Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.103855
Revised: January 12, 2025
Accepted: February 7, 2025
Published online: April 15, 2025
Processing time: 95 Days and 8.6 Hours
Gastric cancer is one of the most common malignant tumors worldwide, with its incidence and mortality rates ranking among the highest in gastrointestinal cancers. The overexpression or gene amplification of human epidermal growth factor receptor 2 (HER-2) occurs in approximately 15%-20% of gastric cancers and serves as a critical molecular target influencing prognosis and treatment out
To analyze the efficacy of ICIs combined with standard treatment regimens and the prognostic factors in patients with advanced HER-2-positive gastric cancer.
Clinical data from 104 patients with advanced HER-2-positive gastric cancer who were treated at our hospital between March 2021 and May 2023 were retrospectively analyzed. Patients were divided into a control group (n = 54, treated with trastuzumab combined with platinum-based chemotherapy as the standard regimen) and an observation group (n = 50, treated with ICIs in addition to the standard regimen). The therapeutic efficacy, survival outcomes, and adverse reactions were compared between the two groups. Univariate and Cox multivariate analyses were performed to identify factors influencing patient prognosis.
With a median follow-up time of 14.6 months, there were no significant differences between the two groups in terms of objective response rate or disease control rate (P > 0.05). The median progression-free survival (mPFS) and mPFS for patients with immunohistochemistry 3 + in the observation group were significantly higher than those in the control group (P < 0.05). Among patients in the observation group, those with positive programmed death-ligand 1 (PD-L1) expression had a significantly higher mPFS than those with negative PD-L1 expression (P < 0.05). Regarding adverse events, significant differences were observed between the two groups in hypothyroidism and neutropenia (P < 0.05). Cox multivariate analysis showed that Eastern Cooperative Oncology Group (ECOG) performance status, peritoneal metastasis, positive programmed death-1 expression, and treatment regimen were independent factors influencing PFS (hazard ratio > 1, P < 0.05).
ICIs combined with standard treatment regimens for patients with advanced HER-2-positive gastric cancer demonstrate favorable clinical efficacy, significantly prolonging PFS with manageable safety. ECOG performance status, peritoneal metastasis, positive PD-L1 expression, and treatment regimen are independent factors influ
Core Tip: Immune checkpoint inhibitors combined with standard treatment regimens for patients with advanced human epidermal growth factor receptor 2-positive gastric cancer demonstrate favorable clinical efficacy, significantly prolonging progression-free survival with manageable safety. Eastern Cooperative Oncology Group performance status, peritoneal metastasis, positive programmed death-ligand 1 expression, and treatment regimen are independent factors influencing progression-free survival, warranting increased clinical attention to patients exhibiting these factors.
- Citation: Zhang SH, Li W, Chen XY, Nie LL. Combining immune checkpoint inhibitors with standard treatment regimens in advanced human epidermal growth factor receptor-2 positive gastric cancer patients. World J Gastrointest Oncol 2025; 17(4): 103855
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/103855.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.103855
Gastric cancer is one of the malignant tumors with high morbidity and mortality worldwide, characterized by complex pathological mechanisms and diverse clinical manifestations. Treatment options for advanced-stage disease are limited, resulting in poor prognosis for these patients[1,2]. According to statistics from the International Agency for Research on Cancer, gastric cancer ranks fifth in global cancer incidence and fourth in mortality, with a five-year survival rate of less than 10% for advanced cases[3]. Molecular pathological studies on gastric cancer[4] have identified human epidermal growth factor receptor 2 (HER-2) as a significant molecular marker. Patients with HER-2 positive gastric cancer exhibit unique molecular characteristics, leading to higher aggressiveness and poorer prognosis[5]. As a result, treatment strategies for HER-2 positive gastric cancer have become a research hotspot in this field.
In recent years, the prevalence of HER-2 positive gastric cancer has been shown to vary significantly across different regions and populations. Studies have indicated that HER-2 overexpression occurs in approximately 15%-20% of gastric cancer cases globally, but this rate may be higher in certain ethnic groups. For instance, higher rates of HER-2 positivity have been reported in Asian populations, with studies from China and Japan showing up to 30% of gastric cancer cases testing positive for HER-2 overexpression. Conversely, the incidence of HER-2 positive gastric cancer appears to be lower in Western populations. These demographic variations underscore the importance of understanding the role of HER-2 as a therapeutic target in different populations and highlight the potential benefits of personalized treatment strategies for HER-2 positive gastric cancer.
In recent years, the development of targeted therapy has offered new treatment opportunities for HER-2 positive gastric cancer patients. Based on the results of the ToGA trial[6], trastuzumab combined with platinum-based chemo
The emergence of tumor immunotherapy has provided new insights into treating various malignancies, including gastric cancer. Immune checkpoint inhibitors (ICIs) enhance the immune system’s ability to recognize and attack tumor cells by reversing immune escape mechanisms, demonstrating good efficacy and safety in multiple solid tumors[8,9]. Notably, the CheckMate 649 trial[10] revealed that the combination of the programmed death-1 inhibitor nivolumab with chemotherapy significantly prolonged median overall survival (mOS) in advanced gastric cancer patients, laying the foundation for further developments in gastric cancer immunotherapy. However, the application of ICIs in HER-2 positive gastric cancer, particularly their combination with existing standard regimens, remains underexplored, with limited data on therapeutic efficacy and potential adverse reactions. Against this background, this study retrospectively analyzed clinical data from 104 advanced HER-2 positive gastric cancer patients treated at our hospital. The aim was to evaluate the efficacy of combining ICIs with standard treatment regimens and to identify factors influencing prognosis, thereby providing a clinical basis for optimizing personalized treatment strategies for HER-2 positive gastric cancer.
A retrospective analysis was conducted on the clinical data of 104 patients with advanced HER-2 positive gastric cancer treated at our hospital between March 2021 and May 2023. Among them, there were 81 males and 23 females, aged 41-76 years, with a median age of 64 years. The median follow-up time was 14.6 months. Inclusion criteria: (1) Age 18-80 years, no gender limitation; (2) Pathologically confirmed adenocarcinoma; (3) HER-2 positive defined as immunohistochemistry (IHC) 3 + or 2 + [in situ hybridization (ISH) positive][11]; (4) Eastern Cooperative Oncology Group (ECOG) performance status score of 0-2[12], and an expected survival of > 3 months; (5) At least two cycles of combination therapy with efficacy confirmed by imaging and clinical evaluation; and (6) Informed consent signed by patients and their families, with explicit agreement to participate in the study. Exclusion criteria: (1) Previous immunotherapy; (2) Recent (within 6 months) history of adverse cardiac events, left ventricular ejection fraction < 40%; (3) Severe dysfunction of other vital organs or severe systemic immune diseases; (4) Coexisting other primary tumors; and (5) Cognitive, communicative, or mental disorders. This study was approved by the Ethics Committee of our hospital and complied with the principles of the Declaration of Helsinki[13] and relevant ethical requirements.
Patients were divided into the control group (n = 54) and observation group (n = 50) based on their treatment regimens. The specific treatment methods and medication regimens are as follows: Control group: Patients received standard treatment, including trastuzumab combined with platinum-based chemotherapy: Trastuzumab (produced by Shanghai Henlius Biotech Co., Ltd., approval No. S20200019) was administered intravenously at a dose of 6 mg/kg on the first day of each treatment cycle. Platinum-based chemotherapy regimens included one of the following options, determined based on tumor type, individual differences, and physician advice: XELOX regimen: Oxaliplatin combined with capecitabine; SOX regimen: Oxaliplatin combined with S-1; Cisplatin + taxane regimen. Observation group: Patients received the control group regimen combined with ICIs therapy. The specific combination regimen was as follows: ICIs selection: Based on patient-specific conditions, one of the following was chosen: Pembrolizumab (produced by MSD Ireland, approval No. SJ20180019), administered intravenously at a dose of 2 mg/kg on the first day of each treatment cycle; Camrelizumab (produced by Jiangsu Hengrui Medicine Co., Ltd., approval No. S20190027), administered intravenously at a dose of 200 mg on the first day of each treatment cycle; Sintilimab (produced by Innovent Biologics, Inc., approval No. S20180016), administered intravenously at a dose of 200 mg on the first day of each treatment cycle. Chemotherapy regimens combined with ICIs included the following three options: XELOX regimen, SOX regimen, or cisplatin + albumin-bound paclitaxel regimen. Both groups underwent standardized treatment cycles of 21 days per cycle, with a minimum of 2 cycles. Some patients received 4 to 6 cycles of combination therapy based on their condition. After completing the primary treatment, patients underwent maintenance therapy or initiated subsequent-line treatments upon disease progression, depending on disease control status. Throughout the treatment period, patients underwent close clinical monitoring, including laboratory tests, imaging evaluations, and adverse event management. The medical team adjusted drug dosages based on treatment efficacy and adverse event severity, with partial treatment suspension if necessary to ensure safety. Summary of treatment regimens for control and observation groups is shown in Table 1.
| Treatment group | Treatment regimen | Chemotherapy regimen | Immunotherapy option | Dosage and administration |
| Control group | Standard treatment | XELOX (Oxaliplatin + Capecitabine) | Trastuzumab (6 mg/kg IV) on Day 1 of each cycle | |
| SOX (Oxaliplatin + S-1) | ||||
| Cisplatin + taxane | ||||
| Observation group | Combination of standard treatment + ICI | XELOX (Oxaliplatin + Capecitabine) | Pembrolizumab (2 mg/kg IV), Camrelizumab (200 mg IV), Sintilimab (200 mg IV) | Pembrolizumab: Day 1 of each cycle; Camrelizumab/Sintilimab: Day 1 of each cycle |
| SOX (Oxaliplatin + S-1) | Same as above | |||
| Cisplatin + Nab-Paclitaxel |
Efficacy evaluation: All patients will undergo follow-up examinations after treatment, including gastroscopy, endoscopic ultrasound, and contrast-enhanced abdominal computed tomography (CT). Treatment efficacy was evaluated using the internationally recognized response evaluation criteria in solid tumors (RECIST 1.1)[14], including the following four categories: Complete response (CR): Disappearance of all target lesions without new lesions for at least 4 weeks; Partial response (PR): A ≥ 30% reduction in the sum of the diameters of target lesions compared to baseline, without new lesions; Stable disease (SD): Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease (PD); PD: A ≥ 20% increase in the sum of the diameters of target lesions compared to the smallest sum recorded or the appearance of new lesions. Short-term efficacy evaluation indicators included: Objective response rate (ORR): ORR = (CR + PR)/total cases × 100%; DCR: DCR = (CR + PR + SD)/total cases × 100%.
Long-term efficacy: PFS was used as the primary evaluation indicator. PFS was defined as the time from the start of treatment to confirmed PD, death, or the last follow-up. Disease progression will be confirmed through imaging exams (such as gastroscopy, endoscopic ultrasound, and contrast-enhanced abdominal CT) and changes in tumor marker levels. Imaging assessments will follow the RECIST 1.1 criteria. Patients will undergo imaging exams at 3 months, 6 months, and every 6 months thereafter, with the frequency adjusted as necessary based on changes in clinical symptoms.
Adverse event evaluation: Adverse events during treatment were graded according to the Common Terminology Criteria for Adverse Events (CTCAE 5.0)[15]. All patients will undergo regular hematological tests (such as complete blood count, liver and kidney function tests, etc.) during the treatment process, with clinical assessments conducted monthly. All adverse events occurring during treatment will be classified according to the CTCAE criteria and recorded in the patient’s follow-up form. Mild (grade 1): Minor symptoms, no interference with daily activities, no treatment required; Moderate (grade 2): Noticeable symptoms interfering with daily activities but not requiring hospitalization; Severe (grade 3): Significant interference with daily activities requiring hospitalization or treatment intervention; Life-threatening (grade 4): Life-threatening symptoms requiring immediate intervention; Death (grade 5): Patient death caused by adverse events. For adverse events of grade 3 or higher, treatment will be temporarily suspended, and interventions will be provided based on the specific situation, such as hospitalization, symptomatic treatment, or medication adjustments. All adverse events will be followed up long-term after treatment completion to ensure patient safety.
Patients were followed up using multiple methods, including reviewing inpatient and outpatient records, regular telephone follow-ups, text message reminders, and interviews with patient families. The main follow-up contents included treatment response, adverse event monitoring, and survival status records. The follow-up cutoff date was set for December 2024 to ensure complete and detailed follow-up data.
GraphPad Prism 8 was used for plotting, and statistical product and service solutions 26.0 software was employed for statistical analysis. Categorical data were analyzed using the χ2 test or Fisher exact test. Survival analysis was performed using the Kaplan-Meier method and Log-rank test for survival curve comparisons. The Cox regression model was used for factor analysis. A P < 0.0 indicated statistically significant differences.
Comparison of baseline characteristics, including gender, age, ECOG score, tumor location, surgical status, liver metastasis, peritoneal metastasis, HER-2 expression, and programmed death-ligand 1 (PD-L1) expression, between the two groups showed no significant differences (P > 0.05). Details are shown in Table 2.
| Control (n = 54) | Observation (n = 50) | χ2 | P value | |
| Gender | 0.198 | 0.655 | ||
| Male | 43 (79.63) | 38 (76.00) | ||
| Female | 11 (20.37) | 12 (24.00) | ||
| Age (years) | 0.025 | 0.873 | ||
| < 65 | 30 (55.56) | 27 (54.00) | ||
| ≥ 65 | 24 (44.44) | 23 (46.00) | ||
| ECOG score | 0.005 | 0.938 | ||
| 0 | 22 (40.74) | 20 (40.00) | ||
| 1-2 | 32 (59.26) | 30 (60.00) | ||
| Tumor location | 1.801 | 0.179 | ||
| Stomach | 21 (38.89) | 26 (52.00) | ||
| Gastroesophageal junction | 33 (61.11) | 24 (48.00) | ||
| Surgical status | 1.874 | 0.171 | ||
| Yes | 10 (18.52) | 15 (30.00) | ||
| No | 44 (81.48) | 35 (70.00) | ||
| Liver metastasis | 0.063 | 0.801 | ||
| Yes | 24 (44.44) | 21 (42.00) | ||
| No | 30 (55.56) | 29 (58.00) | ||
| Peritoneal metastasis | 0.068 | 0.793 | ||
| Yes | 16 (29.63) | 16 (32.00) | ||
| No | 38 (70.37) | 34 (68.00) | ||
| HER-2 expression | 0.497 | 0.480 | ||
| IHC2 + (ISH positive) | 18 (33.33) | 20 (40.00) | ||
| IHC3 + | 36 (66.67) | 30 (60.00) | ||
| PD-L1 expression | 0.292 | 0.588 | ||
| Positive (CPS ≥ 1%) | 22 (40.74) | 23 (46.00) | ||
| Negative (CPS < 1%) | 32 (59.26) | 27 (54.00) | ||
| Chemotherapy regimen | ||||
| XELOX | 23 (42.59) | 31 (62.00) | ||
| SOX | 26 (48.15) | 13 (26.00) | ||
| Cisplatin + Taxanes | 5 (9.26) | |||
| Cisplatin + Nab-Paclitaxel | 6 (12.00) |
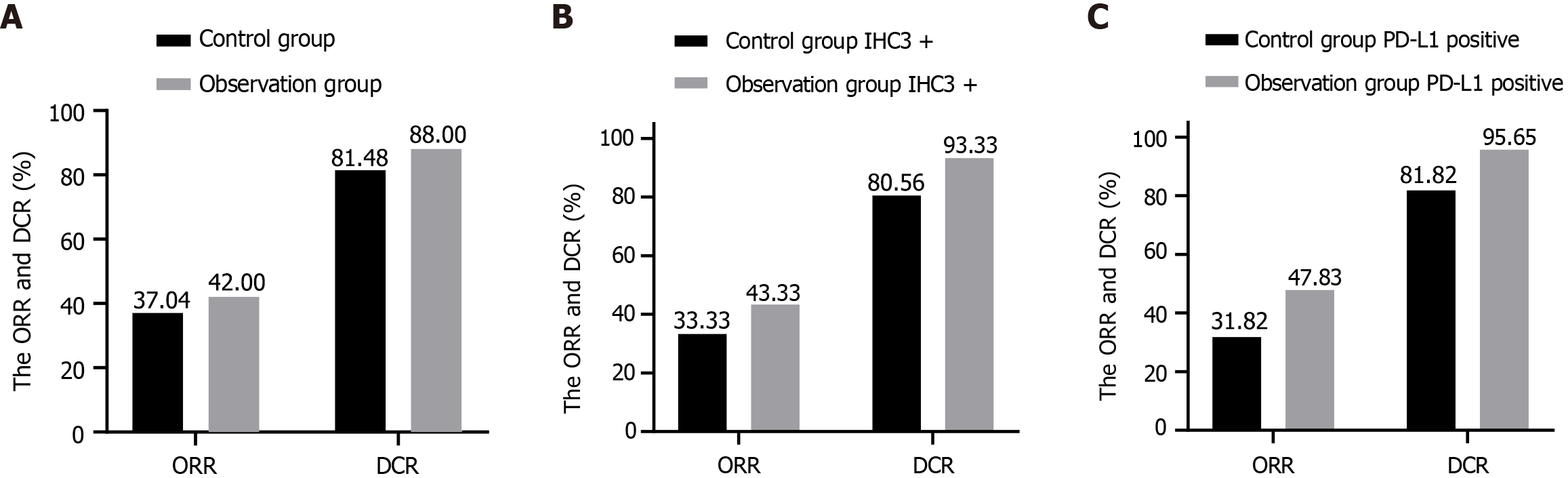
The ORR and DCR in the control group were 37.04% (20/54) and 81.48% (44/54), respectively, while in the observation group, these were 42.00% (21/50) and 88.00% (44/50). Details are shown in Figure 1A. For IHC3 + patients, the ORR and DCR were 33.33% (12/36) and 80.56% (29/36) in the control group and 43.33% (13/30) and 93.33% (28/30) in the observation group, as shown in Figure 1B. For PD-L1-positive patients, the ORR and DCR were 31.82% (7/22) and 81.82% (18/22) in the control group, and 47.83% (11/23) and 95.65% (22/23) in the observation group, as shown in Figure 1C. In the observation group, 14 patients received pembrolizumab, 17 received camrelizumab, and 19 received sintilimab. The ORR and DCR for pembrolizumab were 78.57% (11/14) and 100.00% (14/14), respectively. For camrelizumab, these were 35.29% (6/17) and 82.35% (14/17), and for sintilimab, 21.05% (4/19) and 84.21% (16/19). Differences in ORR and DCR were not statistically significant (P > 0.05).
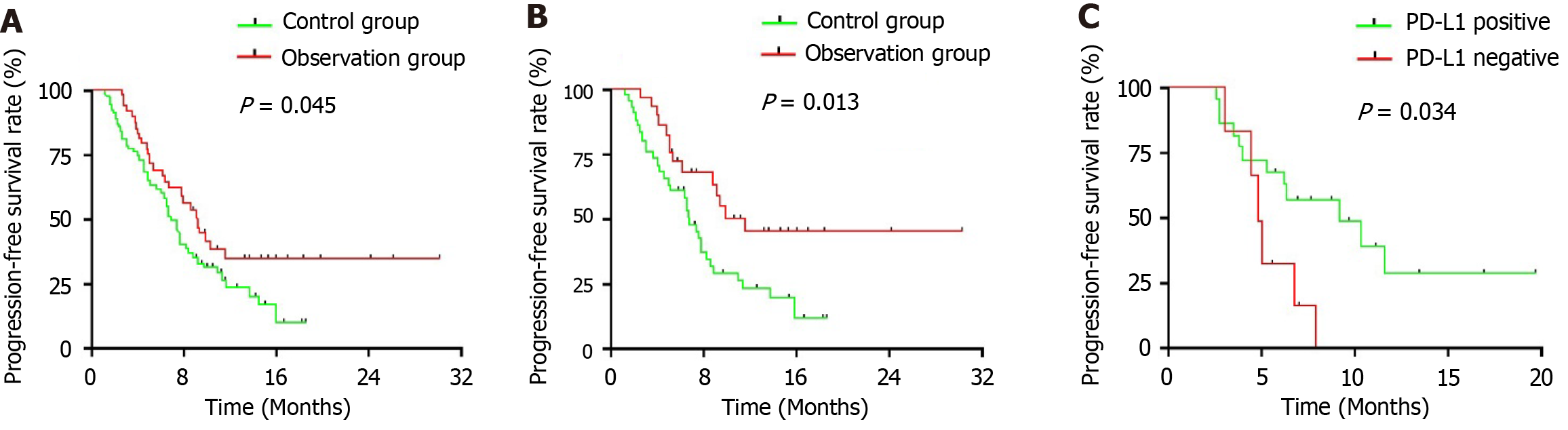
The mPFS of the control and observation groups was 6.9 months and 9.3 months, respectively [hazard ratio (HR) = 0.627, 95% confidence interval (CI): 0.403-0.976; P = 0.045, see Figure 2A]. Among IHC3 + patients, the mPFS in the control and observation groups was 7.0 months and 11.7 months, respectively (HR = 0.459, 95%CI: 0.258-0.821; P = 0.013, see Figure 2B). For IHC2 + (ISH positive) patients, the mPFS was 7.4 months and 7.9 months, respectively (P = 0.815). In the observation group, the mPFS for IHC3 + and IHC2 + (ISH positive) patients was 11.5 months and 8.0 months, respectively (P = 0.052). For patients with PD-L1 expression, the mPFS in the control and observation groups was 6.6 months and 9.3 months, respectively (P = 0.402). Among PD-L1-positive [combined positive score (CPS) ≥ 1%] and negative (CPS < 1%) patients in the observation group, the mPFS was 9.3 months and 4.8 months, respectively (HR = 0.369, 95%CI: 0.102-1.325; P = 0.034, see Figure 2C). Additionally, the mPFS of the observation group using pembrolizumab, camrelizumab, and sintilimab was not reached, 8.6 months, and 6.2 months, respectively. The 1-year PFS rates in the control and observation groups were 12.96% and 24.00%, respectively.
Adverse reactions in both groups were mostly grade 1-2, with statistically significant differences in hypothyroidism (P < 0.05). Among grade 3-4 adverse reactions, the incidence of neutropenia was higher in the control group than in the observation group (P < 0.05). All adverse events were well-controlled through symptomatic management, with no treatment-related deaths due to severe adverse reactions (see Table 3 and Table 4).
| Adverse reaction | Control (n = 54) | Observation (n = 50) | χ2 | P value |
| Leukopenia | 22 (40.74) | 20 (40.00) | 0.005 | 0.938 |
| Neutropenia | 13 (24.07) | 19 (38.00) | 2.363 | 0.124 |
| Lymphopenia | 28 (51.85) | 29 (58.00) | 0.396 | 0.529 |
| Thrombocytopenia | 29 (53.70) | 25 (50.00) | 0.142 | 0.705 |
| Hemoglobin reduction | 23 (42.59) | 22 (44.00) | 0.020 | 0.885 |
| Gastrointestinal reaction | 20 (37.04) | 17 (34.00) | 0.104 | 0.746 |
| Abnormal liver function | 9 (16.67) | 8 (16.00) | 0.008 | 0.927 |
| Pneumonia | 4 (7.41) | 3 (6.00) | 0.011 | 0.916 |
| Hypothyroidism | 0 (0.00) | 6 (12.00) | 4.846 | 0.027 |
| Adverse reaction | Control (n = 54) | Observation (n = 50) | χ2 | P value |
| Leukopenia | 5 (9.26) | 2 (4.00) | 0.459 | 0.497 |
| Neutropenia | 9 (16.67) | 2 (4.00) | 4.404 | 0.035 |
| Lymphopenia | 7 (12.96) | 6 (12.00) | 0.022 | 0.882 |
| Thrombocytopenia | 9 (16.67) | 10 (20.00) | 0.193 | 0.660 |
| Hemoglobin reduction | 4 (7.41) | 2 (4.00) | 0.104 | 0.746 |
| Gastrointestinal reaction | 2 (3.70) | 0 (0.00) | 0.000 | 1.000 |
| Abnormal liver function | 0 (0.00) | 2 (4.00) | 0.000 | 1.000 |
| Pneumonia | 0 (0.00) | 1 (2.00) | 0.000 | 1.000 |
| Hypothyroidism | 0 (0.00) | 0 (0.00) | 0.000 | 1.000 |
Univariate analysis identified ECOG score, peritoneal metastasis, PD-L1 expression, and treatment regimen as factors affecting PFS. Further multivariate Cox regression analysis confirmed these factors as independent predictors of PFS (HR > 1, P < 0.05, see Table 5).
| Factor | n = 104 | mPFS (months) | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |||
| Gender | 0.961 | 0.558-1.657 | 0.894 | |||||
| Male | 81 | 7.8 | ||||||
| Female | 23 | 7.4 | ||||||
| Age (years) | 0.746 | 0.469-1.181 | 0.203 | |||||
| < 65 | 57 | 6.6 | ||||||
| ≥ 65 | 47 | 9.1 | ||||||
| ECOG score | 1.972 | 1.208-3.199 | 0.005 | 2.014 | 1.231-3.265 | 0.004 | ||
| 0 | 42 | 11.6 | ||||||
| 1-2 | 62 | 6.7 | ||||||
| Tumor location | 1.048 | 0.663-1.652 | 0.847 | |||||
| Stomach | 47 | 7.9 | ||||||
| Gastroesophageal Junction | 57 | 7.6 | ||||||
| Surgical status | 1.299 | 0.765-2.214 | 0.335 | |||||
| Yes | 25 | 6.6 | ||||||
| No | 79 | 7.8 | ||||||
| Liver metastasis | 1.125 | 0.708-1.767 | 0.623 | |||||
| Yes | 45 | 6.8 | ||||||
| No | 59 | 7.9 | ||||||
| Peritoneal metastasis | 3.782 | 2.256-6.343 | < 0.001 | 3.767 | 2.249-6.285 | < 0.001 | ||
| Yes | 32 | 4.2 | ||||||
| No | 72 | 9.3 | ||||||
| HER-2 expression | 0.779 | 0.485-1.248 | 0.300 | |||||
| IHC2 + (ISH positive) | 38 | 7.6 | ||||||
| IHC3 + | 66 | 7.9 | ||||||
| PD-L1 expression | 1.847 | 1.159-2.874 | 0.046 | 2.158 | 1.325-3.131 | 0.017 | ||
| Positive (CPS ≥ 1%) | 45 | 8.9 | ||||||
| Negative (CPS < 1%) | 59 | 4.5 | ||||||
| Chemotherapy regimen | 1.592 | 1.003-2.569 | 0.049 | 1.731 | 1.074-2.775 | 0.026 | ||
| Combined immunotherapy | 50 | 9.3 | ||||||
| Non-combined Immunotherapy | 54 | 6.7 | ||||||
The occurrence and progression of gastric cancer are closely related to multiple genomic alterations and the aberrant activation of molecular pathways, among which HER-2 overexpression plays a significant role in its treatment[16]. HER-2, encoded by the ERBB2 gene, is typically defined as HER-2 positive when overexpression is detected by IHC as 3 + or 2 + with ISH positivity[17]. Targeted therapies against HER-2, such as the use of trastuzumab, have significantly improved the OS of patients with advanced gastric cancer. However, subsequent studies[18] have shown that the continued use of trastuzumab after disease progression fails to provide significant clinical benefits, posing challenges to further optimizing therapeutic strategies.
In recent years, immunotherapy has garnered widespread attention in the treatment of advanced gastric cancer. ICIs have provided durable survival benefits to patients with gastric cancer[19], particularly when combined with HER-2-targeted therapies, demonstrating potential synergistic antitumor effects. The results of this study align with those observed in previous studies, such as PANACEA[20] and ATTRACTION-2[21], which also explored the combination of trastuzumab with immunotherapy in HER-2 positive gastric cancer. However, a notable difference was observed in the response rates and PFS between this study and the Keynote-811 trial. Specifically, the Keynote-811 trial reported a CR rate of 11% and an ORR of 74.4% in the pembrolizumab group, which is significantly higher than the ORR of 42% in the present study.
The differences in treatment outcomes may be due to differences in patient populations. In particular, the Keynote-811 trial included a larger proportion of patients with high PD-L1 expression (84.8%) and IHC3 + HER-2 expression (82.9%), which may have contributed to the higher ORR observed in that study. Moreover, the ATTRACTION-2 study demonstrated a modest ORR of 16.9% with nivolumab and trastuzumab in patients who had previously received trastuzumab. In contrast, the present study showed that combination therapy with ICIs and trastuzumab yielded an ORR of 42%. This suggests a potential benefit of combining immunotherapy with trastuzumab even after initial progression, although the response rate in this study was lower than in studies using pembrolizumab.
Another phase II study[22] demonstrated significant clinical outcomes with a first-line pembrolizumab combination regimen in HER-2 positive advanced gastric cancer, achieving an ORR of 91%, a mPFS of 13.0 months, and a mOS of 27.2 months. These findings are largely consistent with the Keynote-811 study[23], which demonstrated the efficacy of pembrolizumab in combination with chemotherapy. However, the present study observed a lower ORR and DCR compared to Keynote-811, where the pembrolizumab group achieved an ORR of 74.4% and a DCR of 96.2%. Several factors may account for these differences, including the patient selection criteria. The Keynote-811 trial included a higher percentage of patients with PD-L1 expression ≥ 1% (84.8%) and IHC3 + positivity (82.9%), whereas the present study had a lower proportion of PD-L1 positive (46%) and IHC3 + (60%) patients. These baseline variations likely contributed to the observed differences in treatment efficacy. Additionally, differences in treatment regimens may explain the divergence. The Keynote-811 trial utilized a fixed combination regimen of pembrolizumab with chemotherapy, while this study employed a more heterogeneous chemotherapy regimen, including XELOX, SOX, and Cisplatin-based regimens.
Moreover, this study adds valuable data to the existing literature by documenting clinical outcomes in a Chinese cohort, a population that has not been extensively studied in earlier trials. The response rate observed in this cohort, while lower than those in some international studies, reflects the current clinical challenges and real-world limitations in treating HER-2 positive gastric cancer in this region.
To date, research on treatment strategies for HER-2 positive gastric cancer after disease progression has not achieved definitive breakthroughs. This may partly be attributed to the significant heterogeneity of HER-2 expression in gastric cancer. HER-2 expression exhibits spatial heterogeneity, where expression levels vary across different tumor regions, and temporal heterogeneity, with dynamic changes in HER-2 expression during disease progression[24,25]. This heterogeneity limits the efficacy of targeted therapies and presents challenges for exploring novel combination therapies.
Currently, apart from trastuzumab, research on other potential targets such as fibroblast growth factor receptor, microsatellite instability-high, mesenchymal to epithelial transition factor amplification, NTRK gene fusion, and claudin18.2 is ongoing. However, definitive clinical results are yet to be achieved. A phase Ib/II study[26] evaluated the efficacy of Margetuximab (a novel HER-2 targeted drug) combined with pembrolizumab in HER-2 positive advanced gastric cancer patients previously treated with trastuzumab. Results showed an ORR of 24.0% and a DCR of 62.0% in patients with high HER-2 expression (IHC3 +), while patients with low HER-2 expression had ORRs and DCRs of 0% and 24.0%, respectively. Similarly, studies like GATSBY[27] have shown that patients with high HER-2 expression benefit significantly more from combination therapies.
Furthermore, the relationship between HER-2 expression levels and immunotherapy efficacy was confirmed in this study. In the observation group, patients with high HER-2 expression (IHC3 +) had a DCR of 93.33% and an mPFS of 11.5 months, while those with low HER-2 expression had a DCR of 80.00% and an mPFS of 8.0 months. This finding suggests that HER-2-high patients benefit more significantly from immunotherapy combinations[28,29]. Although the efficacy of targeted therapy in HER-2-positive breast cancer has been well-established, in gastric cancer, the results have been less promising. The failure of small-molecule drugs like lapatinib, large-molecule drugs like pertuzumab, and antibody-drug conjugates (ADCs) such as T-DM1 may be attributed to the unique resistance mechanisms of HER-2 positive gastric cancer. Notably, the heterogeneity of HER-2 expression, along with alterations in HER-2 signaling pathways (e.g., phosphoinositide 3-kinase-akt), plays a significant role in the reduced efficacy of HER-2-targeted therapies[30]. Recent developments in new HER-2-targeted therapies, such as RC48-ADC (Disitamab Vedotin) and DS-8201 (T-DXd, Trastuzumab Deruxtecan), offer promising clinical benefits, regardless of HER-2 expression levels. Future research should focus on optimizing biomarker screening strategies to improve patient stratification accuracy and exploring individualized treatment approaches[31].
In conclusion, while combination therapies with ICIs and trastuzumab have shown promising efficacy, several challenges remain. Future studies should focus on refining biomarker-based patient selection, optimizing treatment regimens, and investigating the long-term safety and efficacy of these therapies. To validate these findings, larger, multi-center, randomized controlled trials are essential to provide robust evidence supporting immunotherapy strategies for HER-2 positive advanced gastric cancer.
| 1. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 847] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 2. | López MJ, Carbajal J, Alfaro AL, Saravia LG, Zanabria D, Araujo JM, Quispe L, Zevallos A, Buleje JL, Cho CE, Sarmiento M, Pinto JA, Fajardo W. Characteristics of gastric cancer around the world. Crit Rev Oncol Hematol. 2023;181:103841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 158] [Reference Citation Analysis (3)] |
| 3. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2827] [Article Influence: 565.4] [Reference Citation Analysis (5)] |
| 4. | Röcken C. Predictive biomarkers in gastric cancer. J Cancer Res Clin Oncol. 2023;149:467-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 96] [Reference Citation Analysis (5)] |
| 5. | Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 452] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 6. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5303] [Article Influence: 353.5] [Reference Citation Analysis (3)] |
| 7. | Zeng Y, Jin RU. Molecular pathogenesis, targeted therapies, and future perspectives for gastric cancer. Semin Cancer Biol. 2022;86:566-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (1)] |
| 8. | Maiorano BA, Maiorano MFP, Ciardiello D, Maglione A, Orditura M, Lorusso D, Maiello E. Beyond Platinum, ICIs in Metastatic Cervical Cancer: A Systematic Review. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Marei HE, Hasan A, Pozzoli G, Cenciarelli C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 2023;23:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 10. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1868] [Article Influence: 467.0] [Reference Citation Analysis (1)] |
| 11. | Zhang L, Wang WW, Jiang GZ, Zhang YP. [Impact and clinical value of the revised 2019 Chinese HER-2 testing guidelines on the detect result evaluation of invasive breast cancer cases with equivocal HER-2 immunostaining by using fluorescence in situ hybridization]. Zhonghua Zhongliu Zazhi. 2021;43:833-837. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Abdel-Rahman O. ECOG performance score 0 versus 1: impact on efficacy and safety of first-line 5-FU-based chemotherapy among patients with metastatic colorectal cancer included in five randomized trials. Int J Colorectal Dis. 2019;34:2143-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16597] [Cited by in RCA: 18230] [Article Influence: 1519.2] [Reference Citation Analysis (0)] |
| 14. | Iannessi A, Beaumont H, Liu Y, Bertrand AS. RECIST 1.1 and lesion selection: How to deal with ambiguity at baseline? Insights Imaging. 2021;12:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 16. | Malla RR, Nellipudi HR, Srilatha M, Nagaraju GP. HER-2 positive gastric cancer: Current targeted treatments. Int J Biol Macromol. 2024;274:133247. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Kahraman S, Yalcin S. Recent Advances in Systemic Treatments for HER-2 Positive Advanced Gastric Cancer. Onco Targets Ther. 2021;14:4149-4162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Van Cutsem E, di Bartolomeo M, Smyth E, Chau I, Park H, Siena S, Lonardi S, Wainberg ZA, Ajani J, Chao J, Janjigian Y, Qin A, Singh J, Barlaskar F, Kawaguchi Y, Ku G. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. 2023;24:744-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 106] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 19. | Pous A, Notario L, Hierro C, Layos L, Bugés C. HER2-Positive Gastric Cancer: The Role of Immunotherapy and Novel Therapeutic Strategies. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 20. | Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, Bartsch R, Rabaglio-Poretti M, Kammler R, Maibach R, Smyth MJ, Di Leo A, Colleoni M, Viale G, Regan MM, André F; International Breast Cancer Study Group and the Breast International Group. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019;20:371-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 347] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 21. | Satoh T, Kang YK, Chao Y, Ryu MH, Kato K, Cheol Chung H, Chen JS, Muro K, Ki Kang W, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Tanimoto M, Chen LT, Boku N. Exploratory subgroup analysis of patients with prior trastuzumab use in the ATTRACTION-2 trial: a randomized phase III clinical trial investigating the efficacy and safety of nivolumab in patients with advanced gastric/gastroesophageal junction cancer. Gastric Cancer. 2020;23:143-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 22. | Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, Chou JF, Segal MF, Simmons MZ, Momtaz P, Shcherba M, Ku GY, Zervoudakis A, Won ES, Kelsen DP, Ilson DH, Nagy RJ, Lanman RB, Ptashkin RN, Donoghue MTA, Capanu M, Taylor BS, Solit DB, Schultz N, Hechtman JF. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 23. | Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz LS, Xu J, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shah S, Bhagia P, Chung HC. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 475] [Article Influence: 118.8] [Reference Citation Analysis (1)] |
| 24. | Ertürk SA, Hasbek Z, Özer H. The Relationship Between HER-2 Expression Levels and (18)F-FDG PET/CT Parameters in Gastric Cancer. Mol Imaging Radionucl Ther. 2021;30:150-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Zhang X, Wang M, Wang Y, Cheng X, Jiang Y, Xiao H. Clinicopathologic significance of Her-2 and P(53) expressions in gastric cancer. Asian J Surg. 2023;46:526-531. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Catenacci DVT, Kang YK, Park H, Uronis HE, Lee KW, Ng MCH, Enzinger PC, Park SH, Gold PJ, Lacy J, Hochster HS, Oh SC, Kim YH, Marrone KA, Kelly RJ, Juergens RA, Kim JG, Bendell JC, Alcindor T, Sym SJ, Song EK, Chee CE, Chao Y, Kim S, Lockhart AC, Knutson KL, Yen J, Franovic A, Nordstrom JL, Li D, Wigginton J, Davidson-Moncada JK, Rosales MK, Bang YJ; CP-MGAH22-5 Study Group. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21:1066-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 27. | Shah MA, Kang YK, Thuss-Patience PC, Ohtsu A, Ajani JA, Van Cutsem E, Hoersch S, Harle-Yge ML, de Haas SL. Biomarker analysis of the GATSBY study of trastuzumab emtansine versus a taxane in previously treated HER2-positive advanced gastric/gastroesophageal junction cancer. Gastric Cancer. 2019;22:803-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Yang T, Xu R, You J, Li F, Yan B, Cheng JN. Prognostic and clinical significance of HER-2 low expression in early-stage gastric cancer. BMC Cancer. 2022;22:1168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Reference Citation Analysis (0)] |
| 29. | Vivekanandhan S, Knutson KL. Resistance to Trastuzumab. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 30. | Xu Y, Wang Y, Gong J, Zhang X, Peng Z, Sheng X, Mao C, Fan Q, Bai Y, Ba Y, Jiang D, Yang F, Qi C, Li J, Wang X, Zhou J, Lu M, Cao Y, Yuan J, Liu D, Wang Z, Fang J, Shen L. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer. 2021;24:913-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 31. | Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Yamaguchi K; DESTINY-Gastric01 Investigators. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med. 2020;382:2419-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 812] [Article Influence: 162.4] [Reference Citation Analysis (0)] |