified Gegenqinlian Fomular for advanced colorectal cancer (damp heat accumulation type): A multicenter randomized controlled trial. Medicine (Baltimore). 2021;100:e27850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Li Y, Zhang C, Ma X, Yang L, Ren H. Identification of the potential mechanism of Radix pueraria in colon cancer based on network pharmacology. Sci Rep. 2022;12:3765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 57. | Deng XQ, Zhang HB, Wang GF, Xu D, Zhang WY, Wang QS, Cui YL. Colon-specific microspheres loaded with puerarin reduce tumorigenesis and metastasis in colitis-associated colorectal cancer. Int J Pharm. 2019;570:118644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 58. | Zhai Y, Sun J, Sun C, Zhao H, Li X, Yao J, Su J, Xu X, Xu X, Hu J, Daglia M, Han B, Kai G. Total flavonoids from the dried root of Tetrastigma hemsleyanum Diels et Gilg inhibit colorectal cancer growth through PI3K/AKT/mTOR signaling pathway. Phytother Res. 2022;36:4263-4277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 59. | Jin J, Chen B, Zhan X, Zhou Z, Liu H, Dong Y. Network pharmacology and molecular docking study on the mechanism of colorectal cancer treatment using Xiao-Chai-Hu-Tang. PLoS One. 2021;16:e0252508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 60. | Shao S, Jia R, Zhao L, Zhang Y, Guan Y, Wen H, Liu J, Zhao Y, Feng Y, Zhang Z, Ji Q, Li Q, Wang Y. Xiao-Chai-Hu-Tang ameliorates tumor growth in cancer comorbid depressive symptoms via modulating gut microbiota-mediated TLR4/MyD88/NF-κB signaling pathway. Phytomedicine. 2021;88:153606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 61. | Zhang Z, Shao S, Zhang Y, Jia R, Hu X, Liu H, Sun M, Zhang B, Li Q, Wang Y. Xiaoyaosan slows cancer progression and ameliorates gut dysbiosis in mice with chronic restraint stress and colorectal cancer xenografts. Biomed Pharmacother. 2020;132:110916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 62. | Yiqian J, Xibin Z, Wenyuan PU, Chunxiang Z. Sanwu Baisan decoction inhibits colorectal cancer progression in mice by remodeling gut microbiota and tumorigenesis. J Tradit Chin Med. 2023;43:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Xu Y, Wang H, Wang T, Chen C, Sun R, Yao W, Ma Y, Zhang Q, Wu L, Zeng S, Sun X. Dahuang Fuzi Baijiang decoction restricts progenitor to terminally exhausted T cell differentiation in colorectal cancer. Cancer Sci. 2022;113:1739-1751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 64. | Fan JH, Xu MM, Zhou LM, Gui ZW, Huang L, Li XG, Ye XL. Integrating network pharmacology deciphers the action mechanism of Zuojin capsule in suppressing colorectal cancer. Phytomedicine. 2022;96:153881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 65. | Huang S, Zhang Z, Li W, Kong F, Yi P, Huang J, Mao D, Peng W, Zhang S. Network Pharmacology-Based Prediction and Verification of the Active Ingredients and Potential Targets of Zuojinwan for Treating Colorectal Cancer. Drug Des Devel Ther. 2020;14:2725-2740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 66. | Dai Y, Wang H, Sun R, Diao J, Ma Y, Shao M, Xu Y, Zhang Q, Gao Z, Zeng Z, Zhang L, Sun X. Modified Shenlingbaizhu Decoction represses the pluripotency of colorectal cancer stem cells by inhibiting TGF-β mediated EMT program. Phytomedicine. 2022;103:154234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Wang Y, Zhao T, Huang C, Liu F, Zhang Y, Kong D, Fan Z. Effect and mechanism of Banxia Xiexin decoction in colorectal cancer: A network pharmacology approach. Phytomedicine. 2024;123:155174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (1)] |
| 68. | Jiang Y, Hu Y, Yang Y, Yan R, Zheng L, Fu X, Xiao C, You F. Tong-Xie-Yao-Fang promotes dendritic cells maturation and retards tumor growth in colorectal cancer mice with chronic restraint stress. J Ethnopharmacol. 2024;319:117069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 69. | Lu S, Sun X, Zhou Z, Tang H, Xiao R, Lv Q, Wang B, Qu J, Yu J, Sun F, Deng Z, Tian Y, Li C, Yang Z, Yang P, Rao B. Mechanism of Bazhen decoction in the treatment of colorectal cancer based on network pharmacology, molecular docking, and experimental validation. Front Immunol. 2023;14:1235575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 70. | Zhou M, Niu H, Cui D, Huang G, Li J, Tian H, Xu X, Liang F, Chen R. Resveratrol triggers autophagy-related apoptosis to inhibit the progression of colorectal cancer via inhibition of FOXQ1. Phytother Res. 2024;38:3218-3239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 71. | Wan C, Ma Q, Anderson S, Zhang QH, Zhang CF, Wang AH, Bell E, Hou L, Yuan CS, Wang CZ. Effects of Curcuminoids and Surfactant-Formulated Curcumin on Chemo-Resistant Colorectal Cancer. Am J Chin Med. 2023;51:1577-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 72. | Bian Y, Yin G, Wang G, Liu T, Liang L, Yang X, Zhang W, Tang D. Degradation of HIF-1α induced by curcumol blocks glutaminolysis and inhibits epithelial-mesenchymal transition and invasion in colorectal cancer cells. Cell Biol Toxicol. 2023;39:1957-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Li J, Chai R, Chen Y, Zhao S, Bian Y, Wang X. Curcumin Targeting Non-Coding RNAs in Colorectal Cancer: Therapeutic and Biomarker Implications. Biomolecules. 2022;12:1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 74. | Song Y, Yu J, Li L, Wang L, Dong L, Xi G, Lu YJ, Li Z. Luteolin impacts deoxyribonucleic acid repair by modulating the mitogen-activated protein kinase pathway in colorectal cancer. Bioengineered. 2022;13:10998-11011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 75. | Yao Y, Rao C, Zheng G, Wang S. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR384/pleiotrophin axis. Oncol Rep. 2019;42:131-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Maugeri A, Calderaro A, Patanè GT, Navarra M, Barreca D, Cirmi S, Felice MR. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int J Mol Sci. 2023;24:2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 77. | Homayoonfal M, Gilasi H, Asemi Z, Khaksary Mahabady M, Asemi R, Yousefi B. Quercetin modulates signal transductions and targets non-coding RNAs against cancer development. Cell Signal. 2023;107:110667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 78. | García-Gutiérrez N, Luna-Bárcenas G, Herrera-Hernández G, Campos-Vega R, Lozano-Herrera SJ, Sánchez-Tusié AA, García-Solis P, Vergara-Castañeda HA. Quercetin and Its Fermented Extract as a Potential Inhibitor of Bisphenol A-Exposed HT-29 Colon Cancer Cells' Viability. Int J Mol Sci. 2023;24:5604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 79. | Tezerji S, Nazari Robati F, Abdolazimi H, Fallah A, Talaei B. Quercetin's effects on colon cancer cells apoptosis and proliferation in a rat model of disease. Clin Nutr ESPEN. 2022;48:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 80. | Wang L, Du X, Yue D, Chen X. Catechin, rutin and quercetin in Quercus mongolica Fisch leaves exert inhibitory effects on multiple cancer cells. J Food Biochem. 2022;46:e14486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 81. | Biswas P, Dey D, Biswas PK, Rahaman TI, Saha S, Parvez A, Khan DA, Lily NJ, Saha K, Sohel M, Hasan MM, Al Azad S, Bibi S, Hasan MN, Rahmatullah M, Chun J, Rahman MA, Kim B. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int J Mol Sci. 2022;23:11746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 82] [Reference Citation Analysis (0)] |
| 82. | Feng Y, Lu J, Jiang J, Wang M, Guo K, Lin S. Berberine: Potential preventive and therapeutic strategies for human colorectal cancer. Cell Biochem Funct. 2024;42:e4033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 83. | Sun Q, Yang H, Liu M, Ren S, Zhao H, Ming T, Tang S, Tao Q, Chen L, Zeng S, Duan DD, Xu H. Berberine suppresses colorectal cancer by regulation of Hedgehog signaling pathway activity and gut microbiota. Phytomedicine. 2022;103:154227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 84. | Chen H, Ye C, Wu C, Zhang J, Xu L, Wang X, Xu C, Zhang J, Guo Y, Yao Q. Berberine inhibits high fat diet-associated colorectal cancer through modulation of the gut microbiota-mediated lysophosphatidylcholine. Int J Biol Sci. 2023;19:2097-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 85. | Liu L, Liang D, Zheng Q, Zhao M, Lv R, Tang J, Chen N. Berbamine dihydrochloride suppresses the progression of colorectal cancer via RTKs/Akt axis. J Ethnopharmacol. 2023;303:116025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Sun Q, Tao Q, Ming T, Tang S, Zhao H, Liu M, Yang H, Ren S, Lei J, Liang Y, Peng Y, Wang M, Xu H. Berberine is a suppressor of Hedgehog signaling cascade in colorectal cancer. Phytomedicine. 2023;114:154792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 87. | Ni L, Sun P, Ai M, Kong L, Xu R, Li J. Berberine inhibited the formation of metastasis by intervening the secondary homing of colorectal cancer cells in the blood circulation to the lung and liver through HEY2. Phytomedicine. 2022;104:154303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | He R, Ou S, Chen S, Ding S. Network Pharmacology-Based Study on the Molecular Biological Mechanism of Action for Compound Kushen Injection in Anti-Cancer Effect. Med Sci Monit. 2020;26:e918520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 89. | Cheng Y, Yu C, Li W, He Y, Bao Y. Matrine Inhibits Proliferation, Invasion, and Migration and Induces Apoptosis of Colorectal Cancer Cells Via miR-10b/PTEN Pathway. Cancer Biother Radiopharm. 2022;37:871-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Sun J, Li M, Lin T, Wang D, Chen J, Zhang Y, Mu Q, Su H, Wu N, Liu A, Yu Y, Liu Y, Wang S, Yu X, Guo J, Yu W. Cell cycle arrest is an important mechanism of action of compound Kushen injection in the prevention of colorectal cancer. Sci Rep. 2022;12:4384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 91. | Wang KX, Du GH, Qin XM, Gao L. Compound Kushen Injection intervenes metabolic reprogramming and epithelial-mesenchymal transition of HCC via regulating β-catenin/c-Myc signaling. Phytomedicine. 2021;93:153781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 92. | Zhang M, Tao Z, Gao L, Chen F, Ye Y, Xu S, Huang W, Li X. Toosendanin inhibits colorectal cancer cell growth through the Hedgehog pathway by targeting Shh. Drug Dev Res. 2022;83:1201-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Chen J, Zhang Y, Chen X, Luo D, Liu D, Yu Z, Lin Y, He X, Huang J, Lian L. Raddeanin A Inhibits Colorectal Cancer Growth and Ameliorates Oxaliplatin Resistance Through the WNT/β-Catenin Signaling Pathway. Cancer Biother Radiopharm. 2025;40:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Saini A, Saini V, Deswal G, Chopra B, Grewal AS, Dhingra AK. Harnessing Therapeutic Potential of Allicin Against Cancer: An Exploratory Review. Anticancer Agents Med Chem. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 95. | Liu R, Zhang B, Zou S, Cui L, Lin L, Li L. Ginsenoside Rg1 Induces Autophagy in Colorectal Cancer through Inhibition of the Akt/mTOR/p70S6K Pathway. J Microbiol Biotechnol. 2024;34:774-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 96. | Zhao L, Zhang Y, Li Y, Li C, Shi K, Zhang K, Liu N. Therapeutic effects of ginseng and ginsenosides on colorectal cancer. Food Funct. 2022;13:6450-6466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 97. | Chang L, Zhou R, He Y, Meng M, Hu J, Liu Y, Pan Y, Tang Z, Yue Z. Total saponins from Rhizoma Panacis Majoris inhibit proliferation, induce cell cycle arrest and apoptosis and influence MAPK signalling pathways on the colorectal cancer cell. Mol Med Rep. 2021;24:542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Zhou H, Sun Y, Zheng H, Fan L, Mei Q, Tang Y, Duan X, Li Y. Paris saponin VII extracted from trillium tschonoskii suppresses proliferation and induces apoptosis of human colorectal cancer cells. J Ethnopharmacol. 2019;239:111903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Li J, Jia J, Zhu W, Chen J, Zheng Q, Li D. Therapeutic effects on cancer of the active ingredients in rhizoma paridis. Front Pharmacol. 2023;14:1095786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 100. | Feng Y, Li Y, Ma F, Wu E, Cheng Z, Zhou S, Wang Z, Yang L, Sun X, Zhang J. Notoginsenoside Ft1 inhibits colorectal cancer growth by increasing CD8(+) T cell proportion in tumor-bearing mice through the USP9X signaling pathway. Chin J Nat Med. 2024;22:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 101. | Zeng JN, Tan JY, Mo L. Naringin Inhibits Colorectal Carcinogenesis by Inhibiting Viability of Colorectal Cancer Cells. Chin J Integr Med. 2023;29:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 102. | Gao Q, Li L, Zhang QM, Sheng QS, Zhang JL, Jin LJ, Shang RY. Monotropein Induced Apoptosis and Suppressed Cell Cycle Progression in Colorectal Cancer Cells. Chin J Integr Med. 2024;30:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 103. | Lu Q, Jiang J, Wang X, Wang R, Han X. Advancements in the Research of Astragalus membranaceus for the Treatment of Colorectal Cancer. Am J Chin Med. 2025;53:119-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 104. | Kong P, Tang X, Liu F, Tang X. Astragaloside IV regulates circ_0001615 and miR-873-5p/LASP1 axis to suppress colorectal cancer cell progression. Chem Biol Drug Des. 2024;103:e14423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 105. | Zhou J, Li L, Pu Y, Li H, Wu X, Wang Z, Sun J, Song Q, Zhou L, Ma X, Yang L, Ji Q. Astragaloside IV inhibits colorectal cancer metastasis by reducing extracellular vesicles release and suppressing M2-type TAMs activation. Heliyon. 2024;10:e31450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 106. | Wei J, Zheng Z, Hou X, Jia F, Yuan Y, Yuan F, He F, Hu L, Zhao L. Echinacoside inhibits colorectal cancer metastasis via modulating the gut microbiota and suppressing the PI3K/AKT signaling pathway. J Ethnopharmacol. 2024;318:116866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 107. | Si XL, Wang Y, Song BN, Zhang Y, Yang QX, Li Z, Luo YP, Duan YQ, Ma XM, Zhang YY. Potential Chemoprevention of Paeoniflorin in Colitis-Associated Colorectal Cancer by Network Pharmacology, Molecular Docking, and In Vivo Experiment. Chem Biodivers. 2022;19:e202200295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 108. | Su Z, Hu B, Li J, Zeng Z, Chen H, Guo Y, Mao Y, Cao W. Paeoniflorin inhibits colorectal cancer cell stemness through the miR-3194-5p/catenin beta-interacting protein 1 axis. Kaohsiung J Med Sci. 2023;39:1011-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 109. | Huang Z, Gan S, Zhuang X, Chen Y, Lu L, Wang Y, Qi X, Feng Q, Huang Q, Du B, Zhang R, Liu Z. Artesunate Inhibits the Cell Growth in Colorectal Cancer by Promoting ROS-Dependent Cell Senescence and Autophagy. Cells. 2022;11:2472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 110. | Bai C, Zhang Z, Zhou L, Zhang HY, Chen Y, Tang Y. Repurposing Ziyuglycoside II Against Colorectal Cancer via Orchestrating Apoptosis and Autophagy. Front Pharmacol. 2020;11:576547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | Zeng A, Hua H, Liu L, Zhao J. Betulinic acid induces apoptosis and inhibits metastasis of human colorectal cancer cells in vitro and in vivo. Bioorg Med Chem. 2019;27:2546-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 112. | Zheng JL, Wang SS, Shen KP, Chen L, Peng X, Chen JF, An HM, Hu B. Ursolic acid induces apoptosis and anoikis in colorectal carcinoma RKO cells. BMC Complement Med Ther. 2021;21:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Chen L, Liu M, Yang H, Ren S, Sun Q, Zhao H, Ming T, Tang S, Tao Q, Zeng S, Meng X, Xu H. Ursolic acid inhibits the activation of smoothened-independent non-canonical hedgehog pathway in colorectal cancer by suppressing AKT signaling cascade. Phytother Res. 2022;36:3555-3570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 114. | Zhao H, Tang S, Tao Q, Ming T, Lei J, Liang Y, Peng Y, Wang M, Liu M, Yang H, Ren S, Xu H. Ursolic Acid Suppresses Colorectal Cancer by Down-Regulation of Wnt/β-Catenin Signaling Pathway Activity. J Agric Food Chem. 2023;71:3981-3993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 115. | Zhang T, Xiang F, Li X, Chen Z, Wang J, Guo J, Zhu S, Zhou J, Kang X, Wu R. Mechanistic study on ursolic acid inhibiting the growth of colorectal cancer cells through the downregulation of TGF-β3 by miR-140-5p. J Biochem Mol Toxicol. 2024;38:e23581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 116. | Zuo Z, He L, Duan X, Peng Z, Han J. Glycyrrhizic acid exhibits strong anticancer activity in colorectal cancer cells via SIRT3 inhibition. Bioengineered. 2022;13:2720-2731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Meng X, Na R, Peng X, Li H, Ouyang W, Zhou W, You X, Li Y, Pu X, Zhang K, Xia J, Wang J, Tang H, Zhuang G, Peng Z. Musashi-2 potentiates colorectal cancer immune infiltration by regulating the post-translational modifications of HMGB1 to promote DCs maturation and migration. Cell Commun Signal. 2024;22:117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 118. | Chen YL, Xu B, Pan ZF, Cai YP, Yang CY, Cao SL, Chen KH, Xie XT, Zhao M, Li PC, Xie XQ, Chen XY, Wang Q, Zhou L, Luo X. Glycyrrhizic acid reduces neutrophil extracellular trap formation to ameliorate colitis-associated colorectal cancer by inhibiting peptidylarginine deiminase 4. J Ethnopharmacol. 2025;341:119337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 119. | Wang G, Wang YZ, Yu Y, Wang JJ, Yin PH, Xu K. Triterpenoids Extracted from Rhus chinensis Mill Act Against Colorectal Cancer by Inhibiting Enzymes in Glycolysis and Glutaminolysis: Network Analysis and Experimental Validation. Nutr Cancer. 2020;72:293-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 120. | Wang G, Wang YZ, Yu Y, Wang JJ. Inhibitory ASIC2-mediated calcineurin/NFAT against colorectal cancer by triterpenoids extracted from Rhus chinensis Mill. J Ethnopharmacol. 2019;235:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 121. | Ruan J, Li H, Lu M, Hao M, Sun F, Yu H, Zhang Y, Wang T. Bioactive triterpenes of jujube in the prevention of colorectal cancer and their molecular mechanism research. Phytomedicine. 2023;110:154639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 122. | Zhang H, Zhao B, Wei H, Zeng H, Sheng D, Zhang Y. Cucurbitacin B controls M2 macrophage polarization to suppresses metastasis via targeting JAK-2/STAT3 signalling pathway in colorectal cancer. J Ethnopharmacol. 2022;287:114915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 123. | Sui H, Tan H, Fu J, Song Q, Jia R, Han L, Lv Y, Zhang H, Zheng D, Dong L, Wang S, Li Q, Xu H. The active fraction of Garcinia yunnanensis suppresses the progression of colorectal carcinoma by interfering with tumorassociated macrophage-associated M2 macrophage polarization in vivo and in vitro. FASEB J. 2020;34:7387-7403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 124. | Sui H, Deng W, Chai Q, Han B, Zhang Y, Wei Z, Li Z, Wang T, Feng J, Yuan M, Tang Q, Xu H. YTE-17 inhibits colonic carcinogenesis by resetting antitumor immune response via Wnt5a/JNK mediated metabolic signaling. J Pharm Anal. 2024;14:100901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 125. | Dai G, Wang D, Ma S, Hong S, Ding K, Tan X, Ju W. ACSL4 promotes colorectal cancer and is a potential therapeutic target of emodin. Phytomedicine. 2022;102:154149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 126. | Shen Z, Zhao L, Yoo SA, Lin Z, Zhang Y, Yang W, Piao J. Emodin induces ferroptosis in colorectal cancer through NCOA4-mediated ferritinophagy and NF-κb pathway inactivation. Apoptosis. 2024;29:1810-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 127. | Varlı M, Ji M, Kim E, Kim SJ, Choi B, Ha HH, Kim KK, Paik MJ, Kim H. Emodin disrupts the KITENIN oncogenic complex by binding ErbB4 and suppresses colorectal cancer progression in dual blockade with KSRP-binding compound. Phytomedicine. 2025;136:156247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 128. | Chen J, Liu J, Liu X, Wang J, Wang X, Ye X, Xie Q, Liang J, Li Y. Shikonin improves the effectiveness of PD-1 blockade in colorectal cancer by enhancing immunogenicity via Hsp70 upregulation. Mol Biol Rep. 2024;51:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 129. | Wang Z, Cui Q, Shi L, Zhang M, Song P, Duan D, Guo W. Network Pharmacology-based Prediction and Verification of Shikonin for Treating Colorectal Cancer. Recent Pat Anticancer Drug Discov. 2022;17:297-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 130. | Liu Y, Cheng DH, Su ZY, Lv JH, Wang L, Deng YY, Li L. Effects of total coumarins from Pileostegia tomentella on exosomal miRNA expression and angiogenesis in colorectal cancer cells. Pharm Biol. 2024;62:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 131. | Hoveizi E, Hushmandi K. Comparison of effects of P-coumaric acid and coumarin on colorectal cancer cell line by inducing apoptosis and autophagy. Avicenna J Phytomed. 2024;14:470-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 132. | Zhou X, Kang J, Zhang L, Cheng Y. Osthole inhibits malignant phenotypes and induces ferroptosis in KRAS-mutant colorectal cancer cells via suppressing AMPK/Akt signaling. Cancer Chemother Pharmacol. 2023;92:119-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 133. | Miao Q, Deng WQ, Lyu WY, Sun ZT, Fan SR, Qi M, Qiu SH, Zhu YR, Lin JP, Chen MF, Deng LJ. Erianin inhibits the growth and metastasis through autophagy-dependent ferroptosis in KRAS(G13D) colorectal cancer. Free Radic Biol Med. 2023;204:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 134. | Liu S, Teng F, Lu Y, Zhu Y, Liang X, Wu F, Liu J, Zhou W, Su C, Cao Y. Ethoxy-erianin phosphate inhibits angiogenesis in colorectal cancer by regulating the TMPO-AS1/miR-126-3p/PIK3R2 axis and inactivating the PI3k/AKT signaling pathway. BMC Cancer. 2024;24:1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 135. | Zheng Y, Zheng Y, Chen H, Tan X, Zhang G, Kong M, Jiang R, Yu H, Shan K, Liu J, Zhang R, Liu Z, Wu J. Erianin triggers ferroptosis in colorectal cancer cells by facilitating the ubiquitination and degradation of GPX4. Phytomedicine. 2025;139:156465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 136. | Wang P, Jia X, Lu B, Huang H, Liu J, Liu X, Wu Q, Hu Y, Li P, Wei H, Liu T, Zhao D, Zhang L, Tian X, Jiang Y, Qiao Y, Nie W, Ma X, Bai R, Peng C, Dong Z, Liu K. Erianin suppresses constitutive activation of MAPK signaling pathway by inhibition of CRAF and MEK1/2. Signal Transduct Target Ther. 2023;8:96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 137. | Wang W, Zhou L, Zhang X, Li Z. Mollugin suppresses proliferation and drives ferroptosis of colorectal cancer cells through inhibition of insulin-like growth factor 2 mRNA binding protein 3/glutathione peroxidase 4 axis. Biomed Pharmacother. 2023;166:115427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 138. | Zhu L, Chen C, Cai Y, Li Y, Gong L, Zhu T, Kong L, Luo J. Identification of a ferritinophagy inducer via sinomenine modification for the treatment of colorectal cancer. Eur J Med Chem. 2024;268:116250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 139. | Yan R, Zhu H, Huang P, Yang M, Shen M, Pan Y, Zhang C, Zhou X, Li H, Ke X, Zhang W, Hao P, Qu Y. Liquidambaric acid inhibits Wnt/β-catenin signaling and colon cancer via targeting TNF receptor-associated factor 2. Cell Rep. 2022;38:110319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 140. | Lu M, Ruan J, Yu R, Zhang Y, Zhao W, Yang D, Wang W, Zhang Y, Wang T. Neolignan derivatives from Penthorum chinense with antitumor activity in human colorectal cancer cells by regulating Wnt/β-catenin signaling pathway. Phytochemistry. 2023;214:113827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 141. | Zhang H, Guo J, Mao L, Li Q, Guo M, Mu T, Zhang Q, Bi X. Up-regulation of miR-24-1-5p is involved in the chemoprevention of colorectal cancer by black raspberry anthocyanins. Br J Nutr. 2019;122:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 142. | Zhang X, Dong Z, Yang Y, Liu C, Li J, Sun W, Zhu Y, Shen Y, Wang Z, Lü M, Cui H. Morusinol Extracted from Morus alba Inhibits Cell Proliferation and Induces Autophagy via FOXO3a Nuclear Accumulation-Mediated Cholesterol Biosynthesis Obstruction in Colorectal Cancer. J Agric Food Chem. 2023;71:16016-16031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 143. | Zhang K, Hu X, Su J, Li D, Thakur A, Gujar V, Cui H. Gastrointestinal Cancer Therapeutics via Triggering Unfolded Protein Response and Endoplasmic Reticulum Stress by 2-Arylbenzofuran. Int J Mol Sci. 2024;25:999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 144. | Wang S, Yang J, Kuang X, Li H, Du H, Wu Y, Xu F, Liu B. Ethyl cinnamate suppresses tumor growth through anti-angiogenesis by attenuating VEGFR2 signal pathway in colorectal cancer. J Ethnopharmacol. 2024;326:117913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 145. | Guo TH, Li YY, Hong SW, Cao QY, Chen H, Xu Y, Dai GL, Shao G. Evidence for Anticancer Effects of Chinese Medicine Monomers on Colorectal Cancer. Chin J Integr Med. 2022;28:939-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 146. | Zhao L, Zhang T, Zhang K. Pharmacological effects of ginseng and ginsenosides on intestinal inflammation and the immune system. Front Immunol. 2024;15:1353614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 147. | Zhang X, Liu S, Wu K, Shu L, Li Y, Li L, Wang D. Structural characteization and anti-colorectal cancer activity of a fucogalactan purified from Ganoderma tsugae. Carbohydr Polym. 2025;352:123203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 148. | Ruan J, Zhang P, Zhang Q, Zhao S, Dang Z, Lu M, Li H, Zhang Y, Wang T. Colorectal cancer inhibitory properties of polysaccharides and their molecular mechanisms: A review. Int J Biol Macromol. 2023;238:124165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 149. | Li J, Shang L, Zhou F, Wang S, Liu N, Zhou M, Lin Q, Zhang M, Cai Y, Chen G, Yang S. Herba Patriniae and its component Isovitexin show anti-colorectal cancer effects by inducing apoptosis and cell-cycle arrest via p53 activation. Biomed Pharmacother. 2023;168:115690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 150. | Zhao X, Xiu J, Yang H, Han W, Jin Y. Network Pharmacology and Bioinformatics Study of Six Medicinal Food Homologous Plants Against Colorectal Cancer. Int J Mol Sci. 2025;26:930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 151. | Li W, Zhou Q, Lv B, Li N, Bian X, Chen L, Kong M, Shen Y, Zheng W, Zhang J, Luo F, Luo Z, Liu J, Wu JL. Ganoderma lucidum Polysaccharide Supplementation Significantly Activates T-Cell-Mediated Antitumor Immunity and Enhances Anti-PD-1 Immunotherapy Efficacy in Colorectal Cancer. J Agric Food Chem. 2024;72:12072-12082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 152. | Zong L, Yang Y, Zhang J, Dai L, Luo Y, Yang J, Luo X. Rhododendron molle G. Don Extract Induces Apoptosis and Inhibits Migration in Human Colorectal Cancer Cells and Potential Anticancer Components Analysis. Molecules. 2021;26:2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 153. | Jiang YL, Xun Y. Molecular Mechanism of Salvia miltiorrhiza in the Treatment of Colorectal Cancer Based on Network Pharmacology and Molecular Docking Technology. Drug Des Devel Ther. 2024;18:425-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 154. | Zheng Q, Wang X, Gao T, Zhang B, Zhao N, Du R, Zhao Z. Exploring the pharmacological and molecular mechanisms of Salvia chinensis Benth in colorectal cancer: A network pharmacology and molecular docking study. Medicine (Baltimore). 2023;102:e36602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 155. | Yang M, Zhu SJ, Shen C, Zhai R, Li DD, Fang M, Xu JN, Gan YN, Yang L, Ren ZY, Zheng RX, Robinson N, Liu JP. Clinical Application of Chinese Herbal Injection for Cancer Care: Evidence-Mapping of the Systematic Reviews, Meta-analyses, and Randomized Controlled Trials. Front Pharmacol. 2021;12:666368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 156. | Zhang S, Lian P, Huang T, Zhou J. Effect of Quxie capsule in patients with colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e24322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 157. | Tao C, Wang J, Gu Z, Ni H, Luo Y, Ling J, Chen Y, Wu Y, Liu X, Zhou Y, Xu T. Network pharmacology and metabolomics elucidate the underlying mechanisms of Venenum Bufonis in the treatment of colorectal cancer. J Ethnopharmacol. 2023;317:116695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 158. | Tang D, Wang H, Deng W, Wang J, Shen D, Wang L, Lu J, Feng Y, Cao S, Li W, Yin P, Xu K, Chen J. Mechanism of bufalin inhibition of colon cancer liver metastasis by regulating M2-type polarization of Kupffer cells induced by highly metastatic colon cancer cells. Apoptosis. 2024;29:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 159. | Yang B, Pan CS, Li Q, Yang Z, Long FX, Fan JY, Wang CS, Han JY, Tang DX. Inhibitory effects of Chanling Gao on the proliferation and liver metastasis of transplanted colorectal cancer in nude mice. PLoS One. 2019;14:e0201504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 160. | Li S, Shen D, Zuo Q, Wang S, Meng L, Yu J, Liu Y, Li W, Chen C, Yin P, Chen T, Wang J. Efficacy and safety of Huachansu combined with adjuvant chemotherapy in resected colorectal cancer patients: a prospective, open-label, randomized phase II study. Med Oncol. 2023;40:358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 161. | Ding L, Yang Y, Lu Q, Qu D, Chandrakesan P, Feng H, Chen H, Chen X, Liao Z, Du J, Cao Z, Weygant N. Bufalin Inhibits Tumorigenesis, Stemness, and Epithelial-Mesenchymal Transition in Colorectal Cancer through a C-Kit/Slug Signaling Axis. Int J Mol Sci. 2022;23:13354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 162. | Chen J, Wang H, Jia L, He J, Li Y, Liu H, Wu R, Qiu Y, Zhan Y, Yuan Z, Cao Y, Li W, Xu K, Yin P. Bufalin targets the SRC-3/MIF pathway in chemoresistant cells to regulate M2 macrophage polarization in colorectal cancer. Cancer Lett. 2021;513:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 163. | Wang H, Chen J, Li S, Yang J, Tang D, Wu W, Yu K, Cao Y, Xu K, Yin P, Chen Y, Li W. Bufalin reverses cancer-associated fibroblast-mediated colorectal cancer metastasis by inhibiting the STAT3 signaling pathway. Apoptosis. 2023;28:594-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 164. | Zhang W, Wang Y, Yu H, Jin Z, Yuan Y, Liu L, Zhou J. Exploring the mechanism of Erteng-Sanjie capsule in treating gastric and colorectal cancers via network pharmacology and in-vivo validation. J Ethnopharmacol. 2024;327:117945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 165. | Li J, Han Y, Zhou M, Liu N, Li H, Huang G, Yu Z, Luo D, Zhang H, Zheng X, Liang F, Chen R. Electroacupuncture ameliorates AOM/DSS-induced mice colorectal cancer by inhibiting inflammation and promoting autophagy via the SIRT1/miR-215/Atg14 axis. Aging (Albany NY). 2023;15:13194-13212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 166. | Shi C, Liu X, Han SS, Tang YF, Zeng HL, Du ML, Yang Y, Jia JN, Shi Q, Hou FG. Mechanism of Preventing Recurrence of Stage II-III Colorectal Cancer Metastasis with Immuno-inflammatory and Hypoxic Microenvironment by a Four Ingredients Chinese Herbal Formula: A Bioinformatics and Network Pharmacology Analysis. Curr Pharm Des. 2024;30:2007-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 167. | Shao C, Zuo Q, Lin J, Yu RJ, Fu Y, Xiao M, Sun LL, Lin L. Effect of Chinese Herbal Medicine on the Survival of Colorectal Cancer Patients With Liver-Limited Metastases: A Retrospective Cohort Study, 2008 to 2017. Integr Cancer Ther. 2019;18:1534735419883687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 168. | Wang ZJ, Zheng SH, Wang XH, Zhang YZ, Hao SL, Liu LK, Wang XX. Buzhong Tiaogan Formula for delaying colorectal liver metastasis (liver depression spleen deficient type): A multicenter randomized controlled trial: A study protocol. Medicine (Baltimore). 2021;100:e28040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 169. | Lee YS, Mun JG, Park SY, Hong DY, Kim HY, Kim SJ, Lee SB, Jang JH, Han YH, Kee JY. Saikosaponin D Inhibits Lung Metastasis of Colorectal Cancer Cells by Inducing Autophagy and Apoptosis. Nutrients. 2024;16:1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 170. | Hao SL, Zhou YC, Li XL, Zhong QM, Liu LK, Gao Y, Wang XX, Yang WH, Yang LF. Efficiency and safety of Buzhong Tiaogan granule for treating the colorectal cancer patients with liver metastasis: study protocol for a multicenter randomized controlled trial. Front Med (Lausanne). 2024;11:1465280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 171. | Chen C, Yao X, Xu Y, Zhang Q, Wang H, Zhao L, Wen G, Liu Y, Jing L, Sun X. Dahuang Zhechong Pill suppresses colorectal cancer liver metastasis via ameliorating exosomal CCL2 primed pre-metastatic niche. J Ethnopharmacol. 2019;238:111878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 172. | Zhao Y, Xiu L, Li Y, Lu Y, Wang X, Wei P. Effects of Xiaotan Tongfu decoction on hepatic metastasis in obesity-associated colorectal cancer. J Int Med Res. 2019;47:915-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 173. | Li Q, Chen JX, Wu Y, Lv LL, Ying HF, Zhu WH, Xu JY, Ruan M, Guo Y, Zhu WR, Zheng L. The mechanism of FZXJJZ decoction suppresses colorectal liver metastasis via the VDR/TGF-β/Snail1 signaling pathways based on network pharmacology-TCGA data-transcriptomics analysis. J Ethnopharmacol. 2022;287:114904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 174. | Pu Y, Han Y, Ouyang Y, Li H, Li L, Wu X, Yang L, Gao J, Zhang L, Zhou J, Ji Q, Song Q. Kaempferol inhibits colorectal cancer metastasis through circ_0000345 mediated JMJD2C/β-catenin signalling pathway. Phytomedicine. 2024;128:155261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 175. | Song YF, Zhou JY, Zhuang Y, Guo J, Wang XD, Wang YH, Zhao TT, Chen L, Chen H, Sun JH, Pei LX. Moxibustion attenuates liver metastasis of colorectal cancer by regulating gut microbial dysbiosis. Am J Cancer Res. 2023;13:394-407. [PubMed] |
| 176. | Liao S, Jia XL, Yang Y, Sun YX, Gong SM, Li M. Efficacy and safety of traditional Chinese medicine decoction combined with chemotherapy in the treatment of advanced colorectal cancer: A protocol of a systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e23952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 177. | Lin LT, Uen WC, Choong CY, Shi YC, Lee BH, Tai CJ, Tai CJ. Paris Polyphylla Inhibits Colorectal Cancer Cells via Inducing Autophagy and Enhancing the Efficacy of Chemotherapeutic Drug Doxorubicin. Molecules. 2019;24:2102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 178. | Mao W, Fan Y, Cheng C, Yuan X, Lan T, Mao K, Wang J. Efficacy and safety of Kanglaite injection combined with chemotherapy for colorectal cancer: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e22357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 179. | Gao W, Zhang K. Network meta-analysis of 8 types of traditional Chinese medicine injection combined with chemotherapy in colorectal cancer treatment. J Cancer Res Clin Oncol. 2023;149:9823-9838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 180. | Chen H, Deng J, Hou TW, Shan YQ. Villosol reverses 5-FU resistance in colorectal cancer by inhibiting the CDKN2A gene regulated TP53-PI3K/Akt signaling axis. J Ethnopharmacol. 2024;325:117907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 181. | Sun L, Xu Y, Chen N, Zhang C, Wu A, Wang H, Fei Y, Shu P, Diao D, Cheng J, Chu Y, Liu T, Wang W, Yuan Y, Zeng B, Cao Y, Cang S, Cao H, Zhang T, Zheng Y, Wu C, Liu S, He B, Yan Y, Yan S, Wu N, Ning C, Peng R, Epstein AS, Cytryn S, Mao JJ, Yang Y. Chinese herbal medicine (JianPi-BuShen) and completion rate of adjuvant chemotherapy for patients with stage II and III colon cancer: A randomized clinical trial. Eur J Cancer. 2024;213:115109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 182. | Deng H, Liu S, Li D, Wang W, Ye L, Xu S, Wang X, Li Y. Investigating the pharmacological mechanism of Zhengyuan jiaonang for treating colorectal cancer via network pharmacology analysis and experimental verification. J Ethnopharmacol. 2024;322:117607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 183. | Jie Y, Yang X, Chen W. Pulsatilla Decoction Combined with 5-Fluorouracil Triggers Immunogenic Cell Death in Colorectal Cancer Cells. Cancer Biother Radiopharm. 2022;37:945-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 184. | Zhang W, Peng C, Shen X, Yuan Y, Zhang W, Yang C, Yao M. A Bioactive Compound from Sanguisorba officinalis L. Inhibits Cell Proliferation and Induces Cell Death in 5-Fluorouracil-Sensitive/Resistant Colorectal Cancer Cells. Molecules. 2021;26:3843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 185. | Yan SH, Feng S, Xu Y, Yan YZ, He B, Sun LY, Pang B, Liu WJ, Xu YY, Zhao N, Tang M, Chen Y, Yu MK, Yang YF. Effectiveness of Herbal Medicine for Leukopenia/Neutropenia Induced by Chemotherapy in Adults with Colorectal Cancer: A Systematic Review and Meta-analysis. Integr Cancer Ther. 2021;20:15347354211021654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 186. | Yang MD, Zhou WJ, Chen XL, Chen J, Ji Q, Li Q, Wang WH, Su SB. Therapeutic Effect and Mechanism of Bushen-Jianpi-Jiedu Decoction Combined with Chemotherapeutic Drugs on Postoperative Colorectal Cancer. Front Pharmacol. 2021;12:524663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 187. | Chien A, Yang CC, Chang SC, Jan YM, Yang CH, Hsieh YL. Ultrasound Acupuncture for Oxaliplatin-induced Peripheral Neuropathy in Patients With Colorectal Cancer: A Pilot Study. PM R. 2021;13:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 188. | Chan K, Lui L, Lam Y, Yu K, Lau K, Lai M, Lau W, Tai L, Mak C, Bian Z, Zhong LL. Efficacy and safety of electroacupuncture for oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: a single-blinded, randomized, sham-controlled trial. Acupunct Med. 2023;41:268-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 189. | Chan K, Lui L, Yu K, Lau K, Lai M, Lau W, Ng B, Zhong LLD, Bian ZX. The efficacy and safety of electro-acupuncture for alleviating chemotherapy-induced peripheral neuropathy in patients with coloreactal cancer: study protocol for a single-blinded, randomized sham-controlled trial. Trials. 2020;21:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 190. | Cohen SA, Veleber S, Siman J, Guthrie KA, McMillen K, Heit M, Wadhera S, Daniels J, Hansen K, Jacoby M, Taromina K, Chin S, Romeo M, Langley BO, Coveler AL, Hannan LM, King G, Purcell T, Safyan RA, Shankaran V, Zhen DB, Chiorean EG, Greenlee H. Use of acupuncture with acupressure in addition to standard-of-care cryotherapy to decrease chemotherapy-associated neuropathy in patients with gastrointestinal malignancies receiving oxaliplatin-based chemotherapy: Study protocol for a randomized, controlled pilot and feasibility study. Contemp Clin Trials. 2023;131:107273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 191. | Lagerstedt K, Efverman A. A Randomized Sham-Controlled Mixed Methods Pilot Study of the Feasibility of Acupuncture for Chemotherapy-Induced Neuropathy: Lessons Learned From Patient Experiences in Integrative Cancer Care. Integr Cancer Ther. 2023;22:15347354231178877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 192. | Huang MC, Chang SC, Liao WL, Ke TW, Lee AL, Wang HM, Chang CP, Yen HR, Chang HH, Chen WT. Acupuncture May Help to Prevent Chemotherapy-Induced Peripheral Neuropathy: A Randomized, Sham-Controlled, Single-Blind Study. Oncologist. 2023;28:e436-e447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 193. | Lee JH, Cho TJ, Park MG, Kim JH, Song SK, Park SY, Sunwoo YY, Lee I, Park TY. Clinical study on concurrent use of electro-acupuncture or Chuna manual therapy with pregabalin for chemotherapy-induced peripheral neuropathy: safety and effectiveness (open-labeled, parallel, randomized controlled trial, assessor-blinded): A study protocol. Medicine (Baltimore). 2020;99:e18830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 194. | Lee JH, Gang J, Yang E, Kim W, Jin YH. Bee Venom Acupuncture Attenuates Oxaliplatin-Induced Neuropathic Pain by Modulating Action Potential Threshold in A-Fiber Dorsal Root Ganglia Neurons. Toxins (Basel). 2020;12:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 195. | Zhou H, Hu D, Zhao X, Qin S, Nong Q, Tian Y, Zhang Z, Dong H, Zhang P, Xu F. An optimal combination of four active components in Huangqin decoction for the synergistic sensitization of irinotecan against colorectal cancer. Chin Med. 2024;19:94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 196. | Huang WL, Wu SF, Xu ST, Ma YC, Wang R, Jin S, Zhou S. Allicin enhances the radiosensitivity of colorectal cancer cells via inhibition of NF-κB signaling pathway. J Food Sci. 2020;85:1924-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 197. | Weng W, Goel A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin Cancer Biol. 2022;80:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 198. | Lee YW, Lee I, Lee JH, Park MG, Kim JH, Sunwoo YY, Hwang MS, Park TY. Chuna Manual Therapy or Electroacupuncture with Pregabalin for Chemotherapy-Induced Peripheral Neuropathy: A Randomized Controlled Pilot Study. J Clin Med. 2024;13:3916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 199. | Lu Y, Qu HQ, Chen FY, Li XT, Cai L, Chen S, Sun YY. Effect of Baduanjin Qigong Exercise on Cancer-Related Fatigue in Patients with Colorectal Cancer Undergoing Chemotherapy: A Randomized Controlled Trial. Oncol Res Treat. 2019;42:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 200. | Li J, Fan S, Li H, Hu Z, Hu Q. Evaluation of efficacy, safety and underlying mechanism on Traditional Chinese medicine as synergistic agents for cancer immunotherapy: A preclinical systematic review and meta-analysis. J Ethnopharmacol. 2025;338:119035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 201. | Wang Y, Liu F, Du X, Shi J, Yu R, Li S, Na R, Zhao Y, Zhou M, Guo Y, Cheng L, Wang G, Zheng T. Combination of Anti-PD-1 and Electroacupuncture Induces a Potent Antitumor Immune Response in Microsatellite-Stable Colorectal Cancer. Cancer Immunol Res. 2024;12:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 202. | Song M, Qian C, Zhang T, Tang Y, Zhou Y, Wei Z, Wang A, Zhong C, Zhao Y, Lu Y. Salvia mitiorrhiza Bunge aqueous extract attenuates infiltration of tumor-associated macrophages and potentiates anti-PD-L1 immunotherapy in colorectal cancer through modulating Cox2/PGE2 cascade. J Ethnopharmacol. 2023;316:116735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 203. | Liu H, Deng R, Zhu CW, Han HK, Zong GF, Ren L, Cheng P, Wei ZH, Zhao Y, Yu SY, Lu Y. Rosmarinic acid in combination with ginsenoside Rg1 suppresses colon cancer metastasis via co-inhition of COX-2 and PD1/PD-L1 signaling axis. Acta Pharmacol Sin. 2024;45:193-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 204. | Lu Y, Yu M, Ye J, Liang Y, Gao J, Ji Z, Wang J. Sauchinone Inhibits the Proliferation and Immune Invasion Capacity of Colorectal Cancer Cells through the Suppression of PD-L1 and MMP2/MM9. Anticancer Agents Med Chem. 2023;23:1406-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 205. | Hu X, Wang J, Shang P, Wang S, Chen L, Ye C, Yao G. Sauchinone inhibits breast cancer cell proliferation through regulating microRNA-148a-3p/HER-2 axis. Thorac Cancer. 2023;14:1135-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 206. | Gao Q, Sheng Q, Yang Z, Zhu Z, Li L, Xu L, Xia J, Qiao Y, Gu J, Zhu X, Xie T, Sui X. Honokiol-Magnolol-Baicalin Possesses Synergistic Anticancer Potential and Enhances the Efficacy of Anti-PD-1 Immunotherapy in Colorectal Cancer by Triggering GSDME-Dependent Pyroptosis. Adv Sci (Weinh). 2025;12:e2417022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 207. | Feng S, Li S, Wu Z, Li Y, Wu T, Zhou Z, Liu X, Chen J, Fu S, Wang Z, Zhong Z, Zhong Y. Saffron improves the efficacy of immunotherapy for colorectal cancer through the IL-17 signaling pathway. J Ethnopharmacol. 2025;337:118854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 208. | Kong X, Li Q, Wang D, Wang M, Yang F, Meng J. Mechanism of Qizhen decoction-mediated maturation of DC cells to activate the IL-12/JAK2/STAT4 pathway to sensitise PD-1 inhibitors in the treatment of colorectal cancer. J Ethnopharmacol. 2024;320:117399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 209. | Cheng L, Xu L, Yuan H, Zhao Q, Yue W, Ma S, Wu X, Gu D, Sun Y, Shi H, Xu J. Jianpi Jiedu Recipe Inhibits Proliferation through Reactive Oxygen Species-Induced Incomplete Autophagy and Reduces PD-L1 Expression in Colon Cancer. Integr Cancer Ther. 2024;23:15347354241268064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 210. | Wang T, Wu L, Wang S, Shi X, Liu H, Deng W. Chang Wei Qing Decoction enhances the anti-tumor effect of PD-1 inhibitor therapy by regulating the immune microenvironment and gut microbiota in colorectal cancer. Chin J Nat Med. 2023;21:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 211. | Shan F, Sun L, Zhang L, Guo K, Yan Q, Feng G, Zhu Y, Shen M, Ruan S. Inhibition to Epithelial-Mesenchymal Transition and Metastatic Potential In Colorectal Cancer Cell By Combination of Traditional Chinese Medicine Formulation Jiedu Sangen Decoction and PD-L1 Inhibitor. Integr Cancer Ther. 2020;19:1534735420972486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 212. | Jeon Y, Sym SJ, Yoo BK, Baek JH. Long-term Survival, Tolerability, and Safety of First-Line Bevacizumab and FOLFIRI in Combination With Ginsenoside-Modified Nanostructured Lipid Carrier Containing Curcumin in Patients With Unresectable Metastatic Colorectal Cancer. Integr Cancer Ther. 2022;21:15347354221105498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 213. | Yang JW, Shao JK, Wang Y, Liu Q, Liang JW, Yan SY, Zhou SC, Yang NN, Wang LQ, Shi GX, Pei W, Liu CZ. Effect of acupuncture on postoperative ileus after laparoscopic elective colorectal surgery: A prospective, randomised, controlled trial. EClinicalMedicine. 2022;49:101472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 214. | Wang Y, Wang L, Ni X, Jiang M, Zhao L. Effect of acupuncture therapy for postoperative gastrointestinal dysfunction in gastric and colorectal cancers: an umbrella review. Front Oncol. 2024;14:1291524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 215. | Chen HT, Hung KC, Huang YT, Wu JY, Hsing CH, Lin CM, Chen IW, Sun CK. Efficacy of electroacupuncture in improving postoperative ileus in patients receiving colorectal surgery: a systematic review and meta-analysis. Int J Surg. 2024;110:1113-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 216. | Zhang X, Yang W, Shang J, Dan W, Shi L, Tong L, Yang G. The lower He-sea points playing a significant role in postoperative ileus in colorectal cancer treated with acupuncture: based on machine-learning. Front Oncol. 2023;13:1206196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 217. | Liang XW, Chen L, Wu JC. Comment on 'Efficacy of electroacupuncture in improving postoperative ileus in patients receiving colorectal surgery: A systematic review and meta-analysis'. Int J Surg. 2024;110:1800-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 218. | Gao W, Li W, Yan Y, Yang R, Zhang Y, Jin M, Luo Z, Xie L, Ma Y, Xu X, Wang G, Kong Z, Gao Y, Li Y, Ruan Z, Zheng J, Ma D, Wang Q. Transcutaneous electrical acupoint stimulation applied in lower limbs decreases the incidence of paralytic ileus after colorectal surgery: A multicenter randomized controlled trial. Surgery. 2021;170:1618-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 219. | Zhao X, Si S, Liu X, Liu J, Zhang D, Mu Y, Hou A. Does invasive acupuncture improve postoperative ileus after colorectal cancer surgery? A systematic review and meta-analysis. Front Med (Lausanne). 2023;10:1201769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 220. | Ru O, Jin X, Qu L, Long D, Liu M, Cheng L, Jiang Y. Low-intensity transcutaneous auricular vagus nerve stimulation reduces postoperative ileus after laparoscopic radical resection of colorectal cancer: a randomized controlled trial. Minerva Anestesiol. 2023;89:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 221. | Huang W, Long W, Xiao J, Zhao G, Yu T. Effect of electrically stimulating acupoint, Zusanli (ST 36), on patient's recovery after laparoscopic colorectal cancer resection: a randomized controlled trial. J Tradit Chin Med. 2019;39:433-439. [PubMed] |
| 222. | Cao LX, Chen ZQ, Jiang Z, Chen QC, Fan XH, Xia SJ, Lin JX, Gan HC, Wang T, Huang YX. Rapid rehabilitation technique with integrated traditional Chinese and Western medicine promotes postoperative gastrointestinal function recovery. World J Gastroenterol. 2020;26:3271-3282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 223. | Kunitomi Y, Nakashima M, Takeuchi M, Kawakami K. Efficacy of Daikenchuto in the prevention of bowel obstruction in patients with colorectal cancer undergoing laparoscopic surgery: An observational study using a Japanese administrative claims database. Support Care Cancer. 2023;31:133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 224. | Xu Y, Liu RR, Yu XJ, Liu XN, Zhang X, Jiang ZH, Cong ZF, Li QQ, Gao P. Quality markers of Dajianzhong decoction based on multicomponent qualitative and quantitative analysis combined with network pharmacology and chemometric analysis. Phytochem Anal. 2024;35:146-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 225. | Liu T, Xu M, Shi Z, Li M, Wang R, Shi Y, Xu X, Shao T, Sun Q. Shenhuang plaster ameliorates the Inflammation of postoperative ileus through inhibiting PI3K/Akt/NF-κB pathway. Biomed Pharmacother. 2022;156:113922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 226. | Jiang Z, Chen Q, Zhang J, Cao L, Chen Z. Wuda granule, a traditional Chinese herbal medicine, ameliorates postoperative ileus by anti-inflammatory action. Pathol Res Pract. 2020;216:152605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 227. | Gan H, Lin J, Jiang Z, Chen Q, Cao L, Chen Z. Xiangbin prescription for the recovery of gastrointestinal function after abdominal surgery (the XBPRS trial): study protocol for a randomized controlled trial. Trials. 2018;19:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 228. | Xu G, Xiao Q, Lei H, Fu Y, Kong J, Zheng Q, Zhao L, Liang F. Effectiveness and safety of acupuncture and moxibustion for defecation dysfunction after sphincter-preserving surgery for rectal cancer: protocol for systematic review and meta-analysis. BMJ Open. 2020;10:e034152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 229. | Fei M, Zhang J, Zhu C, Luo M, Zhang L, Wu Y. Effects of Modified Baizhu Shaoyao San on Postoperative Diarrhea in Colorectal Cancer Patients: A Single-Blind, Randomized Controlled Trial. Complement Med Res. 2023;30:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 230. | Dulskas A, Aukstikalnis T, Kavaliauskas P, Samalavicius NE. The Role of Traditional Acupuncture in Low Anterior Resection Syndrome Treatment: A Pilot Study. Dis Colon Rectum. 2022;65:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 231. | Xu G, Lei H, Zhou Y, Huang L, Tian H, Zhou Z, Zhao L, Liang F. Acupuncture for Quality of Life of Patients with Defecation Dysfunction after Sphincter Preserving Surgery for Rectal Cancer: A Systematic Review. Evid Based Complement Alternat Med. 2021;2021:7858252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 232. | Jiang Y, Liang D, He Y, Wang J, Xu G, Wang J. Acupuncture and moxibustion for cancer-related psychological disorders: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2022;101:e28860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 233. | Hou Y, Lu J, Xie J, Zhu R, Wu M, Wang K, Zhou J, Li J. Effects of electroacupuncture on perioperative anxiety and stress response in patients undergoing surgery for gastric or colorectal cancer: Study protocol for a randomized controlled trial. Front Psychiatry. 2023;14:1095650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 234. | Hu Q, Chen XP, Tang ZJ, Zhu XY, Liu C. Therapeutic effects of Buzhong Yiqi decoction in patients with spleen and stomach qi deficiency after routine surgery and chemotherapy for colorectal cancer. World J Gastrointest Surg. 2024;16:2183-2193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (5)] |
| 235. | He L, Xu D, Jia C, Xu N, Lin L, Lin J. Effect of Fatigue Three-Needle acupuncture therapy in a patient with cancer-related fatigue: A case report. Medicine (Baltimore). 2019;98:e15659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 236. | Li Z, Xiong Q, Li S, Chen W, Xu N, Qiu F. Quantitative analysis and visualization of literature on acupuncture and related TCM therapies for the treatment of colorectal cancer based on CiteSpace. Front Oncol. 2023;13:1290588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 237. | Jin Y, Liu H, Wang Y, Zhang R, Wang Q, Wang Y, Cui H, Wang X, Bian Y. Pathogenesis and treatment of colitis-associated colorectal cancer: Insights from Traditional Chinese Medicine. J Ethnopharmacol. 2025;338:119096. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 238. | Wang YN, Zou M, Wang D, Zhang ZK, Qu LP, Xu J, Shi CD, Gao F. An exploratory study on TCM syndrome differentiation in preoperative patients with colorectal cancer assisted by laboratory indicators. Heliyon. 2022;8:e10207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 239. | Wang P, Ding S, Sun L, Feng Y, Guo K, Zhu Y, Huang D, Ruan S. Characteristics and differences of gut microbiota in patients with different Traditional Chinese Medicine Syndromes of Colorectal Cancer and normal population. J Cancer. 2020;11:7357-7367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 240. | Ming-Bin G, Ya-Nan W, Yong-Ting X, Min Z, Hao T, Lian-Ping Q, Feng G. TCM syndrome differentiation in colorectal cancer patients assisted by differences in gut microbiota: An exploratory study. Heliyon. 2023;9:e21057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 241. | Chen Q, Huang X, Zhang H, Jiang X, Zeng X, Li W, Su H, Chen Y, Lin F, Li M, Gu X, Jin H, Wang R, Diao D, Wang W, Li J, Wei S, Zhang W, Liu W, Huang Z, Deng Y, Luo W, Liu Z, Zhang B. Characterization of tongue coating microbiome from patients with colorectal cancer. J Oral Microbiol. 2024;16:2344278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 242. | Li Z, Teng L, Pan Z, Yang Y, Zhu J, Wu X, Qian Y, Qian H, Bian Y, Chen Y, Chen W, Bi L. Identification of Comprehensive Biomarkers in Patients With Mismatch Repair-Deficient Colon Adenocarcinoma Based on Parallel Multiomics. Lab Invest. 2024;104:100306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 243. | Zou M, Zhang YS, Feng JK, Tu H, Gui MB, Wang YN, Yang ZJ, Yang ZQ, Xu M, Wu WQ, Gao F. Serum metabolomics analysis of biomarkers and metabolic pathways in patients with colorectal cancer associated with spleen-deficiency and qi-stagnation syndrome or damp-heat syndrome: a prospective cohort study. Front Oncol. 2023;13:1190706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 244. | Hu XQ, Wei B, Song YN, Ji Q, Li Q, Luo YQ, Wang WH, Su SB. Plasma metabolic profiling on postoperative colorectal cancer patients with different traditional Chinese medicine syndromes. Complement Ther Med. 2018;36:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 245. | Lu Y, Zhou C, Zhu M, Fu Z, Shi Y, Li M, Wang W, Zhu S, Jiang B, Luo Y, Su S. Traditional chinese medicine syndromes classification associates with tumor cell and microenvironment heterogeneity in colorectal cancer: a single cell RNA sequencing analysis. Chin Med. 2021;16:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 246. | Yan Y, Yang Y, Ning C, Wu N, Yan S, Sun L. Role of Traditional Chinese Medicine Syndrome Type, Gut Microbiome, and Host Immunity in Predicting Early and Advanced Stage Colorectal Cancer. Integr Cancer Ther. 2023;22:15347354221144051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |