Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.103632
Revised: March 19, 2025
Accepted: March 20, 2025
Published online: June 15, 2025
Processing time: 121 Days and 5.7 Hours
This retrospective study aimed to define associations between Helicobacter pylori
To define associations between H. pylori in childhood and risk factors for gastric cancer with special emphasis on the role of family history of cancer.
Details of 600 children who were subjected to upper gastrointestinal endoscopies at our institution are analyzed. Children were classified into positive and negative groups for H. pylori infection based on biopsy and rapid urease tests. The oc
In our study, among the overall population, 330 children tested positive for H. pylori, which constituted 55% of the study population. The group denoting H. pylori positivity was found to have strikingly higher frequencies of chronic superficial gastritis (78.8% vs 5.9%), gastric atrophy (39.4% vs 7%), and intestinal metaplasia (0.9% vs 0%), as compared to the H. pylori-negative group. It is interesting to observe that there were a few but statistically significant cases of H. pylori-positive children having a family history of gastric cancer (1.2%), whereas no such cases were reported in children who were H. pylori-negative.
Our study finds that H. pylori infection in childhood is associated with an increased risk of precancerous gastric conditions and that family history might provide an additional risk. These insights recommend the necessity of early H. pylori detection and intervention and management strategies in childhood, especially in those families with histories of gastric cancer.
Core Tip: Our investigation exposes a potent correlation: Children afflicted with Helicobacter pylori (H. pylori) exhibit an augmented vulnerability to gastric cancer, notably when there's a genetic lineage of the ailment. This underscores the pressing requirement for preemptive diagnostics and intervention within the juvenile demographic to avert potential gastric malignancies. By highlighting the pivotal role of heredity in pediatric gastric health, our study paves the way for tailored preventative measures, enhancing clinical practices in the eradication of H. pylori and fortifying the frontline against gastric cancer in youth. This insight is crucial for the surgical community, potentially revolutionizing how we approach early cancer detection and prevention.
- Citation: Gong X, Chen C, Shen JF. Gastric cancer in children infected with Helicobacter pylori. World J Gastrointest Oncol 2025; 17(6): 103632
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/103632.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.103632
Helicobacter pylori (H. pylori), recognized globally as the most pervasive chronic bacterial infection, casts a wide net across both adult and pediatric populations[1,2]. This bacterium, classified as a class 1 carcinogen[3], exhibits a staggering prevalence, affecting an estimated 80% of individuals in developing nations and a significant proportion, ranging from 18.2% to 63%, in children within the age bracket of 3 to 12 years[4]. In the pediatric realm, H. pylori can manifest with a spectrum of nonspecific symptoms such as epigastric discomfort, queasiness, lack of appetite, iron deficiency anemia, and even vomiting blood[5]. A considerable number of infected children, however, may remain free of noticeable symptoms[2]. H. pylori is a known instigator of gastritis and peptic ulcer disease, with the potential to induce gastric mucosa atrophy, metaplasia, and carcinogenesis[6]. The existing body of literature presents a fragmented understanding, particularly regarding the long-term implications of early-life H. pylori infection and its potential to evolve into gastric cancer in pediatric populations.
Adenocarcinoma is the most often seen histological variety of gastric malignant solid tumors, which pose a serious health risk. This kind of cancer is the fourth most common disease diagnosed globally and the second most common cause of cancer-related fatalities[7]. H. pylori infection is known to be a significant risk factor for gastric cancer in adults, and the association between the two conditions is well-established[8]. However, these solid tumors might present with a wide range of symptoms when compared to the pediatric population. Abdominal discomfort, fever, anemia, and gastrointestinal bleeding are among the symptoms that children may encounter[9]. Children with gastric adenocarcinoma may also have other symptoms such as vomiting, diarrhea, neck and abdominal masses, and severe weight loss, according to supplementary information from Okuda et al[10]. Furthermore, the prevalence of fundic adenocarcinoma may be indicated by the presence of dysphagia, which is defined as trouble swallowing[10].
While H. pylori's role as a primary pathogenic factor in gastric cancer among adults is well-established, its specific influence on the developing pediatric population warrants further exploration[11,12]. A survey of 80 pediatric gastric cancer patients revealed that 3 out of these children tested positive for H. pylori, with a positivity rate of 66.7%[10]. In another study involving 750 Turkish children who underwent endoscopy, an impressive 52% tested positive for H. pylori, with a mere 2% diagnosed with intestinal metaplasia in gastric mucosal biopsies; in contrast, none of the H. pylori-negative group exhibited this condition[13]. Emerging research suggests that H. pylori infection during childhood might trigger an upregulation in the expression of carcinogenic molecules within the gastric mucosa, indicating that timely eradication therapy could be instrumental in curbing the incidence of gastric cancer[14,15]. Thus, deciphering H. pylori's role in childhood gastric cancer is pivotal for devising effective prevention and treatment strategies[16,17].
The novelty of this study lies in its focused investigation on the incidence of H. pylori infection among pediatric patients undergoing diagnostic upper gastrointestinal endoscopy. It aims to identify early mucosal changes that could potentially lead to malignant transformations in the future. By exploring the connection between H. pylori infection and the risk of gastric cancer in children, this study aims to provide a foundation for clinical prevention strategies to reduce the incidence of childhood gastric cancer. Additionally, we strive to illuminate the relationship between H. pylori, gastric pathology, and genetic predisposition to cancer in young patients, offering a comprehensive perspective that is currently missing in this area of research.
For this retrospective exploration, we meticulously selected our study cohort, focusing on 3250 underage children, all below the age of 18, who had been subjected to an upper gastrointestinal endoscopy at our hospital's pediatric department. This period spanned from the early summer of June 2021 to the late summer of August 2024. The primary reason for endoscopic examination was a constellation of gastrointestinal symptoms, which included but were not limited to nausea, vomiting, retrosternal discomfort, and recurrent abdominal pain.
In our quest for a homogeneous study group, we meticulously excluded individuals who presented without gastrointestinal symptoms, those with a documented history of chronic gastrointestinal diseases such as active upper gastrointestinal bleeding or idiopathic inflammatory bowel disease, and those who had been administered antibiotics, acid suppressants, immunosuppressants, nonsteroidal anti-inflammatory drugs, or steroids within the month preceding their enrollment. This exclusion was critical to prevent any confounding effects on H. pylori testing accuracy. After careful selection, a group of 600 children met the study’s criteria and were included. For these children, a wealth of information was gathered, encompassing demographic details, clinical narratives, and histological findings, all of which were documented anonymously to safeguard confidentiality.
Given the retrospective nature of this study, the requirement for informed consent was waived, aligning with the ethical standards of our institution. The research design and its execution were reviewed and endorsed by our hospital’s Ethics Committee, ensuring alignment with the principles of the Declaration of Helsinki.
A skilled team of pediatric gastroenterologists conducted the endoscopic procedures, each member bringing a wealth of experience in pediatric gastroenteroscopy. During these procedures, at least three biopsy samples were obtained from both the stomach's antrum and its body. One of the antral specimens was designated for the rapid urease test, utilizing the CLOTM method, a recognized diagnostic standard. The other biopsy samples were processed for staining with hematoxylin, eosin, and modified Giemsa, using the advanced Leica BONDMAX automated immunohistochemistry system. Children who returned positive results on both the urease and rapid urease tests were classified as infected with H. pylori, whereas those with negative results on both assessments were considered uninfected. Any samples that exhibited conflicting results were excluded from subsequent analysis. A panel of two seasoned pathologists performed blinded evaluations on all samples.
We conducted a thorough examination of the prevalence and severity of chronic superficial gastritis, gastric atrophy, and intestinal metaplasia. The severity of gastritis was classified according to the updated Sydney system, which defines severity levels as follows: 0 for absent, 1 for mild, 2 for moderate, and 3 for severe[18]. The most severe lesion found in each patient determined the overall severity classification. Gastric atrophy was recognized as the loss of gastric glandular tissue, while intestinal metaplasia was characterized by the presence of intestinal-type epithelial cells in the gastric mucosa, resembling that of the small or large intestine.
For those who tested negative for H. pylori, treatment involved proton pump inhibitors (PPIs) in conjunction with mucosal protectors. On the other hand, patients with positive H. pylori results were administered a more intensive quadruple therapy regimen that included PPIs, bismuth, and two different antibiotics[19]. Our main objective throughout this process was to effectively eliminate the H. pylori infection and ensure proper follow-up care for the children after their treatment.
SPSS version 26 for Windows (IBM Corp., Armonk, NY, United States) was used to analyze the data. Whereas categorical data were displayed as frequencies or percentages, continuous variables were shown as mean ± SD or median (interquartile range). To examine the pathological alterations in the stomach mucosa between patients with and without an H. pylori infection, we used the χ2 test, often known as Fisher's exact test. The Wilcoxon signed-rank test, which took into account the levels of inflammatory cell infiltration, was used to assess grade data. According to two-tailed tests, statistical significance was indicated by a P value of less than 0.05.
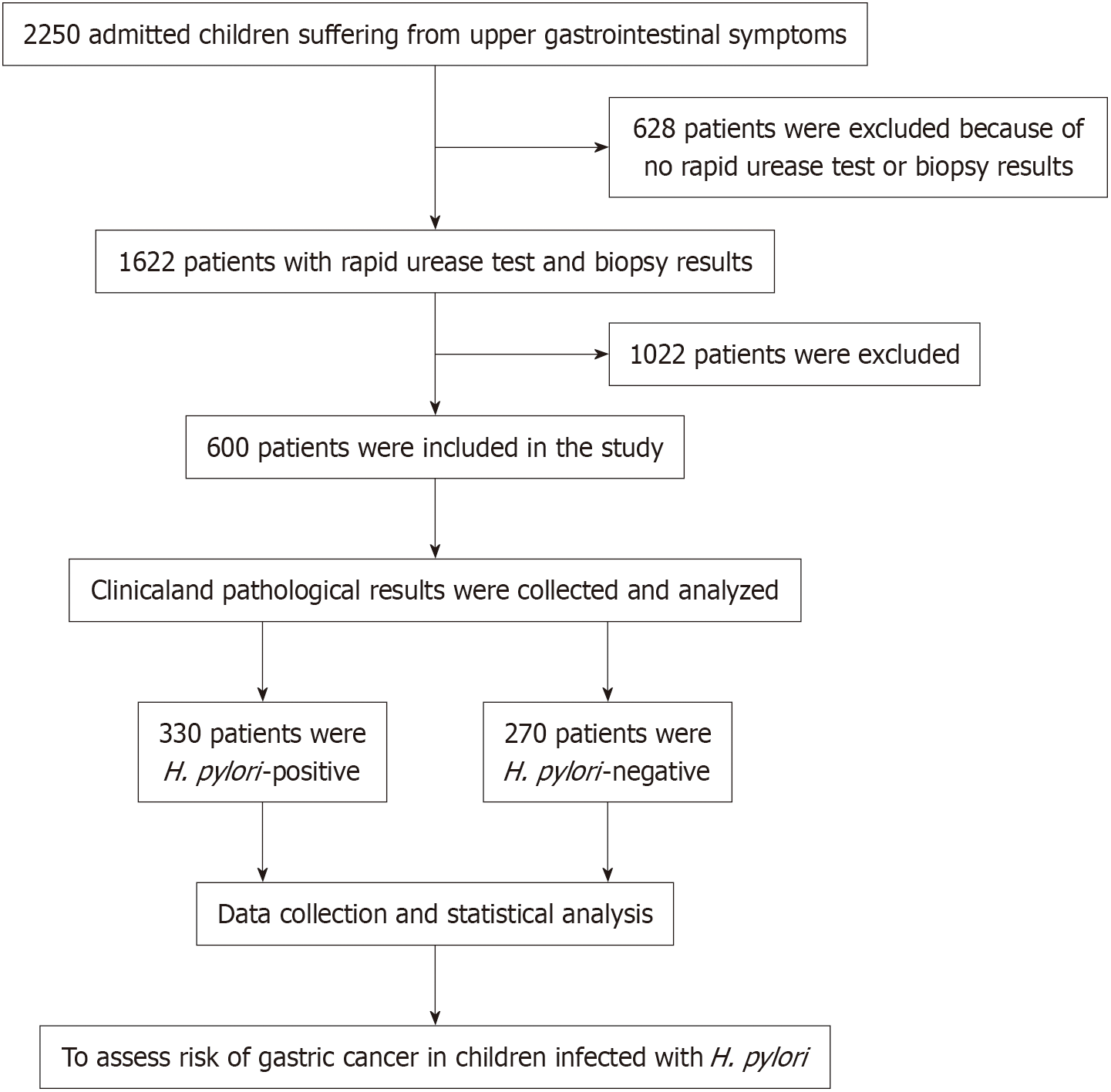
A detailed review involving 2250 pediatric ward patients was performed, as depicted in Figure 1. Following a systematic exclusion process, we removed 628 individuals who had not undergone rapid urease testing or biopsy, along with 1022 patients who did not meet the established criteria. Ultimately, this led to the inclusion and analysis of 600 eligible patients. The sample consisted of nearly equal numbers of males (340) and females (260), with an average age of 9.2 years. Within this cohort, 330 participants, representing 55% of the total, were found to be infected with H. pylori, while the remaining 270, or 45%, did not have the bacterium present. The selection process was rigorous, resulting in the exclusion of 628 patients who either did not undergo the required rapid urease testing or biopsy, in addition to 1022 others who did not fulfill our study criteria. As illustrated in Figure 1, these exclusions culminated in a final analysis of 600 eligible participants. Notably, the median age of those who tested positive for H. pylori was significantly higher at 13 years, compared to 9 years for those who tested negative, with this difference reaching statistical significance (P < 0.05). The incidence of H. pylori infection increased with age, beginning at a mere 5.2% in children aged 1 to 3 years and escalating to a striking 74.8% in those aged 15 to 18 years. Nevertheless, no significant disparities were noted between the two groups regarding gender, parental education, or household income, as summarized in Table 1.
| Variables | H. pylori-positive (n = 330) | H. pylori-negative (n = 270) | P value |
| Sex | |||
| Male | 198 (60.0) | 160 (59.3) | 0.673 |
| Female | 132 (40.0) | 110 (40.7) | |
| Median age (25th-75th IQR) (years) | 13 (8-15) | 9 (5-11) | 0.012a |
| Age group, years | |||
| 1-3 | 17 (5.2) | - | |
| 4-9 | 26 (7.9) | - | |
| 10-14 | 40 (12.1) | - | |
| 15-18 | 247 (74.8) | - | |
| Parental education level | 0.417 | ||
| Low educational attainment | 185 (56.1) | 146 (54.1) | |
| High educational attainment | 145 (43.9) | 124 (45.9) | |
| Annual household income | 0.568 | ||
| Low income | 110 (33.3) | 102 (37.8) | |
| Medium income | 162 (49.1) | 132 (48.9) | |
| High income | 58 (17.6) | 36 (13.3) |
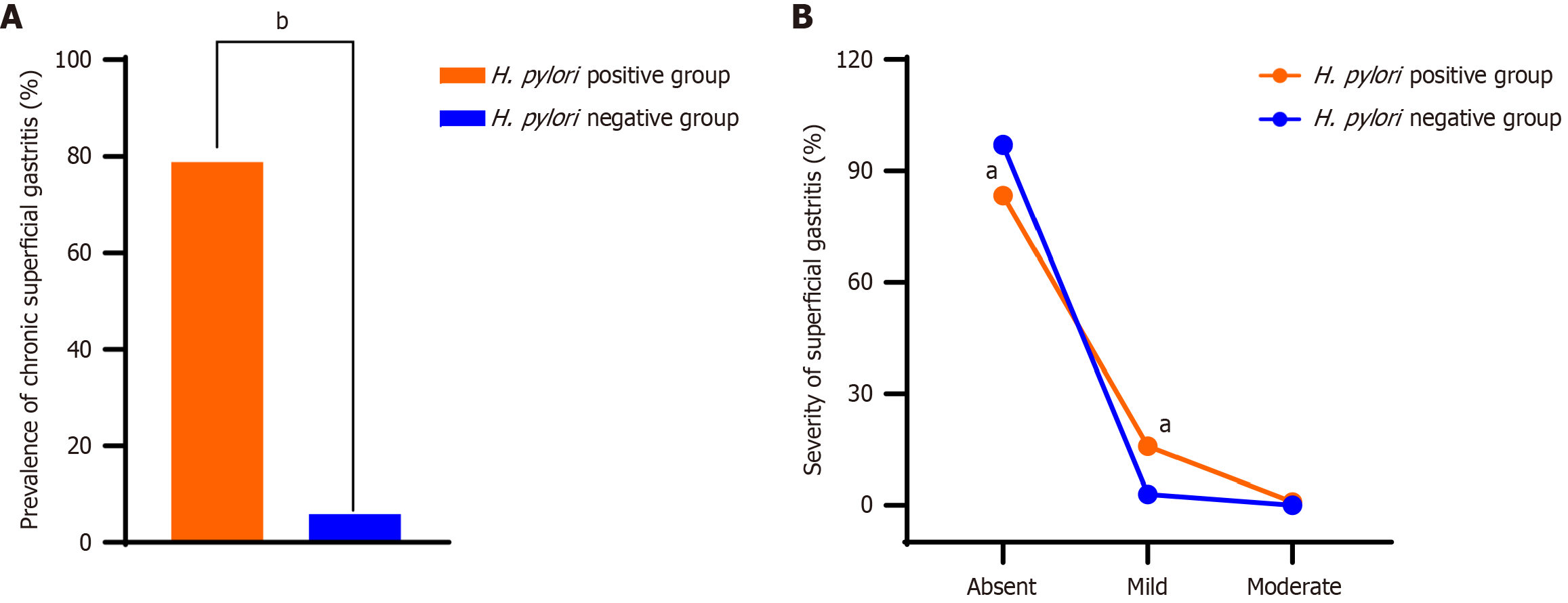
Diving deeper into the data, we discovered that the incidence of chronic superficial gastritis within our cohort of children was a concerning 46.0%. However, this figure was significantly higher within the H. pylori-positive group, affecting 78.8% of these patients compared to a mere 5.9% within the H. pylori-negative group. As shown in Figure 2A, this stark contrast was not only evident but also statistically significant (P < 0.05). Furthermore, when assessing the severity of gastric mucosal inflammation, we found that within the H. pylori-positive faction, the majority, 275 patients (83.3%), had no inflammation, 52 (15.8%) had mild inflammation, and 3 (0.91%) had moderate inflammation. In contrast, within the H. pylori-negative group, the gastric mucosa was without inflammation in 262 patients (97.0%), mild inflammation was present in 8 patients (2.96%), and there were no moderate cases. Figure 2B illustrates these findings, with the severity in the H. pylori-positive group significantly outpacing that of the H. pylori-negative group (Z = 5.782, P < 0.05).
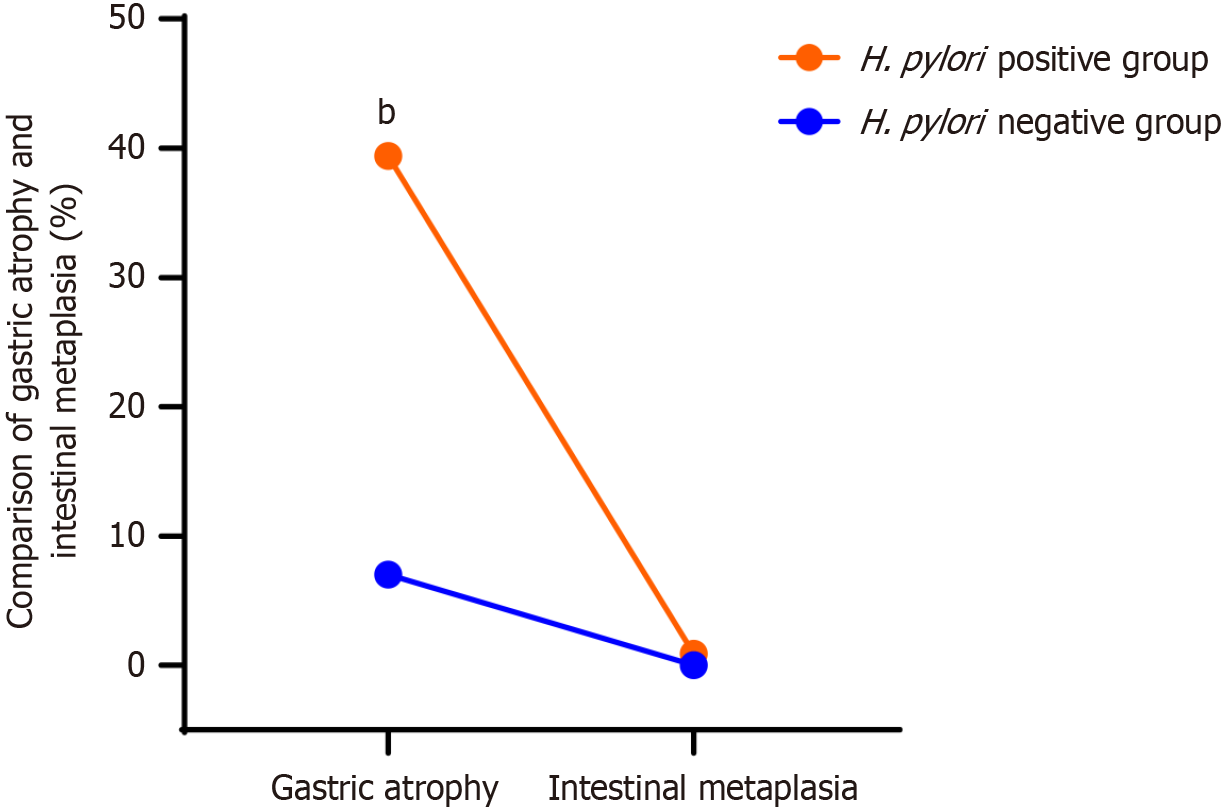
Shifting our focus to gastric atrophy and intestinal metaplasia, we found that the overall prevalence of gastric atrophy within our 600 patients was 24.8%. Within the H. pylori-positive group, a significant 130 (39.4%) had gastric atrophy, and an alarming 3 (0.9%) had intestinal metaplasia. Conversely, within the H. pylori-negative group, only 19 (7.0%) had gastric atrophy, and no cases of intestinal metaplasia were observed. The difference in gastric atrophy between the two groups, as highlighted in Figure 3, was not only pronounced but also statistically significant (P < 0.001). The presence of intestinal metaplasia was exclusive to the H. pylori-positive group, underscoring the association between H. pylori infection and the development of precancerous gastric conditions.
Lastly, we observed that among the parents of the H. pylori-positive children, there were 4 cases, or 1.2%, of gastric cancer. This is in stark contrast to the parents of the H. pylori-negative children, among whom no gastric cancer cases were reported. As detailed in Table 1, this difference between the two groups was statistically significant (P < 0.05), suggesting a higher predisposition to gastric cancer among families with H. pylori-positive children.
All children with H. pylori gastritis underwent eradication therapy. Patients with gastric atrophy and glandular metaplasia were re-evaluated endoscopically one year later, and all findings had regressed, with no evidence of gastric atrophy or metaplasia.
H. pylori represents the most prevalent chronic bacterial infection in developing nations[20]. Typically acquired during childhood, this infection often persists into adulthood[21]. Research highlights a notable risk for transmission within families[22]. This bacterium is linked to conditions such as gastritis and peptic ulcers and may induce long-lasting alterations in the gastric mucosa, potentially elevating the risk of gastric cancer over time[23].
As children mature, the prevalence of H. pylori infection tends to rise. Vanderpas et al[24] found that the infection rate was 18.2% among children younger than 6 years, escalating to 49.3% in adolescents aged 12 to 17. In a similar vein, Ertem et al[4] reported varying prevalence rates: 18.2% for those under 4 years, 41% for ages 4 to 6, 48.6% for ages 6 to 8, 50% for ages 8 to 10, and 63% for children between 11 and 12 years. In our study focusing on children experiencing upper gastrointestinal issues, we observed a biopsy-confirmed H. pylori infection rate of 55%. Furthermore, within the H. pylori-positive group, the infection rate increased with age, starting at 5.2% for those aged 1 to 3 years and reaching 74.8% among those aged 15 to 18 years, which aligns closely with findings from the aforementioned studies.
The gastric mucosa of children infected with H. pylori demonstrates a number of pathological alterations: Bacterial colonization, progressive inflammation, glandular atrophy, and intestinal metaplasia. Both atrophy and intestinal metaplasia can be considered the first signs of potential gastric cancer development[25]. In a study in China, the recurrence figures for chronic superficial gastritis were found to be 41.9% among children with H. pylori and atrophic gastritis in 21.7% of cases[26]. Research indicates that H. pylori infection is a significant risk for gastric cancer and plays a role in other gastrointestinal disorders, including gastritis, in the pediatric population[27]. Moreover, a notable association has been demonstrated between H. pylori infection and gastrointestinal immune responses, possibly influencing allergic disease onset in children[27]. The severity of chronic gastrointestinal inflammation is said to be very closely related to the incidence of atrophic gastritis and intestinal metaplasia among individuals who were reported as positive for H. pylori[28].
Our results showed that 46.0% of H. pylori-positive children exhibited chronic superficial gastritis, while gastric atrophy was present in 24.8%. In our cohort of 330 H. pylori-positive patients, only 3 (0.9%) had intestinal metaplasia, and none was detected in those without H. pylori. The rates of chronic superficial gastritis, gastric atrophy, and intestinal metaplasia were significantly higher in the H. pylori-positive cohort compared to their negative counterparts, which aligns with earlier studies and underscores the strong association between H. pylori infection and gastric lesions[29]. Consequently, early diagnosis and treatment of H. pylori in children are vital to mitigate the risk of potential gastric lesions[30].
The timely detection and removal of H. pylori can significantly diminish inflammation and promote the recovery of the gastric mucosa. Early intervention in diagnosing H. pylori infections in children is particularly crucial for preventing gastric cancer, given that the period of mucosal exposure to carcinogens tends to be shorter and the related mucosal conditions are usually less severe compared to those seen in adults. If H. pylori remains untreated, it can persist indefinitely, causing changes in the mucosa that may eventually lead to cancer. Adopting measures for early identification, effective eradication, and preventive actions could greatly reduce the prevalence of H. pylori infections and gastritis, particularly in regions where this infection is common. A recent meta-analysis indicated that proactive screening and successful eradication of H. pylori can lower the risk of gastric cancer in otherwise healthy, asymptomatic individuals[31]. It has been proposed that effectively managing and monitoring H. pylori infections in children could prevent 70%-80% of future cases of gastric malignancies linked to this bacterium. Additionally, it is vital to enhance awareness among families of gastric cancer patients, both adults and children, regarding H. pylori infections and their potential consequences.
As a retrospective study, this research has several limitations. Firstly, it concentrated exclusively on the relationship between H. pylori infection and chronic superficial gastritis, gastric atrophy, and intestinal metaplasia, without delving into the genetic factors associated with H. pylori. Secondly, due to the rarity of gastric cancer in children, no cases were identified in this study, limiting our ability to assess the direct relationship between H. pylori infection and gastric cancer occurrence; thus, the analysis relied on indirect comparisons with family members, which may impact the accuracy of the results. Thirdly, this single-center, retrospective analysis involved pediatric patients from our hospital, which may restrict the generalizability of our findings to the wider population. In addition, the incidence of H. pylori infection in this study increases with age, and age as a confounding factor may have potential implications on the results. To address this issue, future studies should adopt multicenter longitudinal research to track the dynamic changes of H. pylori infection in patients of different age groups and its long-term effects on patients. Furthermore, studies should be conducted in different regions and populations to verify the relationship between H. pylori infection and age, as well as its impact on clinical outcomes. To overcome these limitations, future large-scale prospective studies are needed to investigate the potential connections between the type and severity of inflammatory responses, various stages of gastric cancer progression, and H. pylori genotypes.
In summary, our study reveals that children testing positive for H. pylori have a higher prevalence of a family history of gastric cancer. Additionally, biopsy samples from these children exhibited a greater incidence of intestinal metaplasia. By identifying and effectively eradicating H. pylori infection in this vulnerable group, we could significantly reduce the risk of future gastric cancer development, as no metaplasia was found in the H. pylori-negative group during endoscopic evaluations.
| 1. | Azrad M, Vazana D, On A, Paritski M, Rohana H, Roshrosh H, Agay-Shay K, Peretz A. Antibiotic resistance patterns of Helicobacter pylori in North Israel - A six-year study. Helicobacter. 2022;27:e12932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Moss SF, Shah SC, Tan MC, El-Serag HB. Evolving Concepts in Helicobacter pylori Management. Gastroenterology. 2024;166:267-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 3. | Abu-Lubad MA, Helaly GF, Haddadin WJ, Jarajreh DAK, Aqel AA, Al-Zeer MA. Loss of p53 Expression in Gastric Epithelial Cells of Helicobacter pylori-Infected Jordanian Patients. Int J Microbiol. 2022;2022:7779770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Ertem D, Harmanci H, Pehlivanoğlu E. Helicobacter pylori infection in Turkish preschool and school children: role of socioeconomic factors and breast feeding. Turk J Pediatr. 2003;45:114-122. [PubMed] |
| 5. | Tallman PS, Miller AA, Brandley SR, Lee CC, Cepon-Robins TJ, Gildner TE, Collins SM. Helicobacter pylori exposure among the Awajún of the Peruvian Amazon: Prevalence and environmental, social, and biological associations. Am J Biol Anthropol. 2024;184:e24941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Dash NR, Khoder G, Nada AM, Al Bataineh MT. Exploring the impact of Helicobacter pylori on gut microbiome composition. PLoS One. 2019;14:e0218274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (2)] |
| 7. | Chen S, Zang Y, Xu B, Lu B, Ma R, Miao P, Chen B. An Unsupervised Deep Learning-Based Model Using Multiomics Data to Predict Prognosis of Patients with Stomach Adenocarcinoma. Comput Math Methods Med. 2022;2022:5844846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Gao W, Teng G, Wang C, Xu Y, Li Y, Cheng H. Eradication rate and safety of a "simplified rescue therapy": 14-day vonoprazan and amoxicillin dual regimen as rescue therapy on treatment of Helicobacter pylori infection previously failed in eradication: A real-world, retrospective clinical study in China. Helicobacter. 2022;27:e12918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 9. | Yang T, Li J, Zhang Y, Deng Z, Cui G, Yuan J, Sun J, Wu X, Hua D, Xiang S, Chen Z. Intracellular presence of Helicobacter pylori antigen and genes within gastric and vaginal Candida. PLoS One. 2024;19:e0298442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Okuda M, Nomura K, Kato M, Lin Y, Mabe K, Miyamoto R, Okumura A, Kikuchi S. Gastric cancer in children and adolescents in Japan. Pediatr Int. 2019;61:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Song ZQ, Zhou LY. Helicobacter Pylori and Gastric Cancer: Clinical Aspects. Chin Med J (Engl). 2015;128:3101-3105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Tepes B. Population based Helicobacter pylori screening and eradication: advances versus side effects. Curr Pharm Des. 2014;20:4501-4509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Cam S. Risk of gastric cancer in children with Helicobacter pylori infection. Asian Pac J Cancer Prev. 2014;15:9905-9908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Obayashi N, Ohtsuka Y, Hosoi K, Ikuse T, Jimbo K, Aoyagi Y, Fujii T, Kudo T, Asaoka D, Hojo M, Nagahara A, Watanabe S, Shimizu T. Comparison of Gene Expression Between Pediatric and Adult Gastric Mucosa with Helicobacter pylori Infection. Helicobacter. 2016;21:114-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Pormohammad A, Mohtavinejad N, Gholizadeh P, Dabiri H, Salimi Chirani A, Hashemi A, Nasiri MJ. Global estimate of gastric cancer in Helicobacter pylori-infected population: A systematic review and meta-analysis. J Cell Physiol. 2019;234:1208-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Liu Y, Zhang B, Zhou Y, Xing Y, Wang Y, Jia Y, Liu D. Targeting Hippo pathway: A novel strategy for Helicobacter pylori-induced gastric cancer treatment. Biomed Pharmacother. 2023;161:114549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 17. | Peng C, Li NS, Hu Y, Lu NH. Impact factors that modulate gastric cancer risk in Helicobacter pylori-infected rodent models. Helicobacter. 2019;24:e12580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Zhang L, Huang MX, Li DY, Zhang YZ, Lan SY, Luo Q, Dai YK, Wu YB, Ye JT, Chen WJ, Li RL, Hu L. The Single-Nucleotide Polymorphism of miR-27a rs895819 and the Expression of miR-27a in Helicobacter pylori-Related Diseases and the Correlation with the Traditional Chinese Medicine Syndrome. Evid Based Complement Alternat Med. 2022;2022:3086205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Weng A, Su X, Yang C, Zheng B, Zheng L, Jian C, Fang J. Telephone follow-up by clinical pharmacists can improve treatment outcomes in patients with peptic ulcers: A prospective randomized study. Medicine (Baltimore). 2022;101:e31150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Gurbuz BC, Inceman HN, Aydemir M, Celtik C, Gerenli N, Zemheri E. Prevalence of Helicobacter pylori among children in a training and research hospital clinic in Istanbul and comparison with Updated Sydney Classification Criteria. North Clin Istanb. 2020;7:499-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Xie C, Li N, Wang H, He C, Hu Y, Peng C, Ouyang Y, Wang D, Xie Y, Chen J, Shu X, Zhu Y, Lu N. Inhibition of autophagy aggravates DNA damage response and gastric tumorigenesis via Rad51 ubiquitination in response to H. pylori infection. Gut Microbes. 2020;11:1567-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 22. | Martínez-Campos C, Torres-Poveda K, Camorlinga-Ponce M, Flores-Luna L, Maldonado-Bernal C, Madrid-Marina V, Torres J. Polymorphisms in IL-10 and TGF-β gene promoter are associated with lower risk to gastric cancer in a Mexican population. BMC Cancer. 2019;19:453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Wang Y, Zheng L. Protocatechuic acid, the main effective monomer in Wuqi Powder, can inhibit gastric ulcers induced by acetic acid and Helicobacter pylori. Am J Transl Res. 2023;15:151-164. [PubMed] |
| 24. | Vanderpas J, Bontems P, Miendje Deyi VY, Cadranel S. Follow-up of Helicobacter pylori infection in children over two decades (1988-2007): persistence, relapse and acquisition rates. Epidemiol Infect. 2014;142:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Liu XM, Ma XY, Liu F, Liu ZL, Tang XY, Ji MZ, Zheng JX. Gastric Cancer Screening Methods: A Comparative Study of the Chinese New Gastric Cancer Screening Score and Kyoto Classification of Gastritis. Gastroenterol Res Pract. 2022;2022:7639968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Yu Y, Su L, Wang X, Wang X, Xu C. Association between Helicobacter pylori infection and pathological changes in the gastric mucosa in Chinese children. Intern Med. 2014;53:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Hojsak I, Kolacek S. Is Helicobacter pylori always a "bad guy"? Curr Pharm Des. 2014;20:4517-4520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Şenocak Taşçı E, Akbaş T. The Relationship between the Sydney Classification and the First-Line Treatment Efficacy in Helicobacter-Associated Gastritis. Med Princ Pract. 2020;29:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Iwańczak B, Francavailla R. Helicobacter pylori infection in pediatrics. Helicobacter. 2014;19 Suppl 1:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Lionetti E, Francavilla R, Castellazzi AM, Arrigo T, Labò E, Leonardi S, Ciprandi G, Miraglia Del Giudice M, Salpietro V, Salpietro C, La Rosa M. Probiotics and Helicobacter pylori infection in children. J Biol Regul Homeost Agents. 2012;26:S69-S76. [PubMed] |
| 31. | Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 442] [Article Influence: 40.2] [Reference Citation Analysis (1)] |