Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.99188
Revised: November 8, 2024
Accepted: January 15, 2025
Published online: April 15, 2025
Processing time: 252 Days and 11.7 Hours
SLC16A8, a lactate efflux transporter, is upregulated in various cancers, but its effects on tumor microenvironments remain understudied. This research explores its role in colorectal cancer (CRC) and the impact on the associated microenvironment consisting of vascular endothelial cells.
To explore the role in CRC and the impact on the associated microenvironment consisting of vascular endothelial cells.
Hypoxic conditions prompted examination of SLC16A8 expression, glycolysis, lactate efflux, and Warburg effect correlations in CRC cell lines. Co-culture with HUVEC allowed for endothelial-mesenchymal transition (EndMT) characterization, revealing lactate efflux's influence. Knockdown of SLC16A8 in CRC cells enabled relevant phenotype tests and tumorigenesis experiments, investigating tumor growth, blood vessel distribution, and signaling pathway alterations.
SLC16A8 expression was significantly upregulated in CRC tissues compared to adjacent normal tissues and correlated with disease progression (P < 0.05). Under hypoxic conditions, HIF-1α induced SLC16A8 expression, leading to enhanced metabolic reprogramming and increased lactate production. siRNA-mediated SLC16A8 knockdown effectively reversed hypoxia-induced changes, including reduced glucose consumption and lactate production. Co-culture experiments revealed that SLC16A8 knockdown significantly inhibited hypoxia-induced EndMT in HUVEC cells. In vivo studies demonstrated that SLC16A8 knockdown suppressed tumor growth, reduced Ki67 expression, and decreased HIF-1α levels. Furthermore, SLC16A8 silencing led to decreased ex
Our findings reveal that SLC16A8 functions as a critical mediator of hypoxia-induced metabolic reprogramming in CRC progression.
Core Tip: Hypoxic SLC16A8 upregulated glycolysis factors in cancer cells. Co-culture with HUVEC increased endothelial-mesenchymal transition in endothelial cells. Knockdown reversed phenotypes in both cell types. In vivo, SLC16A8 in
- Citation: Tian HP, Xiao ZX, Su BW, Li YX, Peng H, Meng CY. Impact of SLC16A8 on tumor microenvironment and angiogenesis in colorectal cancer: New therapeutic target insights. World J Gastrointest Oncol 2025; 17(4): 99188
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/99188.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.99188
Colorectal cancer (CRC) is a frequent gastrointestinal malignancy worldwide. There were 1.9 million new CRC cases and 935000 deaths in 2020, accounting for 1/10 of all cancers[1,2]. And most cases of CRC are not caused by a single factor[3]. Most patients with CRC have no obvious symptoms in the early stage, and about 40%-50% of patients are already in the advanced stage when diagnosed, who have distant metastases with a 5-year survival rate of only 12.5%[4]. The most familiar sites of metastasis are the liver, peritoneum and lung[5]. Currently, therapy is based on radical surgery and radiotherapy[2,6]. While the recurrence and metastasis rates are high after surgery[7]. Therefore, studying the mechanism of CRC development is beneficial to the accurate diagnosis, precise treatment and early prevention of the disease, which is also is an effective way to reduce CRC mortality.
Cancer cells undergo metabolic reprogramming to promote growth, survival, proliferation and long-term maintenance[8]. A common feature of this metabolic alteration is increased glucose uptake and metabolism of glucose to lactate in the presence of perfectly normal mitochondrial function, a phenomenon known as the Warburg effect, or aerobic glycolysis[9]. In recent years, research on cancer metabolism has progressively delved into understanding how metabolic al
The SLC16 gene family consists of 14 members, which are also known as the monocarboxylate transporter family[13]. SLC16 family members are involved in a wide range of metabolic pathways, including energy metabolism, gluconeogenesis, T-lymphocyte activation, intestinal metabolism, spermatogenesis, pancreatic p-cell dysfunction, thyroid hormone metabolism and drug transport in brain, skeletal muscle, heart and tumor cells[14,15]. SLC16A8, a member of this gene family, is mainly responsible for the transport of monocarboxylic acid metabolites such as pyruvate, L-lactate and ketone bodies[16]. SLC16A8 also can participate in intercellular lactate transport across membranes. However, it is not clear that SLC16A8 promotes CRC malignant behavior by altering the Warburg effect.
In our study, to investigate the role of SLC16A8 in CRC, we first confirmed the expression and prognosis of SLC16A8 in CRC. Besides, we investigated the impacts of hypoxia on the proliferation, epithelial-mesenchymal transition (EMT), metastasis, glycolysis, and angiogenesis of CRC cells. And we further confirmed the effect of SLC16A8 silencing on these malignant behaviors of CRC under hypoxia condition. This study was conducted to provide potential targets for the therapy and diagnosis of CRC.
CRC tissues and paired para-cancerous tissues were collected at Nanchong Central Hospital from January 2022 to December 2023. Inclusion criteria: None of them received radiotherapy or chemotherapy before surgery and post
FHC, SW480, RKO, HCT116 and LoVo cell lines were purchased from China National Collection of Authenticated Cell Culture. Briefly, cells were cultured in RPMI-1640 medium with 10% FBS, 1% Penicillin-Streptomycin solution under 37 °C with 5% CO2. During cell passage, the cells were pre-treated with 0.25% trypsin to digest them into a single-cell suspension, and the digestion reaction was terminated with complete culture medium. The cells were then subcultured at a ratio of 1:3. Co-culturing of CRC cells with HUVEC is conducted using a transwell chamber. During the co-culture process, HUVEC are seeded in the lower chamber, and CRC cells are placed in the upper chamber, maintaining a ratio of 5:1 between the two. The culturing conditions are set at 37°C, 5% carbon dioxide, and 100% humidity.
CRC cells were placed in hypoxia chamber at 0.5% O2 with a gas mixture consisting of 95% N2/5% CO2 for 1, 6, and 12 hours, and cells cultured under 5% O2 were set as control. SLC16A8 siRNA1 (AGCAGUUGGUGGCGACAGCCAdTdT), SLC16A8 siRNA2 (AGCACAACGCAGGCAGCAGUUdTdT), SLC16A8 siRNA3 (UUAGCACAACGCAGGCAG
For clinical tissues, they were ground into powder after treatment with liquid nitrogen, followed by total RNA extraction using Trizol reagent. For cells, they were directly lysed using Trizol solution. The extraction process was conducted according to the manual. RNA concentration was quantified using a NanoDrop spectrophotometer, and 1 μg of total RNA was reverse transcribed to obtain a cDNA template. Specific primers were used, and real-time PCR was performed using SYBR Green to obtain the Ct values of each sample. The relative mRNA expression levels in each sample were calculated using the 2-ΔΔCt method. ACTB was used as an internal reference in this experiment. The primers’ sequences were listed in Table 1.
| Name | Sequence (5’-3’) | Product length (bp) |
| ACTB F | CATGTACGTTGCTATCCAGGC | 154 |
| ACTB R | CTCCTTAATGTCACGCACGAT | |
| SLC16A8 F | TGCCTGCGTTGTGCTAAAG | 119 |
| SLC16A8 R | GGTTCCTCTGCAACAACAGG |
For the CCK-8 assay, CRC cells (4 × 105 cells/mL) were seeded in 96-well plates and transfected for 48 hours according to the experimental design. Subsequently, 10 μL of CCK-8 reagent (Dojindo, Tokyo, Japan) was added to each well. After a 2-hour incubation period, the optical density (OD) value at 450 nm was measured. Cells (approximately 1 × 105 cells) were subjected to hypoxia or siRNA treatment for 48 hours, followed by detection using the EdU staining kit provided by Beyotime (Shanghai, China). Briefly, EdU solution was added to the cells and then incubated for an additional 2 hours. Subsequently, the EdU staining solution was discarded, and the cells were fixed with paraformaldehyde and permeabilized with Triton X-100. The fluorescent detection solution was added, followed by a 30-minute incubation in the dark at room temperature. Finally, DAPI nuclear staining solution was added to stain the cell nuclei, and the proliferative activity signals were observed under a laser confocal microscope.
Cell migration and invasion capacities were assessed using Transwell chambers (8 μm pore size, Corning). For migration analysis, CRC cells were harvested and suspended in serum-free medium at a density of 5 × 104 cells per well. The cells were seeded in the upper chamber, while the lower chamber contained 500 μL complete medium supplemented with 10% FBS as a chemoattractant. Following 24-hour incubation under standard conditions, non-migrated cells were removed from the upper surface using cotton swabs. The migrated cells were fixed with 4% paraformaldehyde (20 minutes), stained with 1% crystal violet (10 minutes), and quantified by counting five random microscopic fields. For invasion assays, the upper chambers were pre-coated with Matrigel (EMD Millipore; Cat. No. 356234, diluted 1:8 in serum-free medium). Briefly, 80 μL of diluted Matrigel was applied to each insert and allowed to polymerize at 37 °C for 1 hour. The subsequent experimental procedures were identical to the migration assay protocol.
In this study, to evaluate the impact of CRC cells on endothelial cell angiogenesis, HUVECs were co-cultured with CRC cells, and their tube formation ability was assessed using a tube formation assay. Matrigel was thawed overnight in advance at 4 °C in the refrigerator, and was diluted with FBS-free medium. The cells were inoculated with 2 × 105 cells/well on the surface of the Matrigel at 37 °C for 24 hours. After a few hours, cells begin to form capillary-like structures. The results were recorded by an inverted microscopy. The degree of tube formation is then quantified by counting the number of tubes or measuring the total tube length under a microscope.
In this experiment, a glucose uptake assay kit provided by Abcam was utilized. Briefly, after subjecting cells to hypoxia or siRNA transfection, they underwent starvation treatment. Subsequently, cells were incubated with 2-deoxyglucose (2-DG), a glucose analog, with or without insulin stimulation. Cells were then lysed to measure the intracellular 2-DG content. The concentration of 2-DG6P was quantified by measuring the absorbance at OD412 nm in the lysate. A standard curve for 2-DG6P was established prior to the experiment based on standard samples.
To perform the L-lactate assay, an L-lactate assay kit (abcam, ab65331) was used. Cells were harvested (approximately 2 × 106 cells), and washed with cold PBS, and homogenize by pipetting. Afterwards, homogenate was centrifuge at 4 °C for 5 minutes to remove insoluble material, then keep the supernatant on ice. The endogenous lactate dehydrogenase was removed using Deproteinizing Sample Preparation Kit – TCA (ab204708). Then 50 µL of reaction mix per reaction was prepared, following the provided amounts for assay buffer, developer solution, and enzyme mix. Besides, standard wells were set up with 50 µL of standard dilutions and sample wells with 2-50 µL of samples, adjusted to 50 µL with lactate assay buffer. Incubate the plate at room temperature for 30 minutes and measure at OD450 nm. Calculate concentrations by comparing with the standard curve, adjusted for any sample dilution.
After hypoxia or siRNA transfection, cells were seeded into a 96-well Seahorse microplate (cell density 2 × 104/mL), at 80 µL per well, and cultured at 37 °C in a 5% CO2 incubator for 16 hours. The calibration plate was equilibrated overnight in a non-CO2 incubator. Prior to measurement, cells were washed twice with assay medium and equilibrated in a non-CO2 incubator. After calibration, the probe plate was replaced with the cell plate. For the ECAR measurement, glucose (10 mmol/L), oligomycin (1 μmol/L), and 2-DG (100 mmol/L) were sequentially injected, and measurements were taken continuously.
The total protein was harvested via RIPA lysate (Beyotime, China), and was monitored by BCA kit (Invitrogen). Proteins (40 μg) were separated in SDS-PAGE gel, transferred to PVDF membrane (Millipore). After blocking, the membranes were exposed to primary antibody, PKM2 (Boster, PB9379, 1:1000), LDHA (Boster, PB10075, 1:1000), E-cadherin (Boster, PB9561, 1:1500), N-cadherin (Boster, BA0673, 1:2000), Vimentin (Boster, BM0135, 1:1000), SLC16A8 (antibodies-online, ABIN630366, 2.5 µg/mL), ACTB (Boster, BA2305, 1:5000) overnight at 4 °C. Afterwards, membranes were treated with a secondary antibody (Abcam, ab7090, 1:5500) for 1 hour, followed by dropwise addition of ECL color development solution for visualization. After ECL chemiluminescence, protein was developed on the gel imager (Bio-rad). ACTB was used as internal reference.
Nude mice (6 weeks old, half male and half female, 20 g) were provided by the Animal Experiment Center of Nanchong Central Hospital, with 5 mice per group. After expansion, LoVo cells were injected subcutaneously into the mice, with each mouse receiving an injection of 5 × 106 cells. Subsequently, siRNA was injected every other day at a dose of 15 nmol/20 g via intravenous injection. During the experiment, the animals were provided with sufficient water and food, the animal laboratory was maintained at 23 ± 2 °C, and the humidity was kept at 60%. The experiment lasted for 4 weeks. The length and width of the tumor were measured weekly, and the tumor volume was calculated using the formula (volume = length × width2 × 0.5). After anesthesia with sodium pentobarbital, the animals were quickly euthanized by cervical dislocation. Tumor tissues were collected for subsequent experimental testing. Ethical approval for all animal experimental procedures was provided by the Animal Ethics Committee of Nanchong Central Hospital.
Tumors from mice were processed through 4% paraformaldehyde fixation, gradient ethanol dehydration, and embedded in paraffin blocks for 4-μm sectioning. After baking, the sections were dewaxed and hydrated with xylene and gradient alcohol, followed by hematoxylin-stained nuclei, eosin-stained cell pulp, ethanol dehydration and xylene transparency, and finally sealed with neutral resin. Morphological changes in tumor tissue were observed under the microscope.
Paraffin sections were subjected to microwave antigen thermal repair using 0.01 mol/L sodium citrate, and endogenous enzymes were blocked by incubation with 3% H2O2. After washing, the sections were closed by 5% BSA for 30 minutes, incubated with the diluted primary antibody (Ki-67, Abcam) at 4 °C overnight, and goat secondary antibody (Abcam) for 1 hour. Then the sections were developed with DAB (Invitrogen, Cat. No. 34002), re-stained with hematoxylin, and sealed with neutral resin. Then the staining results were observed under a microscope. And five high magnification fields (× 200) were selected for each mouse for immunohistochemistry analysis.
For Figure 1A, significance testing was performed using the t-test method. For experiments involving three or more groups, a post-hoc analysis followed by Tukey's test was employed. Data visualization was represented in the form of mean ± SD, and GraphPad (Ver 9) was used for the creation of bar graphs and line charts. Data significance analysis was conducted using SPSS 22.0. A P value of < 0.05 was considered statistically significant. Each experiment was repeated three times.
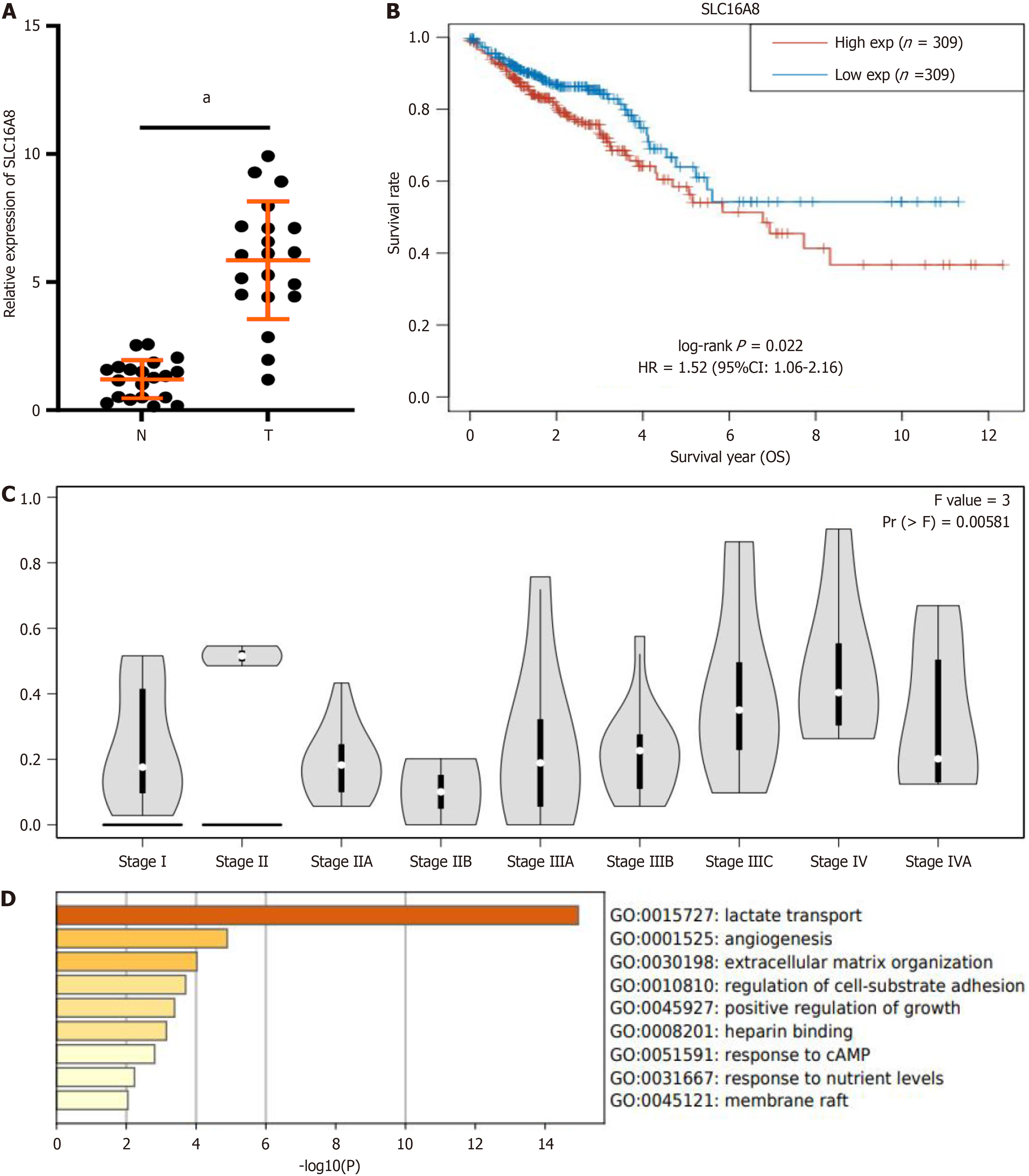
To analyze the expression characteristics of SLC16A8 in CRC, cancerous and adjacent non-cancerous tissues were collected from patients during surgery, and qPCR experiments were conducted. As shown in Figure 1A, SLC16A8 was significantly upregulated in cancerous tissues, exhibiting a marked difference compared to expression in adjacent non-cancerous tissues. Further database analysis revealed that low SLC16A8 expression was associated with favorable prognosis and survival in CRC patients, demonstrating a significant correlation (Figure 1B). Moreover, as CRC pro
HIF-1α is a crucial transcription factor that regulates cellular responses under hypoxic conditions, and its role in modu
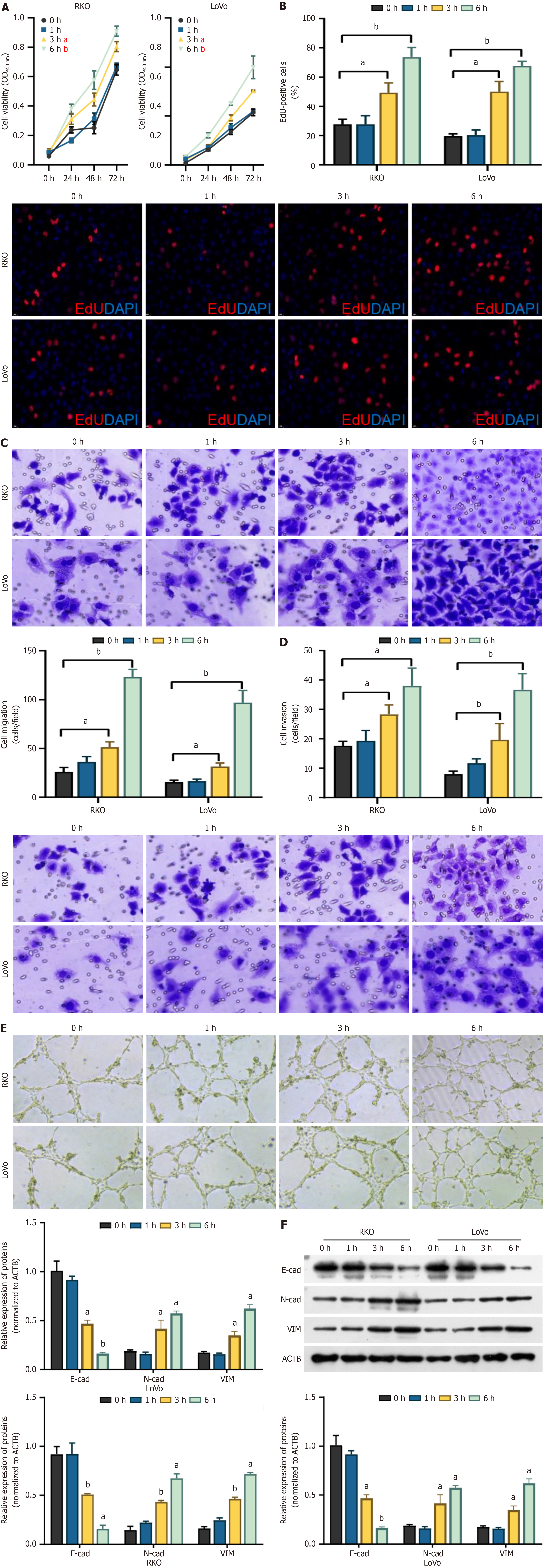
To determine the malignant biological behavior of CRC cell lines RKO and LoVo under hypoxia, we examined their effects on HUVEC cells under different hypoxia times. As shown in Figure 3A and B, cell proliferation activity of HUVEC cells was significantly enhanced with prolonged hypoxia when co-cultured with RKO and LoVo cells, and positively correlated with time (Figure 3A and B). Further Transwell chamber experiments showed that hypoxia also enhanced the migration and invasion abilities of HUVEC cells in the presence of RKO and LoVo cells (Figure 3C and D). The results demonstrated that the angiogenesis ability of the corresponding HUVEC cells was gradually enhanced with prolonged hypoxia when co-cultured with CRC cells (Figure 3E).and caused the upregulation of N-Cadherin, Vimentin expression and a decreasing of E-cadherin levels (Figure 3F). These data demonstrated that hypoxic conditions significantly en
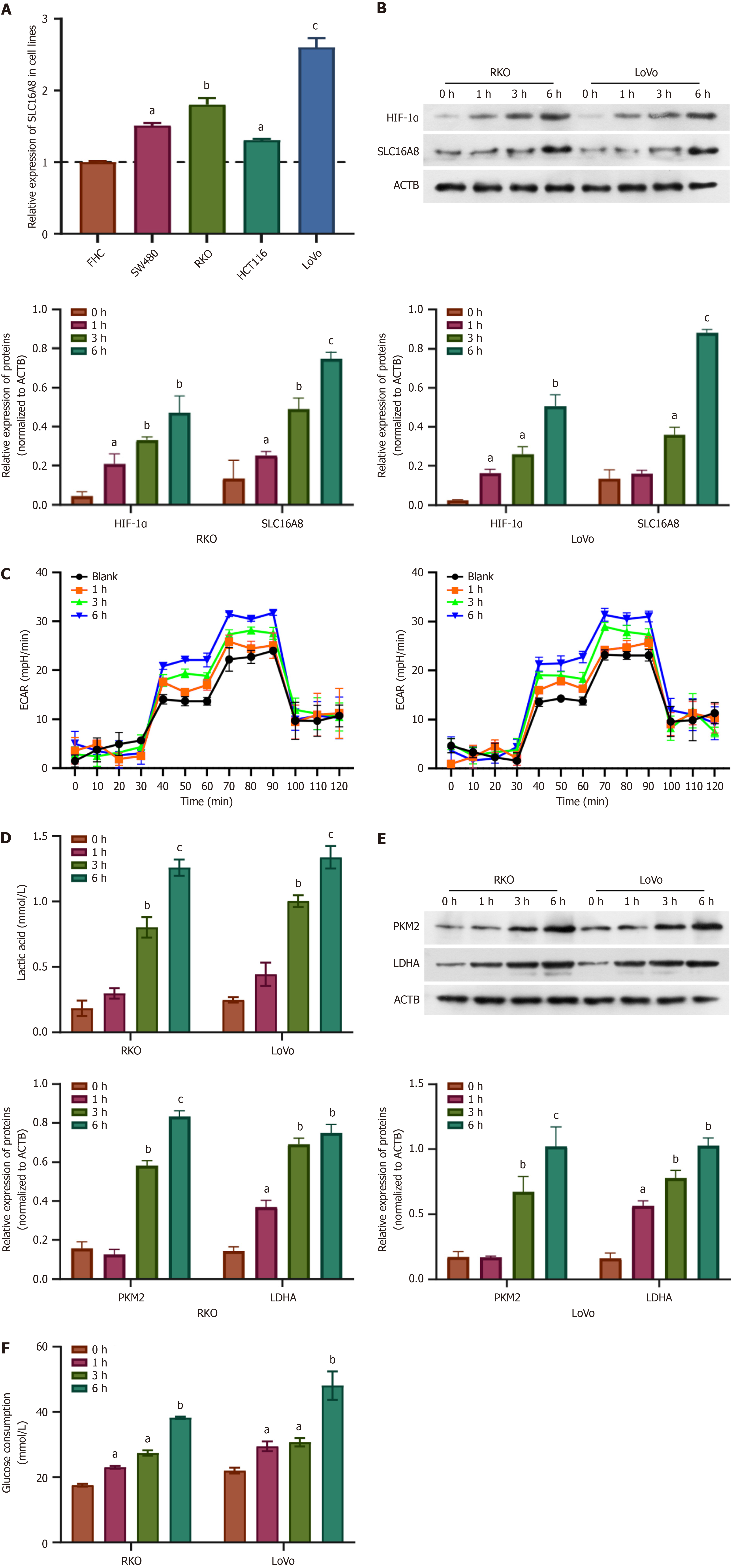
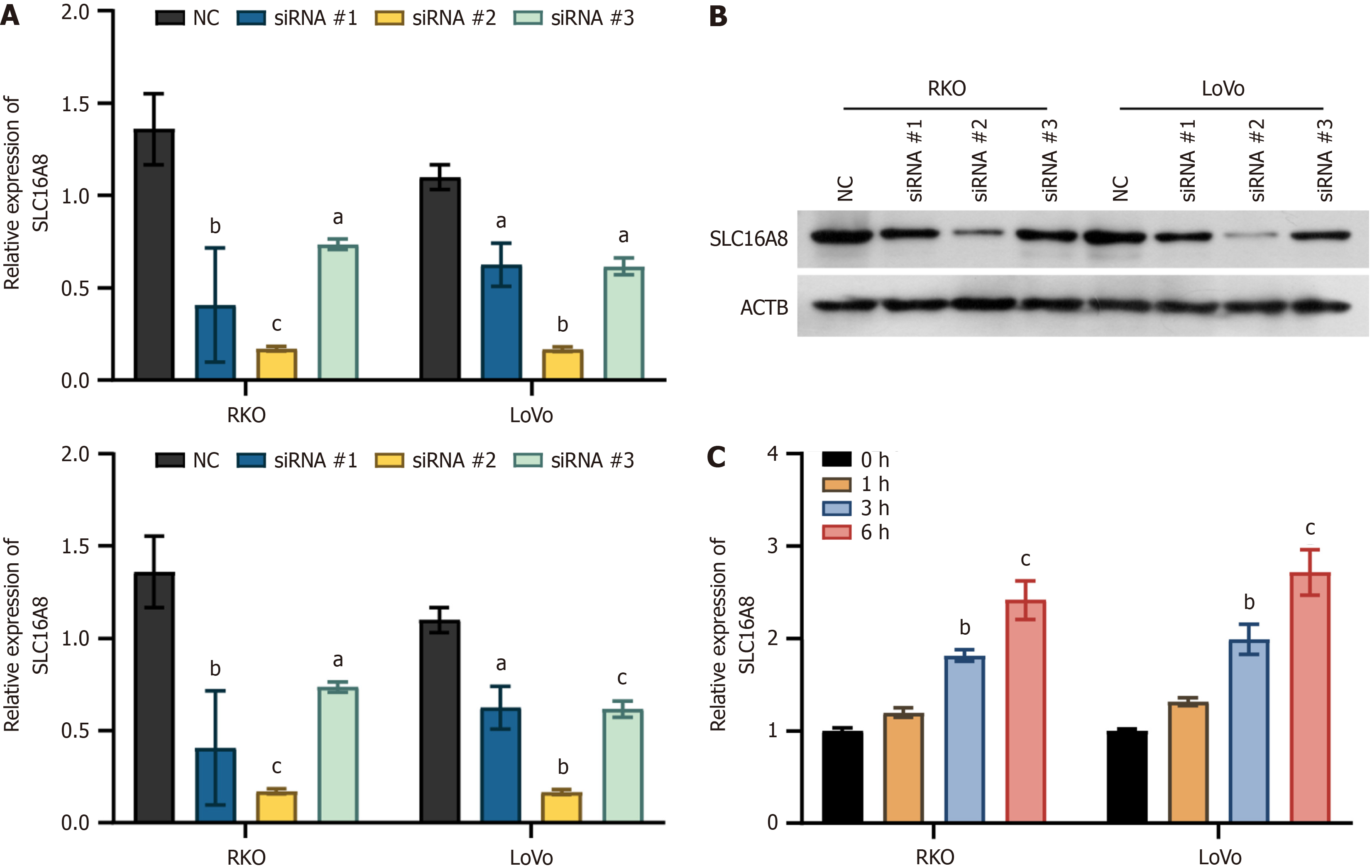
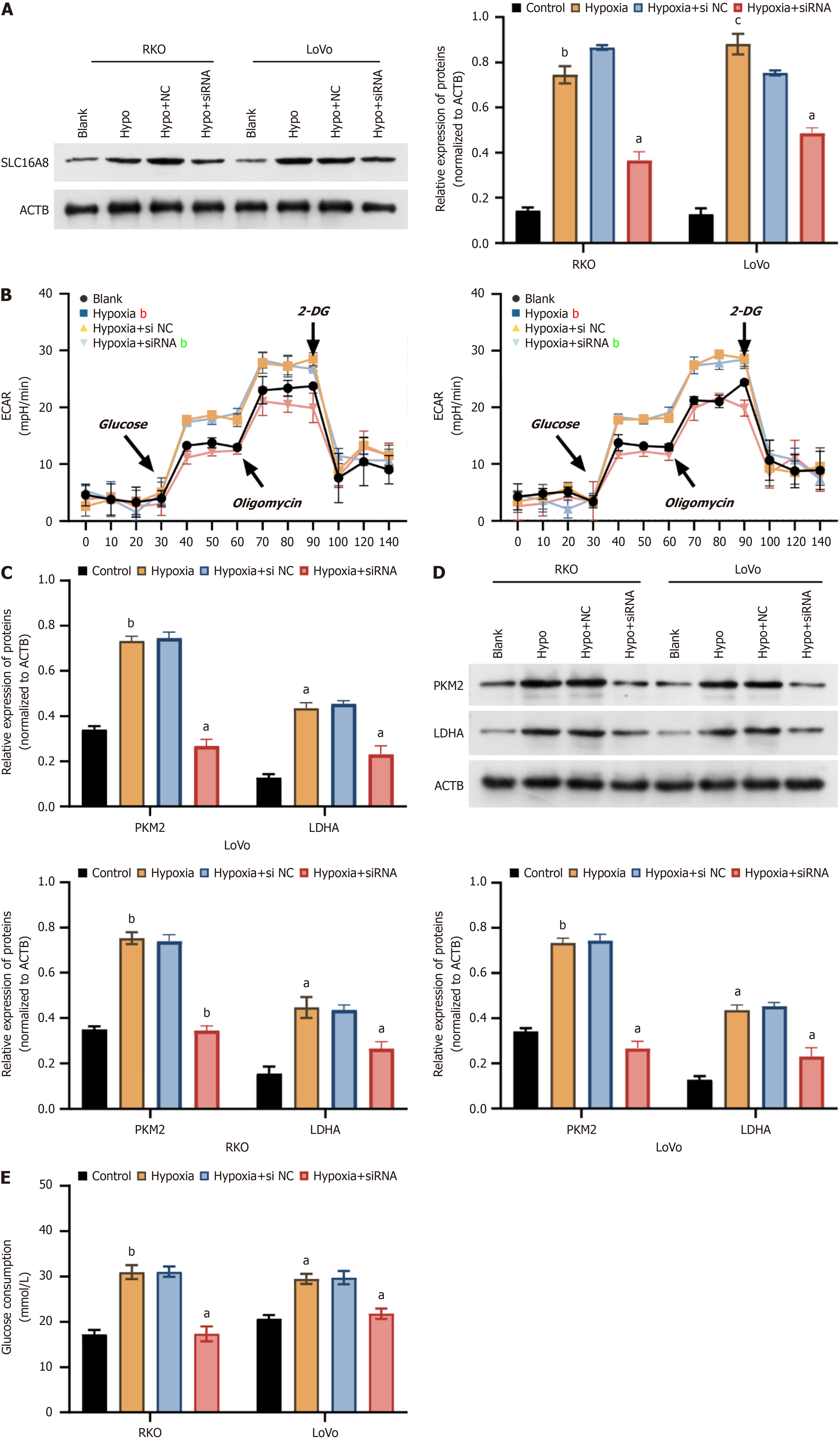
In order to further explore the mechanism of SLC16A8 in CRC cells, siRNAs targeting SLC16A8 were designed and synthesized. After transfection of SLC16A8 siRNA into RKO and LoVo cells, qPCR and Western blot results showed that all three siRNAs inhibited SLC16A8 expression. Among them, siRNA 2 had the highest knockdown efficiency (Figure 4A and B), so siRNA 2 was selected for subsequent experiments. Results also showed that the SLC16A8 expression increased with duration of hypoxia (Figure 4C). These findings identified the most effective siRNA for SLC16A8 knockdown, providing a tool for subsequent studies.
To clarify the regulation of hypoxia on SLC16A8-mediated tumor metabolic reprogramming, SLC16A8 was further interfered in CRC cells treated with hypoxia. As shown in Figure 5A, SLC16A8 siRNA significantly suppressed the upregulation of SLC16A8 expression induced by hypoxia. The results of extracellular acidification rate showed that the extracellular acidification induced by hypoxia was mitigated by SLC16A8 siRNA (Figure 5B), accompanied by a sig
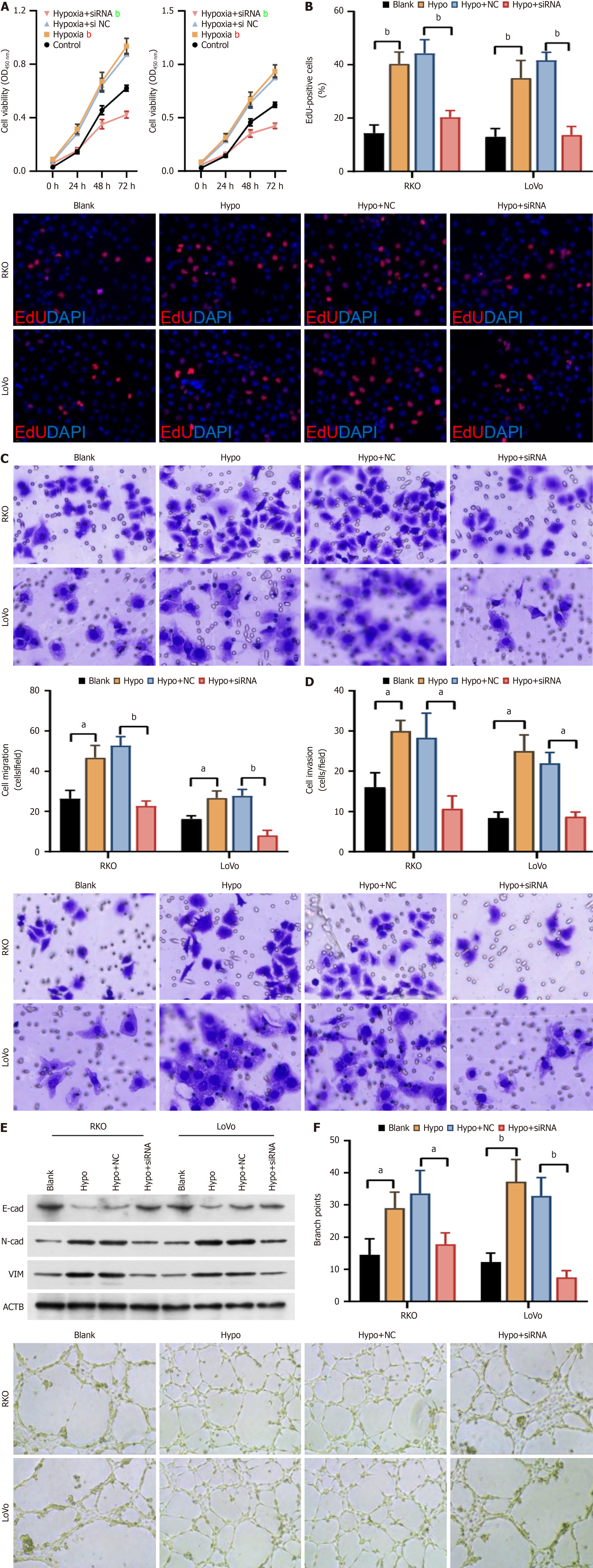
In order to unveil the mechanism of SLC16A8 in CRC, CRC cells under hypoxic conditions were subjected to SLC16A8 knockdown, co-cultured with HUVEC cells, and the occurrence of endothelial-mesenchymal transition (EndMT) in HUVEC cells was examined. The results showed that SLC16A8 siRNA significantly inhibited the increase in proliferative activity induced by hypoxia in HUVEC cells when co-cultured with RKO and LoVo cells (Figure 6A and B). Transwell chamber experiments showed that the hypoxia-induced enhancement of migration and invasion of HUVEC cells in the presence of RKO and LoVo cells was significantly reversed by SLC16A8 siRNA (Figure 6C and D), accompanied by changes in E-Cadherin, N-Cadherin, and Vimentin expression (Figure 6E). Finally, after the CRC cells of each group were co-cultured with vascular endothelial cells (HUVEC), the results were as shown in Figure 6F. Hypoxia-induced CRC cells could significantly induce vascular endothelial cells (HUVEC) to form tubes; In contrast, SLC16A8 siRNA treatment of CRC cells significantly reversed the ability of endothelial cells (HUVEC) to form tubes (Figure 6F). SLC16A8 siRNA effectively inhibited the EMT induced by hypoxia in CRC cells, highlighting the role of SLC16A8 in the tumor microenvironment.
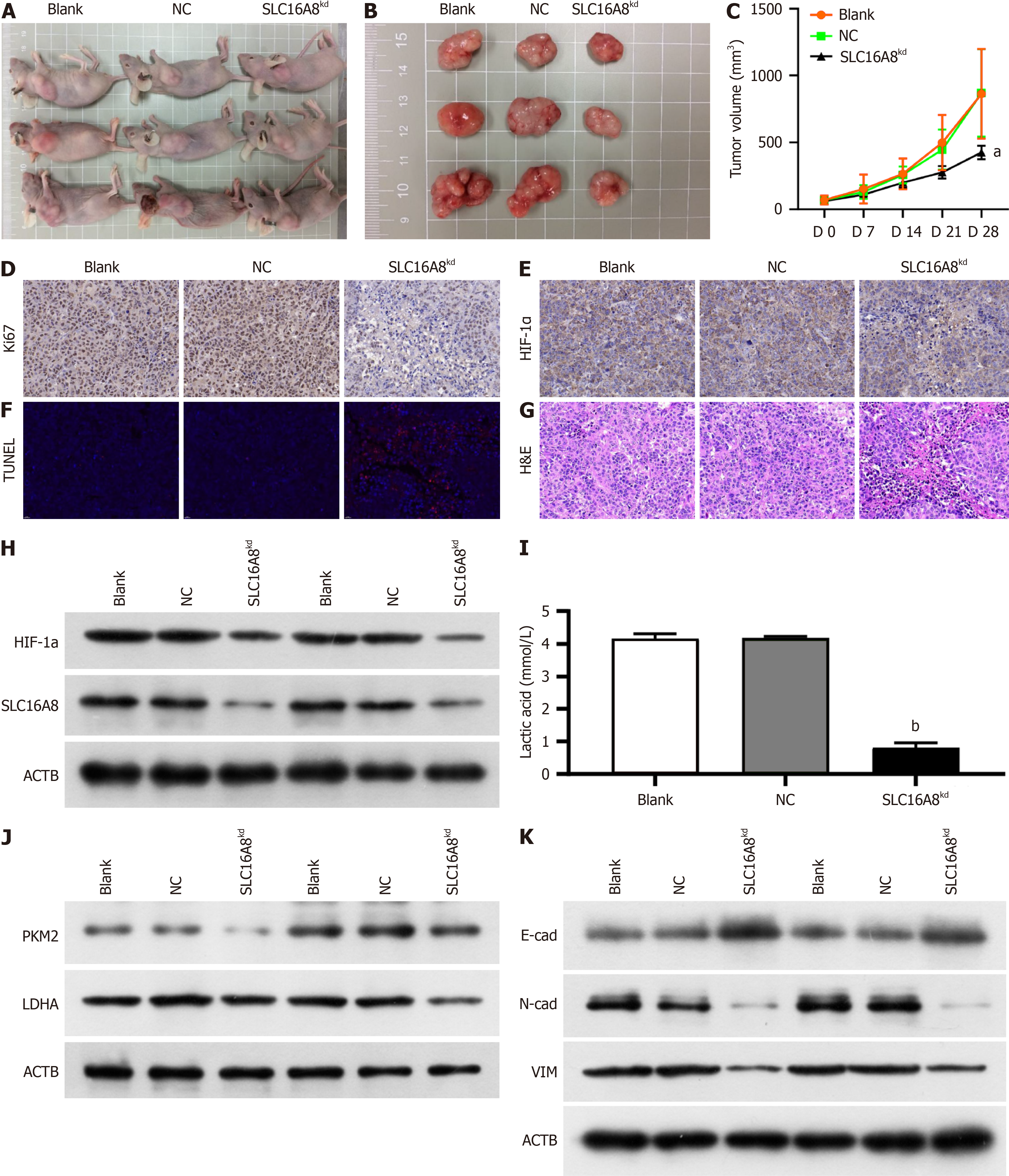
In order to investigate that effect of SLC16A8 on the growth of tumor, the nude mice bearing tumor model was established. As shown in Figure 7, SLC16A8 knockdown significantly suppressed tumor growth (Figure 7A and B) and significantly suppressed tumor volume (Figure 7C). There was a significant decrease in the Ki67 proliferation index (Figure 7D), indicating reduced tumor cell proliferation. Knockdown of SLC16A8 also led to a marked reduction in HIF-1α expression, as shown in Figure 7E and F. Additionally, apoptosis levels increased (Figure 7G), along with notable histological changes (Figure 7H). The study found that the knockdown of SLC16A8 significantly reduced the lactate levels in animal serum (Figure 7I). Simultaneously, changes in the expression of proteins related to the Warburg effect and EndMT in the tissues were observed (Figure 7J and K). The knockdown of SLC16A8 suppressed in vivo tumor growth and glycolysis, emphasizing its potential as a therapeutic intervention target in CRC.
CRC is a frequent malignant tumor of the gastrointestinal tract, and its incidence is on the rise year by year[17]. Most patients with CRC are already in the progressive stage at the time of diagnosis[17]. For this group of patients, surgery and adjuvant therapy have limited effectiveness and high adverse effects, leading to poor prognosis[18]. Understanding the molecular mechanisms that drive CRC development and progression remains crucial for advancing therapeutic stra
Tissue hypoxia affects tumor metabolism, angiogenesis and intrinsic immunity, and is also considered one of the important microenvironmental factors that promote tumor metastasis[19]. Hypoxia, or reduced oxygen availability, is a common feature of the tumor microenvironment[20]. It affects angiogenesis and metabolism and promotes tumorigenesis and progression[21]. Study have also shown that under hypoxic conditions, CRC cells exhibit resistance to multiple therapeutic agents and enhanced angiogenesis and EMT capacity[22]. Thus, the hypoxic microenvironment is essential for tumor growth. In our study, we proved that hypoxia could enhance CRC cell proliferation, migration, invasion, angiogenesis, EMT suggesting that hypoxia can accelerate the malignant process of CRC.
The main energy source for tumor cell growth metabolism is glucose metabolism[23]. The body metabolizes glucose primarily through two pathways: oxidative phosphorylation and glycolysis. Research has demonstrated that glycolysis plays a significant role in modulating tumor cell behavior[24]. Glucose enters the cell through membrane-bound glucose transporters and is converted to pyruvate through the process of glycolysis[25]. Under hypoxic conditions, pyruvate is converted to lactate, while in aerobic conditions, it undergoes mitochondrial oxidative phosphorylation to generate energy. The metabolic activity of tumor cells is directly linked to this energy production[26]. Even in the presence of adequate oxygen, tumor cells exhibit a preference for increased glucose consumption and energy production through glycolysis - a phenomenon known as the Warburg effect[27]. Our findings showed that hypoxia increased glucose uptake and lactate production in CRC cells. Additionally, hypoxia elevated ECAR in CRC cells, confirming that hypoxia enhances glycolysis in CRC cells.
The unique energy metabolism of malignant tumors is a vital aspect in exploring the mechanisms of tumor carcinogenesis[28]. The aberrant energy metabolism of tumors not only provides sufficient material and energy for the malignant expansion of tumors, but also plays a key role in maintaining tumor cell survival, resisting stressful stressful envi
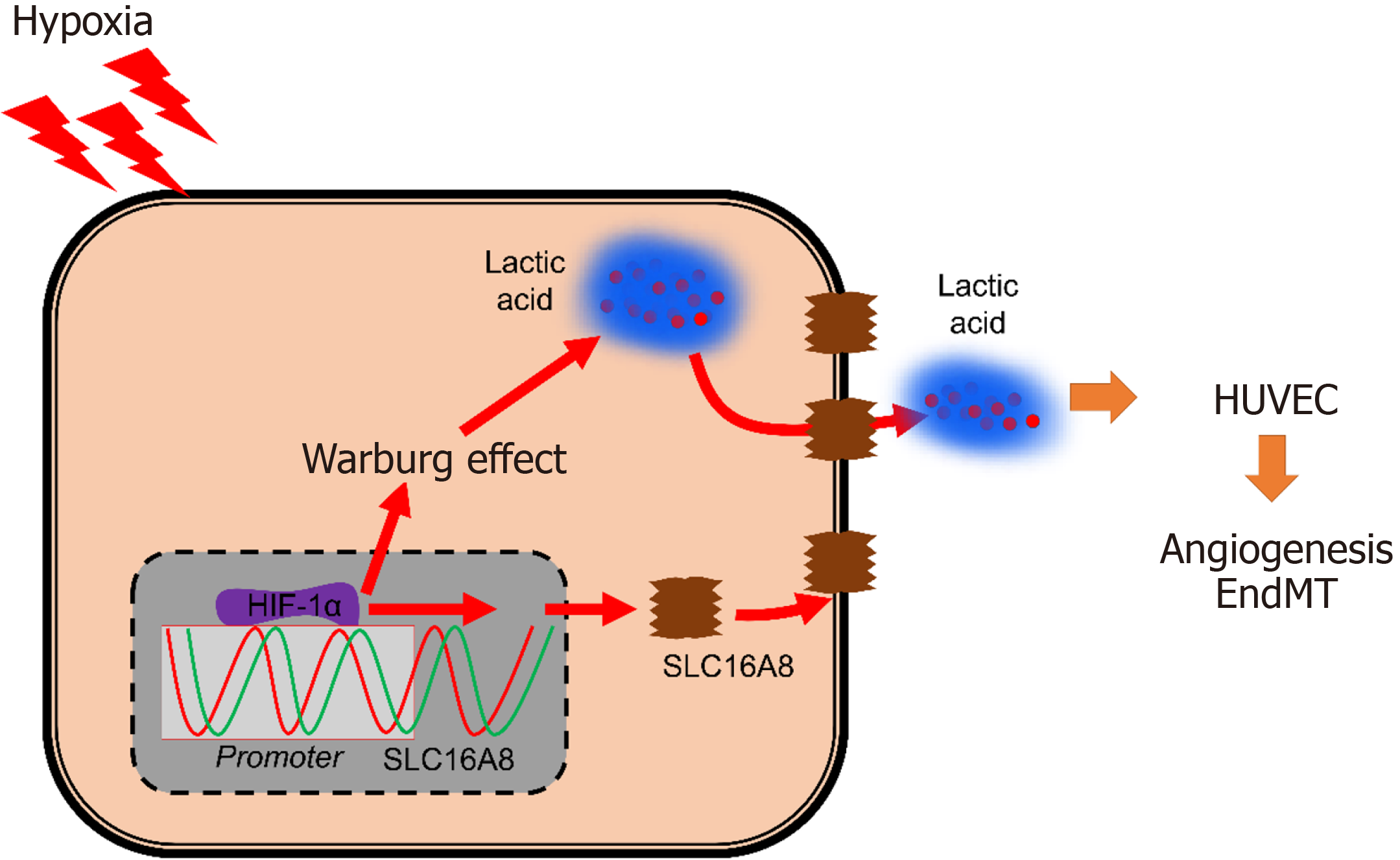
This study demonstrated that SLC16A8, as an oncogene, could accelerate proliferation, EMT, metastasis, angiogenesis, and glycolysis of CRC cells in the absence of oxygen. Therefore, we suggested that inhibition of SLC16A8 might weaken the Warburg effect to achieve the therapeutic effect of CRC (Figure 8).
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64390] [Article Influence: 16097.5] [Reference Citation Analysis (176)] |
| 2. | Nanda N, Dhawan DK. Role of Cyclooxygenase-2 in colorectal cancer patients. Front Biosci (Landmark Ed). 2021;26:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Li J, Ma X, Chakravarti D, Shalapour S, DePinho RA. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021;35:787-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 303] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 4. | Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 592] [Article Influence: 84.6] [Reference Citation Analysis (1)] |
| 5. | Stewart CL, Warner S, Ito K, Raoof M, Wu GX, Kessler J, Kim JY, Fong Y. Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr Probl Surg. 2018;55:330-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 6. | Johdi NA, Sukor NF. Colorectal Cancer Immunotherapy: Options and Strategies. Front Immunol. 2020;11:1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 7. | Xu J, Meng Q, Sun H, Zhang X, Yun J, Li B, Wu S, Li X, Yang H, Zhu H, Aschner M, Relucenti M, Familiari G, Chen R. HER2-specific chimeric antigen receptor-T cells for targeted therapy of metastatic colorectal cancer. Cell Death Dis. 2021;12:1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984-4001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1081] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 9. | Poff A, Koutnik AP, Egan KM, Sahebjam S, D'Agostino D, Kumar NB. Targeting the Warburg effect for cancer treatment: Ketogenic diets for management of glioma. Semin Cancer Biol. 2019;56:135-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Zanotelli MR, Zhang J, Reinhart-King CA. Mechanoresponsive metabolism in cancer cell migration and metastasis. Cell Metab. 2021;33:1307-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 203] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 11. | Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 1102] [Article Influence: 110.2] [Reference Citation Analysis (0)] |
| 12. | Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5:e10250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 505] [Cited by in RCA: 722] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 13. | Halestrap AP. The SLC16 gene family - structure, role and regulation in health and disease. Mol Aspects Med. 2013;34:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 554] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 14. | Felmlee MA, Jones RS, Rodriguez-Cruz V, Follman KE, Morris ME. Monocarboxylate Transporters (SLC16): Function, Regulation, and Role in Health and Disease. Pharmacol Rev. 2020;72:466-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 255] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 15. | Bosshart PD, Charles RP, Garibsingh RA, Schlessinger A, Fotiadis D. SLC16 Family: From Atomic Structure to Human Disease. Trends Biochem Sci. 2021;46:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Klipfel L, Cordonnier M, Thiébault L, Clérin E, Blond F, Millet-Puel G, Mohand-Saïd S, Goureau O, Sahel JA, Nandrot EF, Léveillard T. A Splice Variant in SLC16A8 Gene Leads to Lactate Transport Deficit in Human iPS Cell-Derived Retinal Pigment Epithelial Cells. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3004] [Article Influence: 500.7] [Reference Citation Analysis (3)] |
| 18. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1418] [Article Influence: 354.5] [Reference Citation Analysis (0)] |
| 19. | Multhoff G, Vaupel P. Hypoxia Compromises Anti-Cancer Immune Responses. Adv Exp Med Biol. 2020;1232:131-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 20. | Yuen VW, Wong CC. Hypoxia-inducible factors and innate immunity in liver cancer. J Clin Invest. 2020;130:5052-5062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 21. | Infantino V, Santarsiero A, Convertini P, Todisco S, Iacobazzi V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 280] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 22. | Sebestyén A, Kopper L, Dankó T, Tímár J. Hypoxia Signaling in Cancer: From Basics to Clinical Practice. Pathol Oncol Res. 2021;27:1609802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Mulukutla BC, Yongky A, Le T, Mashek DG, Hu WS. Regulation of Glucose Metabolism - A Perspective From Cell Bioprocessing. Trends Biotechnol. 2016;34:638-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Bose S, Le A. Glucose Metabolism in Cancer. Adv Exp Med Biol. 2018;1063:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 25. | Lu YY, Wu CH, Hong CH, Chang KL, Lee CH. GLUT-1 Enhances Glycolysis, Oxidative Stress, and Fibroblast Proliferation in Keloid. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21:162-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 568] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 27. | Schwartz L, Supuran CT, Alfarouk KO. The Warburg Effect and the Hallmarks of Cancer. Anticancer Agents Med Chem. 2017;17:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 286] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Yang JM. Altered energy metabolism in cancer: a unique opportunity for therapeutic intervention. Cancer Biol Ther. 2013;14:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Yu S, Wu Y, Li C, Qu Z, Lou G, Guo X, Ji J, Li N, Guo M, Zhang M, Lei L, Tai S. Comprehensive analysis of the SLC16A gene family in pancreatic cancer via integrated bioinformatics. Sci Rep. 2020;10:7315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Daniele LL, Sauer B, Gallagher SM, Pugh EN Jr, Philp NJ. Altered visual function in monocarboxylate transporter 3 (Slc16a8) knockout mice. Am J Physiol Cell Physiol. 2008;295:C451-C457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Cascella R, Strafella C, Longo G, Ragazzo M, Manzo L, De Felici C, Errichiello V, Caputo V, Viola F, Eandi CM, Staurenghi G, Cusumano A, Mauriello S, Marsella LT, Ciccacci C, Borgiani P, Sangiuolo F, Novelli G, Ricci F, Giardina E. Uncovering genetic and non-genetic biomarkers specific for exudative age-related macular degeneration: significant association of twelve variants. Oncotarget. 2018;9:7812-7821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |