Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.105170
Revised: March 11, 2025
Accepted: April 18, 2025
Published online: June 15, 2025
Processing time: 151 Days and 4.3 Hours
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related deaths worldwide and currently lacks effective treatment options. This is particularly true for advanced HCC, for which conventional therapies often lead to a poor prognosis.
To assess the safety and efficacy of transarterial chemoembolization (TACE) with donafenib and immune checkpoint inhibitors (ICIs) for unresectable HCC.
We retrospectively assessed the data of patients with HCC who underwent TACE combined with donafenib and an ICI (tislelizumab or cedilimumab). Patients received oral donafenib daily for 2 weeks before TACE, followed by tislelizumab or cedilimumab 200 mg intravenously on day 1 of a 21-day therapeutic cycle. The primary endpoints were objective response rate, disease control rate, and duration of response according to the modified RECIST criteria. The secondary endpoint was presence of treatment-related adverse events (TRAEs).
The median follow-up was 7.8 months (95%CI: 5.0-11.8 months). The objective response rate was 60.0% (18/30), while the disease control rate was 93.3%. The median duration of response in confirmed responders was 6.6 months (95%CI: 1.3-12.9 months). The median progression-free survival was 11.8 months (95%CI: 8.3-15.4 months). More than half of the patients survived until the end of the study. Grade > 3 TRAEs occurred in 40% of the patients with no grade 5 TRAEs reported. The most common grade 3/4 TRAE was palmar-plantar erythrodysesthesia, a dermatologic condition characterized by painful redness and swelling of the palms and soles, with an incidence of 56.7%. No ICI-related adverse effects were observed.
TACE combined with donafenib and ICI is a promising and safe therapeutic regimen for unresectable HCC.
Core Tip: Transarterial chemoembolization combined with donafenib and immune checkpoint inhibitors has been clinically applied in patients with unresectable hepatocellular carcinoma. This study assesses the safety and efficacy of this treatment strategy to better promote the adoption thereof in clinical practice.
- Citation: Yang ZH, Liu SN, Chu FY, Yang C, Chen X. Efficacy and safety of donafenib plus transarterial chemoembolization and immunotherapy for hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(6): 105170
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/105170.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.105170
The incidence of liver cancer varies significantly worldwide and ranks among the top three causes of cancer-related deaths in 46 countries and within the top five in 90 countries. We predict that the number of cases and deaths will increase over the next 20 years with growth of the world population[1]. Hepatocellular carcinoma (HCC) is the primary type of liver cancer and the fourth leading cause of cancer-related death worldwide[2]. The treatment for early HCCs is affirmative, with resection being recommended for patients without liver cirrhosis, and ablation and transplantation for those with liver cirrhosis, both of which can achieve a favorable prognosis. However, treatment choices for intermediate HCCs are heterogeneous and personalized. Moreover, in late-stage HCCs with portal vein invasion or extrahepatic metastasis, transarterial chemoembolization (TACE) and systemic therapy are the mainstream treatment modalities, although the prognosis remains unsatisfactory[3].
In recent years, systemic therapy for intermediate- and late-stage HCCs has improved with novel tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs). Donafenib is a novel TKI approved as a first-line monotherapy for advanced HCC in China[4]. Additionally, ICIs, including anti-programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) inhibitors, are being increasingly used in the clinical management of HCC. Although monotherapy with ICI does not demonstrate a survival benefit[5], the combination treatment of atezolizumab and bevacizumab (T + A) has displayed an astonishing effect and is the mainstream therapy for advanced HCC[6].
Due to the efficacy of the T + A regimen and results from phase II trials[7,8], clinicians have paid considerable attention to the combination treatment of TACE with TKIs and ICIs. Furthermore, previous studies have reported interesting results for lenvatinib-based regimens. However, few studies have investigated the effects of donafenib. Therefore, this retrospective study aimed to review the data of patients with HCC who received a donafenib-based triad therapy regimen. The results presented here provide evidence of the efficacy and safety of combination treatment for unresectable HCC, which can be widely applied to prolong the survival time of patients with HCC. This also provides clinical doctors with more treatment options.
In this retrospective study, the medical records and imaging data of patients with HCC were obtained at the Shanghai Eastern Hepatobiliary Surgery Hospital and Nantong First People’s Hospital between June 2021 and February 2023. Inclusion criteria were as follows: (1) Diagnosis of HCC with pathological or radiological evidence; (2) At least one measurable target lesion; (3) Eastern Cooperative Oncology Group Performance Status (ECOG-PS) score of 0-1; and (4) Child–Pugh class A score of 5-6. Exclusion criteria were as follows: (1) Prior history of or simultaneous cancer in other organs; (2) Tumor burden > 70% of the liver volume; (3) History of autoimmune disease; and (4) Platelet count < 50 × 109/L. This study was approved by the hospital ethics committee. All the patients provided written informed consent before inclusion in the study.
TACE was performed by an interventional radiologist with more than 10 years of experience. After puncturing the femoral artery, celiac trunk, and superior mesenteric artery, angiography was performed selectively using a 5-F catheter (RH catheter; Cook, Bloomington, IN, USA). When tumor-feeding arteries were identified, the catheter was advanced into these arteries individually. Moreover, a 3-F microcatheter (SP microcatheter; Terumo, Tokyo, Japan) was utilized for selective catheterization if necessary. Oxaliplatin (75 mg/m2) was infused via the catheter and iodized oil (Lipiodol Ultrafluid; Guerbet, Aulnay-sous-Bois, France) mixed with epirubicin (30-50 mg/m2) was administered to embolize the tumor-feeding arteries. Subsequent TACE procedures were determined based on the follow-up results.
Donefenib was prescribed orally at a dose of 200 mg twice daily. ICI (tislelizumab or cedilimumab) was prescribed at 200 mg, intravenously infused every 3 weeks. In case of grade 3 treatment-related adverse events (TRAEs), the donefenib dose was reduced to 100 mg twice daily.
Follow-up visits were performed 4-6 weeks after TACE. The patients underwent chest computed tomography (CT), abdominal multiphase CT or magnetic resonance imaging, and laboratory examinations during each follow-up visit. The laboratory examinations encompassed liver function tests, including bilirubin, aspartate aminotransferase, alanine aminotransferase, albumin, and g-glutamyl transpeptidase levels, along with prothrombin time and serum alpha-fetoprotein (AFP) level evaluation. Overall survival (OS) was defined as the duration from the first TACE treatment to death or last follow-up. Progression-free survival (PFS) was defined as the duration from the first TACE treatment to disease recurrence or last follow-up. Intrahepatic tumor progression (25% increase from baseline) and transient deterioration of liver function to Child–Pugh C, macrovascular invasion, or extrahepatic metastasis were considered indicators of disease progression[9].
Tumor response was evaluated using the mRECIST Criteria for Solid Tumors. The primary endpoints were objective response rate (ORR), disease control rate (DCR), and duration of response (DOR). Furthermore, the secondary endpoint was the incidence of TRAEs. Other indicators including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were also assessed.
This retrospective pilot study aimed to evaluate the feasibility and preliminary efficacy of TACE combined with donafenib and ICIs in unresectable HCC. Due to the exploratory nature of the study and limited prior data on this regimen, a formal sample size calculation was not conducted a priori. However, a post hoc power analysis indicated that the observed ORR of 60% (vs a historical control of ≤ 40%) achieved 80% statistical power (α = 0.05) with the included cohort (n = 30). The Kaplan–Meier method was used to estimate the DOR, PFS, and OS. Patients with a confirmed CR or PR were analyzed for DOR. All statistical analyses were performed using SPSS version 23.0.
Thirty eligible patients were enrolled between June 2021 and February 2023. The baseline characteristics of the enrolled patients are summarized in Table 1. The median age was 56 years; most patients were male (29/30), and 28 had Child–Pugh class A liver function, with hepatitis B viral infection being the main etiology (29/30). Additionally, 1, 4, and 25 patients were classified as Barcelona Clinic Liver Cancer stages A, B, and C, respectively.
| Parameters | No. |
| Age (years) | |
| ≤ 65 | 23 |
| > 65 | 7 |
| Gender | |
| Male | 29 |
| Female | 1 |
| HBV infection | |
| Yes | 29 |
| No | 1 |
| HCV infection | |
| Yes | 0 |
| No | 30 |
| Child–Pugh score | |
| A | 28 |
| B | 2 |
| AFP (ng/mL) | |
| ≤ 400 | 17 |
| > 400 | 13 |
| PIVKA (mAU/mL) | |
| Median level | 1423.50 |
| ECOG-PS | |
| 0 | 29 |
| 1 | 1 |
| BCLC stage | |
| B | 4 |
| C | 26 |
All the patients received donafenib, ICI, or TACE as combination therapies. In particular, nine patients received combination therapy as a second-line regimen due to disease progression after lenvatinib or sorafenib treatment. At the data cutoff in July 2023, 10 patients (33.3%) were still receiving donafenib treatment. Reasons for discontinuation included disease progression, adverse effects (AEs), and withdrawal of consent. The median number of treatments administered was two (95%CI: 1-2 times), with the median treatment duration of donafenib being 211 days. The median follow-up was 7.8 months (95%CI: 5.0-11.8 months).
A total of 28 patients (93.3%) experienced TRAEs of any grade. The most common TRAEs were palmar-plantar erythrodysesthesia (PPE, 56.7%), rash (40%), diarrhea (16.7%), hypertension (13.3%), and fatigue (6.7%) (Table 2). No ICI TRAEs, including autoimmune thyroiditis or colitis, were observed. During the study, 40% of patients experienced grade 3 AEs (n = 12), with PPE being the most common grade 3 event (23.3%). Additionally, 3.3% of patients experienced grade 4 AEs (n = 1). A total of 3.3% (n = 1) of patients discontinued treatment due to AEs. No grade 5 TRAEs were reported, and one patient discontinued donafenib due to TRAEs.
| AEs | Any grade No. | Grade 1 No. (%) | Grade 2 No. (%) | Grade 3 No. (%) | Grade 4 No. (%) | Grade 5 No. (%) |
| Palmar-plantar erythrodysesthesia | 17 (56.7) | 7 | 8 | 2 | 0 | 0 |
| Rash | 12 (40) | 5 | 5 | 2 | 0 | 0 |
| Diarrhea | 5 (16.7) | 0 | 2 | 0 | 1 | 0 |
| Hypertension | 4 (13.3) | 3 | 1 | 0 | 0 | 0 |
| Hair loss | 4 (13.3) | 2 | 2 | 1 | 0 | 0 |
| Fatigue | 2 (6.7) | 0 | 1 | 1 | 0 | 0 |
| Gastrointestinal hemorrhage | 1 (3.33) | 0 | 0 | 1 | 0 | 0 |
All patients were eligible for objective response evaluation. Three and 15 patients achieved CR and PR, respectively, with an ORR of 60.0% (18/30). Ten patients were rated as having SD and two as having PD, resulting in a DCR of 93.3% (Table 3).
| Tumor response | Value |
| CR | 10% (3/30) |
| PR | 50% (15/30) |
| ORR | 60% |
| SD | 33.3% (10/30) |
| PD | 6.7% (2/30) |
| DCR | 93.3% |
| Median TTP | 6.3 |
| Median DOR | 6.6 |
| Median PFS | 11.8 |
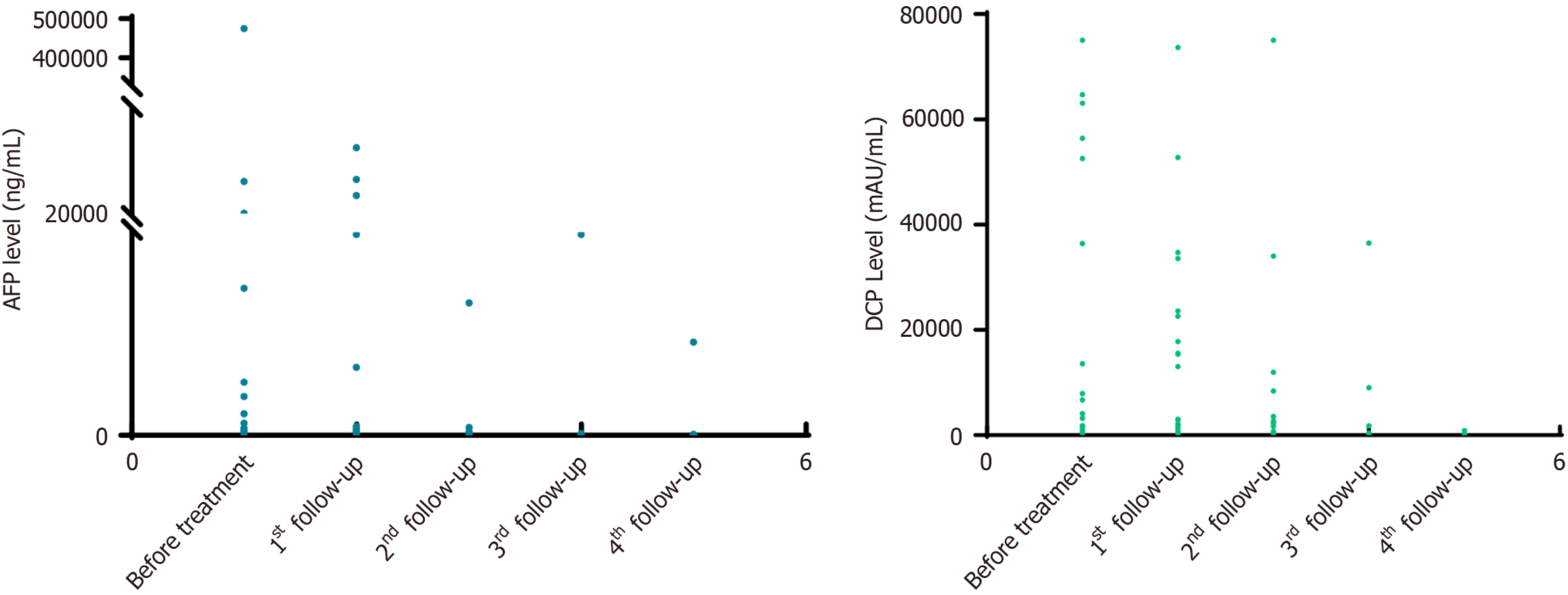
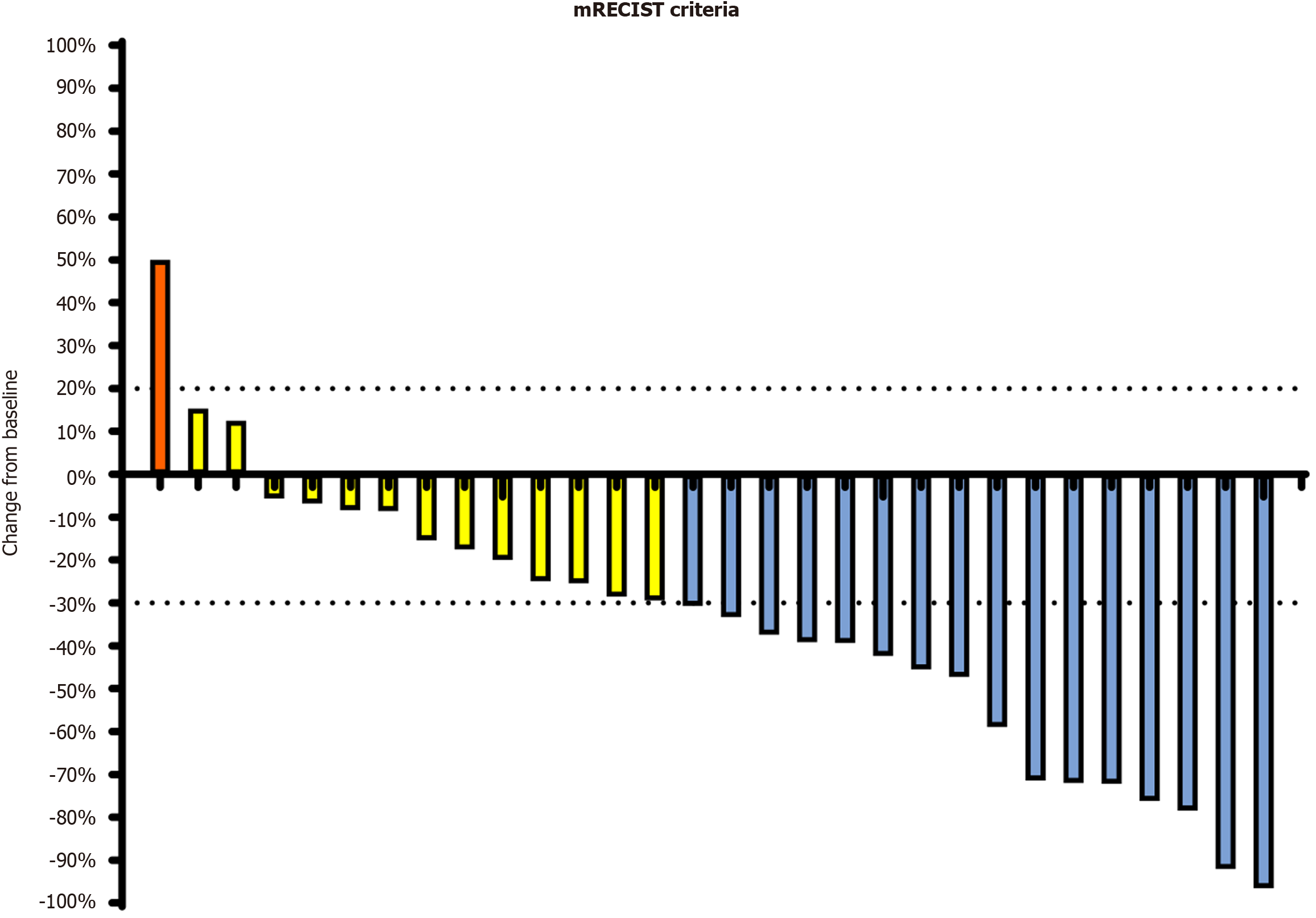
The median baseline levels of AFP and protein induced by vitamin K absence-II (des-gamma carboxyprothrombin) were 168 ng/mL and 1423.5 mAU/mL, respectively. Figure 1 displays the dynamics of the biomarker values at different follow-up periods. Tumor assessment was based on the mRECIST criteria. The optimal percentage change in the size of the target lesion per patient is illustrated in Figure 2.
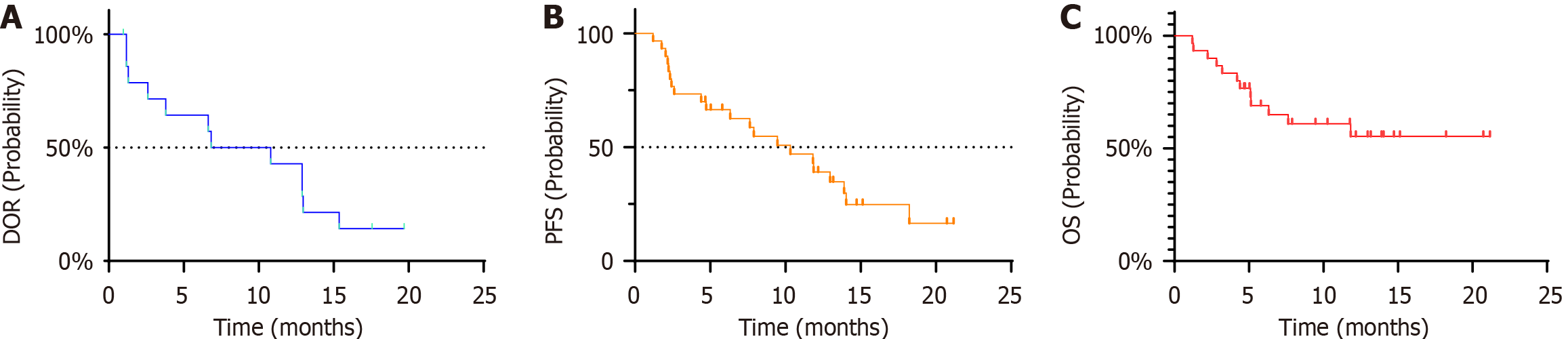
The median time to progression was 6.3 months (95%CI: 2.3-11.3), and the DOR in confirmed responders was 6.8 months (95%CI: 2.6-13.0; Figure 3A). The median PFS was 11.8 months (95%CI: 8.3-15.4 months, Figure 3B). During the study period, 11 patients died, and the remaining patients experienced SD. The median OS was not reached and the 2-year survival probability was estimated (Figure 3C).
HCC is a highly heterogeneous malignant tumor with treatment varying based on liver function, tumor stage, and physical status. Even for tumors at the same stage, including intermediate HCC, treatment can be personalized. In general, TACE is often used in patients with unresectable or marginally resectable HCCs with the aim of tumor downstaging. Targeted drugs are another viable option in addition to TACE. Sorafenib, the first TKI approved for unresectable HCC in 2006[10] remained the sole option for a decade until lenvatinib demonstrated comparable efficacy. Donafenib demonstrates survival superiority over sorafenib in improving OS, and has favorable safety and tolerability in Chinese patients with advanced HCC[4]. Additionally, this drug has become the first-line treatment of choice for unresectable HCC in China.
Immunotherapy for HCC has garnered significant attention in recent years. Numerous anti-PD-1 and anti-PD-L1 antibodies have been developed and tested in clinical trials. Although ICI monotherapy failed to achieve positive results, the combination of ICI with TACE, ICI with vascular endothelial growth factor (VEGF) monotherapy, or dual immunotherapy demonstrated survival benefits. In the IMbrave150 trial[6], T + A resulted in significantly better OS and PFS than those observed with sorafenib alone in patients with unresectable HCC. This also raises the question of whether TACE combined with TKI and ICI can achieve improved treatment outcomes.
The use of TKI alone is not satisfactory; however, a combination of ICIs and TKI has demonstrated superior effects[11]. The mechanism of combination therapy remains unclear, and it is surmised that changes in the tumor microenvironment play an important role in this process. HCC develops and evolves through continuous crosstalk with the surrounding microenvironment, and emerging evidence indicates that immunosuppression often occurs concomitantly in response to this crosstalk. Therefore, combinatorial strategies with immunotherapy are likely to disrupt the balance of the tumor environment and improve treatment responses. For instance, VEGF may play a significant role in maintaining the immunosuppressive microenvironment of HCC, and blocking VEGF enhances the efficacy of ICI[12]. Therefore, we retrospectively evaluated the prognosis of patients with advanced HCC managed with TACE or donafenib plus ICI to explore the superiority of donafenib.
TACE is a viable treatment option for intermediate-stage (multinodular, preserved liver function, and ECOG-PS = 0) HCC, although repeated procedures may disrupt the tumor microenvironment, potentially contributing to adverse outcomes. Repeated TACE can be detrimental to liver function, portal invasion, and extrahepatic spread[13]. Therefore, TACE, combined with TKIs and ICIs, is widely used to treat unresectable HCC. The mechanism of combination therapy may involve TACE inducing hypoxia in cancer cells, which leads to the upregulation of VEGF during angiogenesis. The use of TKI before or after TACE normalizes abnormal tumor vessels and down-regulates VEGF[14]. In this study, all patients received donafenib 2 weeks before TACE to obtain the optimum results.
In the present study, the ORR was 63.3% (19/30), whereas CR and PR were observed in three and 16 patients, respectively. The DOR was 6.6 months (95%CI: 1.3-12.9 months). PFS was 11.8 months (95%CI: 8.3-15.4 months) with over half of patients surviving at the end of the study. Compared with the outcomes of IMbrave150, the PFS was longer (11.8 months vs 6.8 months) indicating that TACE combined with TKI and ICI helps improve prognosis. The median PFS and OS were longer than those reported in a similar study of lenvatinib combined with TACE and ICI[15]. A comparison of PFS with that in Mbrave150 also demonstrated that PFS slowed progression of the disease more than that seen in Mbrave150, leading to prolonged patient survival[16]. Therefore, this study demonstrated that TACE combined with donafenib and an ICI has good efficacy in unresectable HCC.
With regard to the safety of combination therapy, although we observed frequent TRAEs, only one patient (3.3%) discontinued donafenib treatment due to grade 3 or 4 AEs. The incidence and severity of AEs were similar to those documented in a previously reported trial and lower than those associated with sorafenib. The most common TRAEs were PPE, rashes, diarrhea, hypertension, and fatigue. Furthermore, donafenib a deuterated derivative of sorafenib, may offer higher stability and reduced susceptibility to hepatic drug-metabolizing enzymes, which increase plasma exposure and reduce toxic metabolite levels. Compared to monotherapy with donafenib, combination treatment does not increase the incidence of AEs[4], making the therapy safe and tolerable for patients with unresectable HCCs.
Several limitations should be noted before further application of this combination regimen. First, potential bias may exist due to the retrospective design of the study and the limited sample size. Second, the small sample size limits the statistical power for subgroup analyses. Additionally, this study did not explore etiology-specific treatment responses [e.g., hepatitis B virus (HBV) vs non-HBV HCC] due to the homogeneity of the cohort and limited sample size. Future larger trials should prioritize etiological stratification to refine therapeutic strategies.
Compared to the latest technologies, this triple therapy may not be particularly effective, but its safety is undeniable. Moreover, this technology is already well-established, and we can effectively implement the treatment plan. However, as this is a retrospective report, the study has the disadvantage of being one-sided, which is unavoidable.
The ORR, DOR, PFS, and OS in this study were not inferior to the previously reported results of other combination regimens, and the AEs were acceptable. TACE combined with donafenib and ICIs is effective and safe in patients with unresectable HCC. This triad regimen may be a feasible option to improve the prognosis of patients with unresectable HCC.
| 1. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1061] [Article Influence: 353.7] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 3. | Erridge S, Pucher PH, Markar SR, Malietzis G, Athanasiou T, Darzi A, Sodergren MH, Jiao LR. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, Ying J, Lu Y, Meng Z, Pan H, Yang P, Zhang H, Chen X, Xu A, Cui C, Zhu B, Wu J, Xin X, Wang J, Shan J, Chen J, Zheng Z, Xu L, Wen X, You Z, Ren Z, Liu X, Qiu M, Wu L, Chen F. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol. 2021;39:3002-3011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 257] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 5. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1339] [Article Influence: 267.8] [Reference Citation Analysis (0)] |
| 6. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4687] [Article Influence: 937.4] [Reference Citation Analysis (2)] |
| 7. | Cao F, Yang Y, Si T, Luo J, Zeng H, Zhang Z, Feng D, Chen Y, Zheng J. The Efficacy of TACE Combined With Lenvatinib Plus Sintilimab in Unresectable Hepatocellular Carcinoma: A Multicenter Retrospective Study. Front Oncol. 2021;11:783480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 8. | Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, Sun JH, Jin ZC, Chen JJ, Ge NJ, Ding WB, Li WH, Huang JH, Mu W, Gu SZ, Li JP, Zhao H, Wen SW, Lei YM, Song YS, Yuan CW, Wang WD, Huang M, Zhao W, Wu JB, Wang S, Zhu X, Han JJ, Ren WX, Lu ZM, Xing WG, Fan Y, Lin HL, Zhang ZS, Xu GH, Hu WH, Tu Q, Su HY, Zheng CS, Chen Y, Zhao XY, Fang ZT, Wang Q, Zhao JW, Xu AB, Xu J, Wu QH, Niu HZ, Wang J, Dai F, Feng DP, Li QD, Shi RS, Li JR, Yang G, Shi HB, Ji JS, Liu YE, Cai Z, Yang P, Zhao Y, Zhu XL, Lu LG, Teng GJ; CHANCE001 Investigators. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 76.5] [Reference Citation Analysis (1)] |
| 9. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 502] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 10. | Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, Taylor I, Moscovici M, Saltz LB. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 908] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 11. | Chen R, Ielasi L, di Carlo A, Tovoli F. Donafenib in hepatocellular carcinoma. Drugs Today (Barc). 2023;59:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 12. | Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 1394] [Article Influence: 199.1] [Reference Citation Analysis (0)] |
| 13. | Ghanaati H, Mohammadifard M, Mohammadifard M. A review of applying transarterial chemoembolization (TACE) method for management of hepatocellular carcinoma. J Family Med Prim Care. 2021;10:3553-3560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Li X, Chen J, Wang X, Bai T, Lu S, Wei T, Tang Z, Huang C, Zhang B, Liu B, Li L, Wu F. Outcomes and prognostic factors in initially unresectable hepatocellular carcinoma treated using conversion therapy with lenvatinib and TACE plus PD-1 inhibitors. Front Oncol. 2023;13:1110689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 15. | Zhao S, Zhou M, Wang P, Yang J, Zhang D, Yin F, Song P. Sorafenib, Lenvatinib, or Lenvatinib Combining PD-1 Inhibitors Plus TACE in Unresectable Hepatocellular Carcinoma: A Retrospective Analysis. Technol Cancer Res Treat. 2022;21:15330338221133640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 16. | Zhong W, Yang C, Zhang Y, Liu Y, Yang D. The Chemical Profiling and Anticancer Potential of Functional Polysaccharides from Flos Sophorae Immaturus. Molecules. 2022;27:5978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |