Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.103776
Revised: January 12, 2025
Accepted: February 7, 2025
Published online: April 15, 2025
Processing time: 116 Days and 2.1 Hours
Intrahepatic cholangiocarcinoma (iCCA) is the second most common liver malignancy with poor prognosis and limited treatment options.
To identify the most effective drug for transarterial chemoembolization (TACE) in cholangiocarcinoma and evaluate the efficacy and safety of combining it with gemcitabine and cisplatin (GemCis) for unresectable iCCA.
Cholangiocarcinoma cell lines (RBE, HuCC-T1) were treated with 10 chemotherapeutic drugs, and cytotoxicity was assessed by cell counting kit-8 assays. Tumor-bearing nude mice were treated with idarubicin or GemCis, and tumor growth was monitored. Clinical data from 85 iCCA patients were analyzed to evaluate the efficacy and safety of idarubicin-TACE combined with GemCis.
Idarubicin demonstrated the highest cytotoxicity, significantly outperforming GemCis, the standard first-line therapies. In tumor-bearing mouse models, idarubicin and GemCis treatments significantly slowed tumor growth, with idarubicin showing particularly pronounced effects on days 12 and 15 (P < 0.05). In retrospective analysis, the median overall survival (OS) and progression-free survival (PFS) in the combination therapy group were significantly longer than those in the GemCis alone group (median OS, 16.23 months vs 10.07 months, P = 0.042; median PFS, 7.73 months vs 6.30 months, P = 0.023). Additionally, major grade 3/4 adverse events (AEs) in the combination therapy group were abdominal pain (26.3% vs 6.5%, P = 0.049) and elevated transaminases (42.1% vs 12.9%, P = 0.038). Most AEs were mild to moderate and manageable.
Idarubicin demonstrated higher cytotoxicity than GemCis, significantly inhibiting tumor growth in tumor-bearing mouse models. Preliminary clinical results suggest that local idarubicin-TACE combined with GemCis may offer improved survival outcomes for iCCA patients with a manageable safety profile.
Core Tip: Intrahepatic cholangiocarcinoma (iCCA) is a liver cancer with poor survival rates and limited treatments, prompting the need for novel therapeutic strategies. This study reveals that idarubicin demonstrates superior cytotoxicity compared to standard treatments and shows improved survival outcomes when combined with gemcitabine and cisplatin in both preclinical and clinical settings. The findings highlight the potential of idarubicin-transarterial chemoembolization as a promising therapeutic agent for unresectable iCCA, offering hope for better management of this challenging disease with a manageable safety profile.
- Citation: Zhao CH, Liu H, Pan T, Xiang ZW, Mu LW, Luo JY, Zhou CR, Li MA, Liu MM, Yan HZ, Huang MS. Idarubicin-transarterial chemoembolization combined with gemcitabine plus cisplatin for unresectable intrahepatic cholangiocarcinoma. World J Gastrointest Oncol 2025; 17(4): 103776
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/103776.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.103776
Intrahepatic cholangiocarcinoma (iCCA) is the second most common liver malignancy in humans after hepatocellular carcinoma, accounting for approximately 20% of all liver cancers. In recent years, the incidence and mortality rates of iCCA have been markedly increasing worldwide[1,2]. Most patients exhibit significant invasion of surrounding organs and distant metastases, rendering them ineligible for complete surgical resection[3,4]. The five-year survival rate (7%-20%) and the post-resection recurrence rate of the tumor are disappointing[5,6].
For unresectable iCCA, the combination of gemcitabine and cisplatin (GemCis) is currently the standard first-line treatment. Compared to gemcitabine alone, GemCis significantly improves the median overall survival (OS) (11.7 vs 8.1 months) and median progression-free survival (PFS) (8 vs 5 months) of patients[7,8]. However, the efficacy of systemic therapy alone remains limited. Many patients experience severe treatment-related adverse reactions, leading to chemotherapy resistance or discontinuation. There is still no consensus on the optimal treatment for unresectable iCCA.
Theoretically, the liver parenchyma has dual blood supply, primarily from the portal vein and partially from the hepatic artery. Liver tumors, however, almost entirely rely on the hepatic artery for blood supply. Transarterial chemoembolization (TACE) utilizes a microcatheter to super-selectively access the tumor-feeding artery, where high concentrations of chemotherapeutic agents are injected and the tumor's nutrient vessels are embolized. This enhances local chemotherapy efficacy, causing tumor necrosis due to ischemia, hypoxia, and localized chemotherapeutic toxicity. Retrospective studies have shown that the OS for unresectable iCCA treated with TACE ranges from 12.0 to 25.2 months[9]. Systemic therapy combined with TACE extends the median OS to 26.2 months, which is 13.1 months longer than systemic chemotherapy alone[10]. A study by Scheuermann et al[11] suggested that for patients not achieving R0 resection, survival rates between those receiving TACE and those undergoing surgery showed no significant difference.
TACE is currently the standard treatment for intermediate-stage hepatocellular carcinoma and has shown good safety and efficacy in treating iCCA, as recommended by several clinical guidelines[7]. However, due to significant differences in chemotherapeutic agents, studies report the radiologic objective response rates of TACE combined with cisplatin, doxorubicin, mitomycin, irinotecan, or combinations of these drugs, ranging from 8.7% to 55%. The long-term efficacy remains uncertain, and how to improve the efficacy of TACE is a major clinical challenge at present[12,13].
Considering the significant heterogeneity of iCCA at the genomic, molecular, and epigenetic levels, this study aims to screen for the most sensitive drugs for TACE treatment of cholangiocarcinoma cell lines. Furthermore, we aim to demonstrate the efficacy and safety of this drug in combination with GemCis for treating unresectable cholangiocarcinoma through clinical research.
Cell culture human hepatobiliary cholangiocarcinoma cells (RBE) (RRID: CVCL_4896) and human cholangiocarcinoma cells (HuCC-T1) (RRID: CVCL_0324) were provided by Wuhan Procell Life Science and Technology Co., Ltd. RBE cells originate from a female liver cholangiocarcinoma patient and produce carcinoembryonic antigen and carbohydrate antigen 199. HuCC-T1 cells are derived from a 56-year-old male cholangiocarcinoma patient with ascites metastasis. I confirm that all human cell lines used in this study have been authenticated using STR profiling within the last three years. Cells were cultured in RPMI 1640 medium (GIBCO, United States) supplemented with 10% fetal bovine serum (FBS) (GIBCO, United States) and 1% streptomycin-penicillin (GIBCO, United States). The culture flasks were incubated in a 37 °C, 5% carbon dioxide (CO2) incubator (Thermo Fisher Scientific). Additionally, all cell lines were tested and confirmed to be free of mycoplasma contamination.
A total of 10 clinically used chemotherapeutic drugs were tested, including: (1) Three anthracyclines: Idarubicin (IDA) (Pfizer, Wuxi, Jiangsu Province, China), epirubicin (Pfizer, Wuxi, Jiangsu Province, China), and doxorubicin (Wanle Pharmaceutical, Shenzhen, China); (2) Three platinum-based drugs: Oxaliplatin (Chia Tai Tianqing, Nanjing, Jiangsu Province, China), lobaplatin (Hainan Chang’an International Pharmaceutical, Hainan, Hainan Province, China), and cisplatin (Qilu Pharmaceutical, Shandong Province, China); (3) Three antimetabolites: Gemcitabine (Vianex S.A. - Plant C, Pallini, Greece), 5-fluorouracil (Pude Pharmaceutical, Shanxi Province, China), and raltitrexed (Chia Tai Tianqing, Nanjing, Jiangsu Province, China); and (4) One DNA topoisomerase I inhibitor: Irinotecan (Hengrui Medicine, Jiangsu Province, China). All drugs used in this study were obtained from the affiliated hospital to ensure data reliability and uniformity. The drugs were reconstituted in RPMI 1640 containing 0.5% FBS to achieve their maximum concentrations (Cmax): Raltitrexed 0.5 mg/mL, oxaliplatin 5 mg/mL, lobaplatin 5 mg/mL, irinotecan 20 mg/mL, IDA 1 mg/mL, gemcitabine 38 mg/mL, epirubicin 2 mg/mL, doxorubicin 2 mg/mL, 5-fluorouracil 50 mg/mL, and cisplatin 5 mg/mL.
Day 1: RBE and HuCC-T1 cells in the logarithmic growth phase were trypsinized, centrifuged, counted, and resuspended to a density of 1 × 105 cells/mL. A 100 μL cell suspension (10000 cells) was seeded into each well of a 96-well plate and incubated for 24 hours.
Day 2: The medium was replaced with 100 μL of the test solution, with the highest concentration in the first column, followed by a serial 1:4 dilution with medium containing 0.5% FBS. Column 9 served as the control group with medium only, and column 10 as the blank group with only medium. Based on previous studies, the highest drug concentration in tumors post-conventional TACE (cTACE) occurs 4 hours post-procedure; thus, the plates were incubated at 37 °C with 5% CO2 for 4 hours[14]. Afterward, the medium was replaced with RPMI 1640 containing 10% FBS and incubated for an additional 24 hours.
Day 3: 100 μL of medium containing 10% cell counting kit 8 (Dojindo, Japan) was added to each well, and the plates were incubated for 2 hours at 37 °C with 5% CO2. Absorbance was measured at 450 nm using a microplate reader (BioTek). Cell viability was calculated, and each group was tested in triplicate, with three independent experiments performed.
The experimental protocol was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University Experimental Animal Center and followed institutional and national guidelines for the care and use of laboratory animals. This study was also reported in accordance with the ARRIVE guidelines[15]. Male BALB/c nude mice (4-5 weeks old) were purchased from GemPharmatech (Nanjing, Jiangsu Province, China). HuCC-T1 cells (5 × 106) were subcutaneously injected into the axilla of each mouse (n = 6 per group). When the tumor size reached 250 cubic millimeters (approximately 4 weeks), the mice were randomly assigned to treatment groups. As described in previous studies, they were administered either IDA (2 mg/kg) via tail vein injection or gemcitabine (50 mg/kg) and cisplatin (5 mg/kg) via intraperitoneal injection[16,17]. The control group received an intraperitoneal injection of an equal volume of saline, administered once a week for two consecutive weeks. Tumor length (L) and width (S) were measured every three days using a caliper, and the formula for calculating tumor volume is:
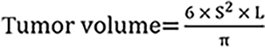
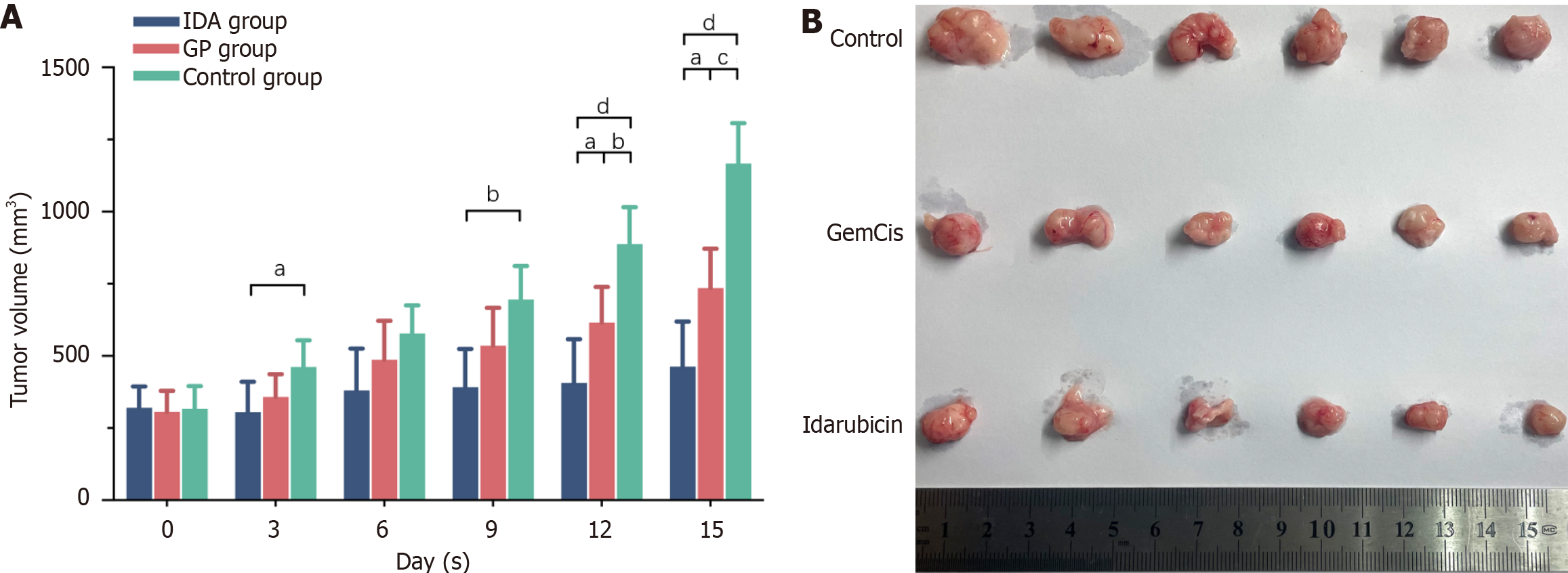
When the tumor volume reached 1000 mm3 (approximately 15 days), the tumors were excised.
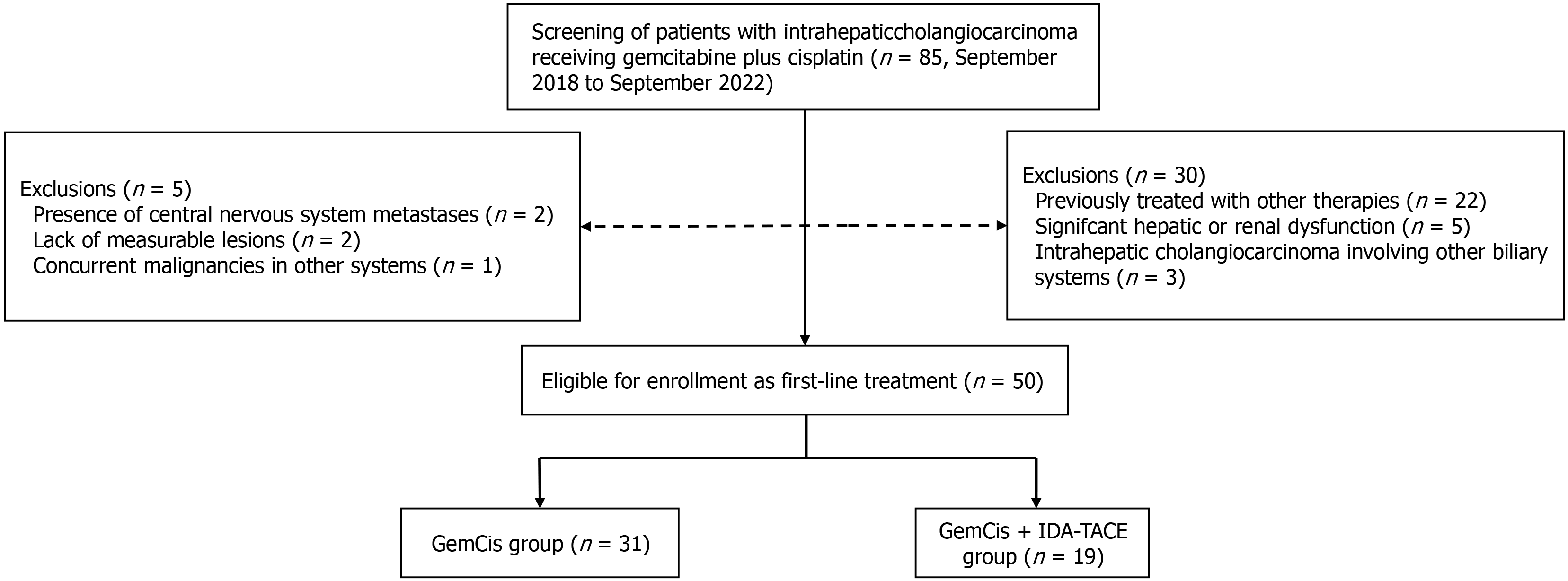
We collected data on iCCA patients treated at the Third Affiliated Hospital of Sun Yat-sen between September 2018 and September 2022. These patients received either a combination of gemcitabine plus cisplatin and IDA-TACE (GemCis + IDA-TACE group) or gemcitabine plus cisplatin alone (GemCis group). A total of 85 patients with complete records were included in the study. The inclusion criteria were as follows: (1) Age 18-80 years; (2) Diagnosed with intrahepatic cholangiocarcinoma according to the EASL-ILCA Clinical Practice Guidelines on the management of iCCA[7]; (3) Child-Pugh grade A or B; (4) Eastern cooperative oncology group performance status of 0-1; (5) No prior treatment or recurrence after radical resection; (6) Received gemcitabine plus cisplatin combined with IDA-TACE or gemcitabine plus cisplatin alone; (7) Received initial systemic therapy within 30 days before or after TACE; and (8) Had at least one measurable lesion. Exclusion criteria included: (1) The presence of cardiac, pulmonary, hepatic, renal, and other important organ disorders; (2) Severe coagulation disorders; (3) Prior treatment with any systemic therapy, including chemotherapy, targeted therapy, or immunotherapy; (4) Combination of targeted or immunotherapy during first-line treatment; (5) Presence of other biliary malignancies; (6) The presence of metastases to the central nervous system; and (7) Concurrent other types of malignant tumors.
All patients underwent preoperative biopsy, confirming ICC pathology. Based on the inclusion and exclusion criteria, 35 patients were excluded: 22 had previous treatments, 5 had significant liver or kidney impairment, 3 had ICC involving other biliary systems, 2 had central nervous system metastasis, 2 lacked measurable lesions, and 1 had another type of malignancy. This retrospective clinical study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University and complied with the 1975 Helsinki Declaration (Ethics number: II2024-238). Informed consent was waived due to the retrospective nature of the study.
Gemcitabine plus cisplatin treatment: The final treatment regimen was determined by a multidisciplinary team. The chemotherapy protocol included gemcitabine (1000 mg/m²) and cisplatin (25 mg/m²), with gemcitabine administered intravenously on days 1 and 8 of each cycle and cisplatin on day 1. Each cycle lasted 21 days. Treatment continued until clinical or radiological progression, unacceptable toxicity, or patient refusal.
TACE treatment: Within 30 days after the initial gemcitabine plus cisplatin treatment, patients underwent TACE. Patients were positioned supine and given local infiltration anesthesia. Using the Seldinger technique, a catheter was inserted through the femoral artery to the hepatic artery for digital subtraction angiography to determine the number, location, size, and arterial supply of the tumors and to check for arteriovenous fistulas. Based on the tumor characteristics and liver function, the attending physician selected the appropriate TACE method. Permitted TACE methods included cTACE and drug-eluting bead TACE (DEB-TACE). In cTACE, 10 mg of IDA was mixed with iodized oil to form an emulsion, which was then injected into the tumor’s target artery branches using a microcatheter, with cone beam computed tomography (CT) used to assess the deposition range and extent if possible. For DEB-TACE, 10 mg of IDA was loaded onto HepaSphere or CalliSpheres microspheres (100 μm-300 μm or 300 μm-500 μm) and injected at a rate of 1 mL/min until tumor staining disappeared. Tumor viability was assessed via contrast-enhanced CT and magnetic resonance imaging (MRI), and the attending physician decided on the need for repeat TACE.
Follow-up and data collection: Patients underwent complete blood counts, biochemical tests, physical examinations, and routine tests before and after each systemic treatment cycle or TACE. Dynamic contrast-enhanced CT or MRI was recommended for efficacy evaluation at 4-6 weeks post-initial treatment and every 2-3 months thereafter. OS was defined as the time from the first gemcitabine plus cisplatin administration to death from any cause or the last follow-up date (December 31, 2023). PFS was defined as the time from the first gemcitabine plus cisplatin administration to disease progression or death. Progressive disease (PD) was defined as: (1) New intrahepatic lesions or major vascular invasion; (2) New extrahepatic metastasis; or (3) An increase of more than 20% in the sum of the longest diameters of the target lesions from the recorded minimum value. PD was independently evaluated by two radiologists and an interventional radiologist. Adverse reactions were assessed after each cycle and graded according to the common terminology criteria for adverse events version 5.0. Follow-up continued until patient loss to follow-up or death, with a cutoff date of December 31, 2023.
Experimental data were statistically analyzed using Prism 9.5 and Origin software. Data were expressed as mean ± SD. Differences between groups were assessed using one-way analysis of variance followed by t-tests. A P value < 0.05 was considered statistically significant. The half maximal inhibitory concentration (IC50) was determined using logistic regression fitted from three independent experiments. The formula used is as follows:
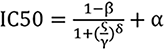
Where 𝜁 is the drug concentration, and α, β, γ, and δ are the four estimated parameters of the dose-response model.
The cytotoxicity index (CyI50) was used to compare the effects of a single molecule on a single cell line. It is calculated as the ratio of the maximum drug concentration to the IC50: CyI50 = CMax/IC50.
Continuous variables were described as mean ± SD and compared between groups using an independent samples t-test. For non-normally distributed data, median (interquartile range) was used, and comparisons were made using the non-parametric Mann-Whitney U test. Categorical variables were described as numbers (percentages) and compared between groups using Pearson’s χ² test or Fisher’s exact test. OS and PFS were calculated using the Kaplan-Meier method, with differences between groups compared using the log-rank test. Statistical analysis was performed using statistical product and service solutions 26.0 (SPSS Inc., Chicago, IL, United States). All statistical tests were two-sided, and a P value < 0.05 was considered statistically significant.
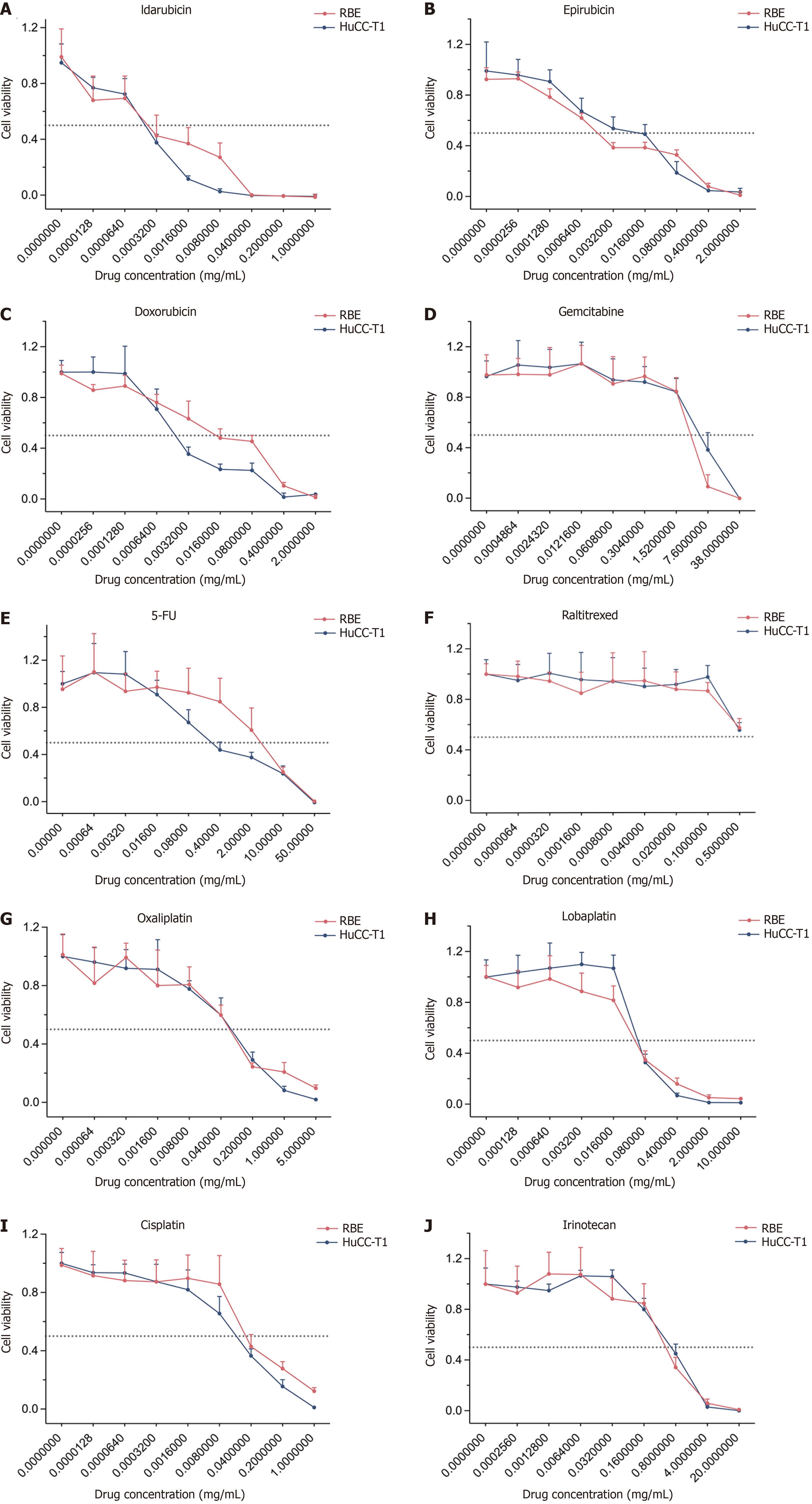
The viability curves of the ten chemotherapeutic drugs on cholangiocarcinoma cell lines (RBE, HuCC-T1) are shown in Figure 1. The concentrations of these drugs required to induce 50% cell death in cholangiocarcinoma cells are summarized in Table 1. Overall, IDA exhibited the lowest IC50 values among the tested drugs, with IC50 = 0.00072 ± 0.00082 mg/mL in the RBE cell line and IC50 = 0.00023 ± 0.00005 mg/mL in the HuCC-T1 cell line.
| Cholangiocarcinoma cell line | ||
| Antitumor drugs | RBE | HuCC-T1 |
| 5-FU | 4.99 ± 2.92 | 0.30 ± 0.24 |
| Gemcitabine | 3.15 ± 0.58 | 7.23 ± 3.12 |
| Lobaplatin | 0.049 ± 0.009 | 0.062 ± 0.006 |
| Oxaliplatin | 0.054 ± 0.028 | 0.076 ± 0.014 |
| Idarubicin | 0.00072 ± 0.00082 | 0.00023 ± 0.00005 |
| Doxorubicin | 0.35 ± 1.13 | 0.0015 ± 0.0005 |
| Epirubicin | 0.0055 ± 0.0075 | 0.0088 ± 0.0069 |
| Irinotecan | 0.50 ± 0.13 | 0.65 ± 0.15 |
| Cisplatin | 0.029 ± 0.007 | 0.030 ± 0.008 |
| Raltitrexed | ||
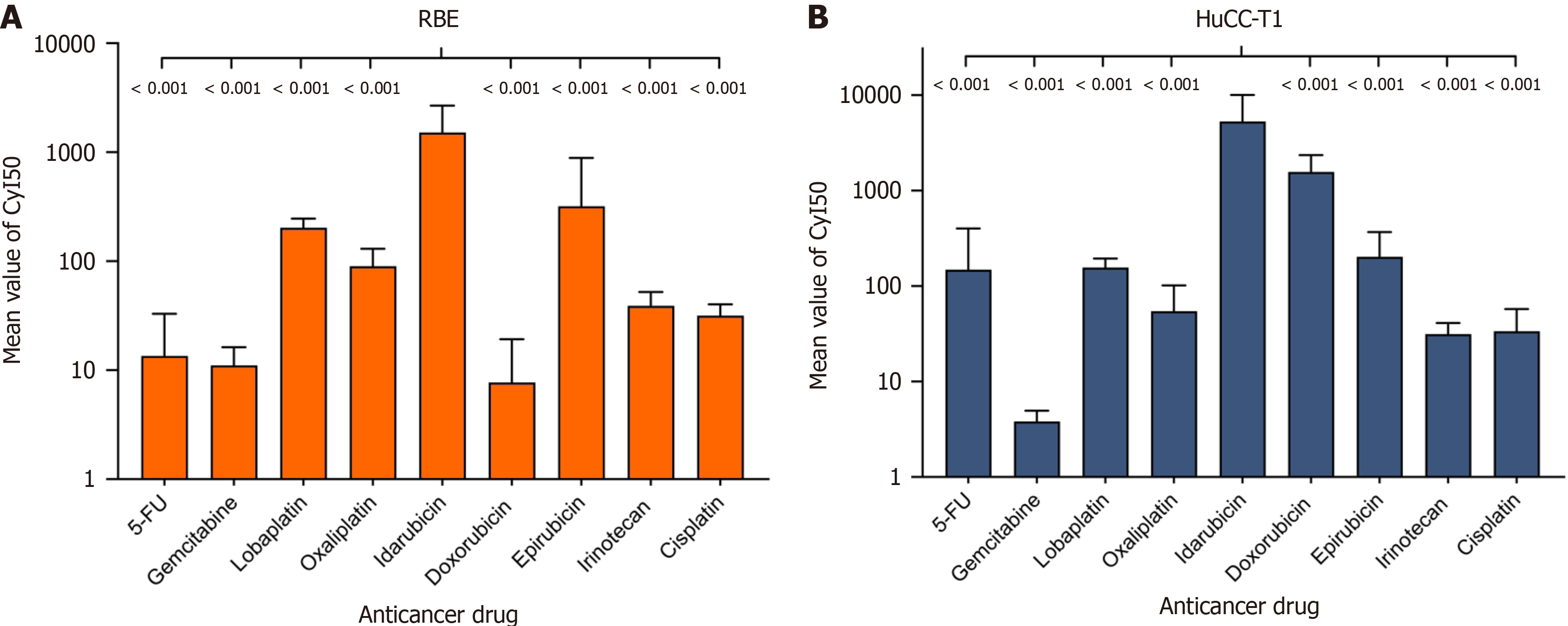
Considering the different molecular weights and Cmax ns of each cytotoxic drug, we introduced a more accurate evaluation standard: The CyI50, which reflects the activity of a single molecule. IDA demonstrated the highest molecular activity, with CyI50 = 1715.32 ± 743.62 in the RBE cell line and CyI50 = 6517.36 ± 4924.53 in the HuCC-T1 cell line (Table 2 and Figure 2). Notably, compared to the clinically common TACE chemotherapeutic agent epirubicin, IDA showed 3.89 times greater efficacy in the RBE cell line and 27.34 times greater efficacy in the HuCC-T1 cell line. Additionally, IDA exhibited 145.49 times greater efficacy than the standard first-line therapy gemcitabine in the RBE cell line, 1658.36 times greater in the HuCC-T1 cell line, and 52.73 times greater efficacy than cisplatin in the RBE cell line, and 171.37 times greater in the HuCC-T1 cell line.
| Cholangiocarcinoma cell line | ||
| Antitumor drugs | RBE | HuCC-T1 |
| 5-FU | 19.51 ± 17.02 | 223.61 ± 194.21 |
| Gemcitabine | 11.79 ± 3.76 | 3.93 ± 0.93 |
| Lobaplatin | 206.93 ± 34.33 | 160.32 ± 35.81 |
| Oxaliplatin | 95.63 ± 31.13 | 62.26 ± 25.04 |
| Idarubicin | 1715.32 ± 743.62 | 6517.36 ± 4924.53 |
| Doxorubicin | 10.75 ± 7.97 | 1697.96 ± 740.25 |
| Epirubicin | 441.10 ± 283.93 | 238.35 ± 139.13 |
| Irinotecan | 40.44 ± 10.32 | 32.51 ± 8.29 |
| Cisplatin | 32.53 ± 6.76 | 38.03 ± 19.38 |
| Raltitrexed | ||
The changes in tumor volume for tumor-bearing mice in each group are shown in Figure 3. Before treatment, there were no significant differences in tumor volume among the groups. Over time, tumor volumes in all groups generally increased. Following intervention with IDA and GemCis, the rate of tumor volume increase significantly slowed, with the inhibitory effect of IDA being particularly notable. In the IDA group, tumor volumes showed significant inhibition compared to the control group at the first measurement after each administration (Day 3, P < 0.05; Day 9, P < 0.01). On Days 12 and 15, the inhibitory effect of the IDA group was more pronounced compared to the GemCis group, displaying significant statistical differences (P < 0.05).
Patient baseline characteristics: A total of 50 patients met the inclusion criteria for this study. The detailed study flowchart is shown in Figure 4. There were 31 patients in the GemCis group and 19 patients in the GemCis + IDA-TACE group. The baseline characteristics of the patients are summarized in Table 3. Overall, there were no significant differences in baseline characteristics between the two groups. At the start of treatment, both cohorts had Child-Pugh grades A or B, with 43 patients (86.0%) in grade A and 7 patients (14.0%) in grade B. Notably, according to American Joint Committee on Cancer (AJCC) staging, 16 patients had distant metastases (AJCC stage IV), with 9 patients (29.0%) in the GemCis group and 7 patients (36.8%) in the GemCis + IDA-TACE group.
| Characteristics | Total (n = 50) | GemCis group (n = 31) | GemCis + IDA-TACE group (n = 19) | P value |
| Age, years, median (IQR) | 56.5 (48.0-63.0) | 57.0 (48.0-63.0) | 56.0 (48.0-63.0) | 0.976 |
| Gender | ||||
| Male | 30 (60.0) | 18 (58.1) | 12 (63.2) | 0.721 |
| Female | 20 (40.0) | 13 (41.9) | 7 (36.8) | |
| ECOG performance status | ||||
| 0 | 16 (32.0) | 9 (29.0) | 7 (36.8) | 0.566 |
| 1 | 34 (68.0) | 22 (71.0) | 12 (63.2) | |
| Nodules | ||||
| Single | 23 (46.0) | 15 (48.4) | 8 (42.1) | 0.665 |
| Multiple | 27 (54.0) | 16 (51.6) | 11 (57.9) | |
| AJCC stage | ||||
| I | 6 (12.0) | 4 (12.9) | 2 (10.5) | 0.951 |
| II | 11 (22.0) | 7 (22.6) | 4 (21.1) | |
| III | 17 (34.0) | 11 (35.5) | 6 (31.6) | |
| IV | 16 (32.0) | 9 (29.0) | 7 (36.8) | |
| Largest nodule | ||||
| ≤ 5 | 28 (56.0) | 19 (61.3) | 9 (47.4) | 0.336 |
| > 5 | 22 (44.0) | 12 (38.7) | 10 (52.6) | |
| Child-Pugh class | ||||
| A | 43 (86.0) | 25 (80.6) | 18 (94.7) | 0.229 |
| B | 7 (14.0) | 6 (19.4) | 1 (5.3) | |
| Extrahepatic spread | ||||
| Absent | 17 (34.0) | 11 (35.5) | 6 (31.6) | 0.900 |
| Present | ||||
| Single metastasis | 24 (48.0) | 15 (48.4) | 9 (47.4) | |
| Multiple metastases | 9 (18.0) | 5 (16.1) | 4 (21.1) | |
| CA199, U/mL | ||||
| ≤ 37 | 12 (24.0) | 6 (19.4) | 6 (31.6) | 0.496 |
| > 37 | 38 (76.0) | 25 (80.6) | 13 (68.4) | |
| CA125, U/mL | ||||
| ≤ 35 | 27 (54.0) | 15 (48.4) | 12 (63.2) | 0.309 |
| > 35 | 23 (46.0) | 16 (51.6) | 7 (36.8) | |
| TBIL, μmol/mL, median (IQR) | 11.2 (7.5-21.6) | 11.2 (7.6-48.5) | 10.3 (7.2-19.6) | 0.556 |
| Albumin, g/L | 41.3 ± 6.4 | 42.0 ± 7.5 | 40.1 ± 4.1 | 0.302 |
| RBC, × 109/L | 4.4 ± 0.8 | 4.4 ± 0.9 | 4.3 ± 0.8 | 0.770 |
| WBC, × 109/L | 7.45 ± 3.1 | 7.7 ± 2.9 | 7.0 ± 3.4 | 0.454 |
| Platelet, × 109/L | 216.8 ± 82.1 | 229.8 ± 89.5 | 195.6 ± 66.2 | 0.156 |
| PT, seconds | 13.3 ± 1.2 | 13.4 ± 1.2 | 13.1 ± 1.2 | 0.537 |
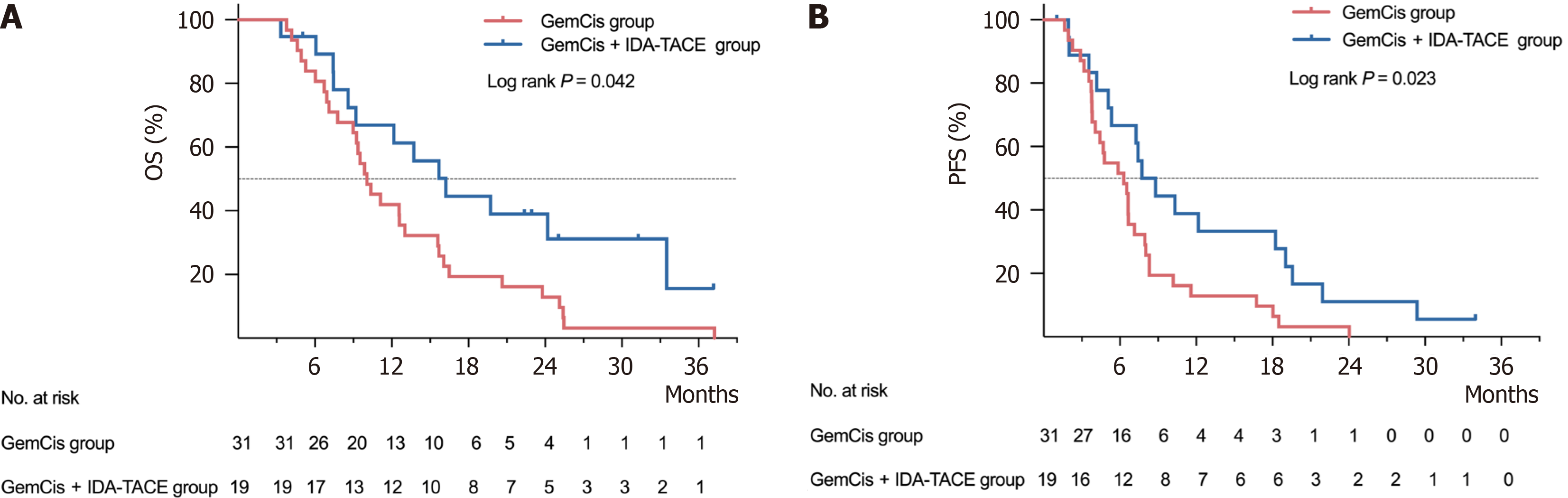
Clinical efficacy: As of December 31, 2023, the median follow-up time for the 50 patients was 37.17 months [95% confidence interval (CI): 25.95-48.39]. During the follow-up period, 44 patients died, with 31 deaths in the GemCis group and 13 in the GemCis + IDA-TACE group. The OS for each cohort is shown in Figure 5A. The median OS was 10.07 months (95%CI: 8.15-11.99) for the GemCis group and 16.23 months (95%CI: 11.08-21.38) for the GemCis + IDA-TACE group. The median OS in the GemCis + IDA-TACE group was approximately 6.16 months longer than that in the GemCis group, showing a significant statistical difference [hazard ratio (HR) = 0.51; 95%CI: 0.27-0.99; P = 0.042].
The PFS for both groups is illustrated in Figure 5B. A total of 48 patients experienced disease progression or death, with 31 cases in the GemCis group and 17 cases in the GemCis + IDA-TACE group. The median PFS was 6.30 months (95%CI: 4.55-8.05) in the GemCis group and 7.73 months (95%CI: 4.88-10.58) in the GemCis + IDA-TACE group. The median PFS in the GemCis + IDA-TACE group was extended by approximately 1.43 months compared to the GemCis group, with a significant statistical difference (HR = 0.49; 95%CI: 0.26-0.92; P = 0.023).
Common adverse events are summarized in Table 4. No treatment-related deaths occurred in either group. Notably, compared to the GemCis group, the GemCis + IDA-TACE group experienced more common adverse events of any grade, primarily post-embolization syndrome, including nausea and vomiting (73.7% vs 42.9%, P = 0.029), abdominal pain (73.7% vs 19.4%, P < 0.001), elevated transaminase levels (63.2% vs 29.0%, P = 0.018), and elevated bilirubin levels (42.1% vs 12.9%, P = 0.038). Additionally, for grade 3/4 adverse events, the GemCis + IDA-TACE group had higher incidences of abdominal pain (26.3% vs 6.5%, P = 0.049) and elevated transaminase levels (42.1% vs 12.9%, P = 0.038) compared to the GemCis group.
| Adverse event | Any grade group | Grades 3-4 group | ||||
| GemCis group (n = 31) | GemCis + IDA-TACE group (n = 19) | P value | GemCis group (n = 31) | GemCis + IDA-TACE group (n = 19) | P value | |
| Nausea and vomiting | 13 (42.9) | 14 (73.7) | 0.029 | 0 (0) | 1 (5.3) | 0.380 |
| Abdominal pain | 6 (19.4) | 15 (78.9) | < 0.001 | 2 (6.5) | 5 (26.3) | 0.049 |
| Myelosuppression | 11 (35.5) | 8 (42.1) | 0.640 | 6 (19.4) | 4 (21.1) | 1.000 |
| Fever | 7 (22.6) | 10 (52.6) | 0.029 | 4 (12.9) | 5 (26.3) | 0.273 |
| Fatigue | 3 (9.7) | 4 (21.1) | 0.404 | 0 (0) | 0 (0) | 1.000 |
| Infections | 5 (16.1) | 6 (31.6) | 0.293 | 1 (3.2) | 2 (10.5) | 0.549 |
| Ascites | 6 (19.4) | 3 (15.8) | 1.000 | 2 (6.5) | 1 (5.3) | 1.000 |
| Elevated transaminases | 9 (29.0) | 12 (63.2) | 0.018 | 4 (12.9) | 8 (42.1) | 0.038 |
| Hyperbilirubinemia | 4 (12.9) | 8 (42.1) | 0.038 | 0 (0) | 1 (5.3) | 0.380 |
The ABC-02 study established gemcitabine combined with cisplatin as the first-line treatment for unresectable iCCA. However, its efficacy remains limited, with a median OS of only 11.7 months[8]. Consequently, many clinical experts are seeking more effective treatment regimens for iCCA. This study differentiates itself from previous clinical studies by first conducting in vivo and in vitro drug sensitivity experiments, which highlighted the potential effectiveness of IDA in cholangiocarcinoma treatment. Additionally, further clinical research was conducted to demonstrate that GemCis combined with IDA-TACE is an effective and safe treatment modality for unresectable iCCA, extending median OS and median PFS by approximately 6.16 months and 1.63 months, respectively.
Over the past decade, several studies have explored the combination of IDA with lipiodol and DEBs in TACE for hepatocellular carcinoma, achieving favorable outcomes[18,19]. Notably, IDA-lipiodol emulsions are more stable than other chemotherapy drug emulsions when used in a volume ratio of 1:2, providing a significant advantage in cTACE applications[20]. Four hours post-cTACE, the drug concentration within the tumor is at its highest, gradually decreasing thereafter[14]. In the first part of this study, we evaluated the CyI50 of IDA on cholangiocarcinoma cell lines at the 4-hour mark, where it was found to be highest. In RBE and HuCC-T1 cell lines, the CyI50 of IDA was 3.89 times and 27.34 times that of epirubicin, 145.49 times and 1658.36 times that of gemcitabine, and 52.73 times and 171.37 times that of cisplatin. As a bi-metabolic drug, IDA and its metabolite idarubicinol both exhibit prolonged anti-tumor effects, with a terminal half-life of approximately 72 hours. Additionally, the lipophilicity of IDA facilitates its easier entry into cells, enhancing its anti-tumor action[21].
The research demonstrates that IDA effectively inhibits the proliferation of cholangiocarcinoma cells both in vitro and in vivo. In addition to in vitro experiments, we evaluated the effect of IDA on tumor growth by tail vein injection in nude mice, finding that IDA significantly inhibited tumor growth. TACE can improve intrahepatic disease control, reduce tumor burden, and enhance liver function, thereby extending the duration of GemCis’s effects. GemCis can enhance systemic disease control, reduce the emergence of new lesions, and inhibit distant metastasis. Combining GemCis with TACE effectively improves PFS, translating into improved OS and long-term outcomes. Several authoritative institutions, including the European association for the study of the liver, have indicated that TACE is a safe and feasible treatment option, making it a reasonable choice for unresectable iCCA[7,22].
Our study has several limitations. Firstly, while the effects and mechanisms of IDA were studied in vitro using nude mouse xenograft models and clinical research demonstrated the efficacy and safety of the combination treatment, ethical constraints prevent direct head-to-head comparisons with other chemotherapeutic agents. Secondly, the application of IDA in TACE for treating iCCA is a novel approach, and the sample size remains limited. We aim to provide preliminary clinically relevant information and will update long-term follow-up data and include more samples at appropriate times. Additional prospective studies are necessary to further validate our findings.
In conclusion, our data provide the first evidence that IDA may exert the most effective inhibitory effect on cholangiocarcinoma compared to other commonly used chemotherapy drugs. Preliminary clinical practice shows that local IDA-TACE combined with systemic GemCis therapy may extend both OS and PFS in iCCA patients more effectively than systemic GemCis therapy alone, with a favorable safety profile. However, further studies with larger sample sizes and randomized controlled trials are needed to confirm these findings.
We would like to express our sincere gratitude to the Department of Interventional Radiology and the Department of Radiology at the Third Affiliated Hospital of Sun Yat-sen University for their support and assistance throughout this study. The patients participating in this study are sincerely acknowledged.
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11394] [Article Influence: 3798.0] [Reference Citation Analysis (4)] |
| 2. | Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 495] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 3. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Ilyas SI, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1527] [Article Influence: 305.4] [Reference Citation Analysis (0)] |
| 4. | El-Diwany R, Pawlik TM, Ejaz A. Intrahepatic Cholangiocarcinoma. Surg Oncol Clin N Am. 2019;28:587-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Kamsa-Ard S, Luvira V, Suwanrungruang K, Kamsa-Ard S, Luvira V, Santong C, Srisuk T, Pugkhem A, Bhudhisawasdi V, Pairojkul C. Cholangiocarcinoma Trends, Incidence, and Relative Survival in Khon Kaen, Thailand From 1989 Through 2013: A Population-Based Cancer Registry Study. J Epidemiol. 2019;29:197-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Alabraba E, Joshi H, Bird N, Griffin R, Sturgess R, Stern N, Sieberhagen C, Cross T, Camenzuli A, Davis R, Evans J, O'Grady E, Palmer D, Diaz-Nieto R, Fenwick S, Poston G, Malik H. Increased multimodality treatment options has improved survival for Hepatocellular carcinoma but poor survival for biliary tract cancers remains unchanged. Eur J Surg Oncol. 2019;45:1660-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. 2023;79:181-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 8. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3157] [Article Influence: 210.5] [Reference Citation Analysis (1)] |
| 9. | Ierardi AM, Angileri SA, Patella F, Panella S, Lucchina N, Petre EN, Pinto A, Franceschelli G, Carrafiello G, Cornalba G, Sofocleous CT. The role of interventional radiology in the treatment of intrahepatic cholangiocarcinoma. Med Oncol. 2017;34:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Gairing SJ, Thol F, Müller L, Hahn F, Thomaidis T, Czauderna C, Bartsch F, Pitton MB, Marquardt JU, Wörns MA, Galle PR, Moehler M, Weinmann A, Kloeckner R, Foerster F. The Addition of Transarterial Chemoembolization to Palliative Chemotherapy Extends Survival in Intrahepatic Cholangiocarcinoma. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Scheuermann U, Kaths JM, Heise M, Pitton MB, Weinmann A, Hoppe-Lotichius M, Otto G. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma--a single-center experience. Eur J Surg Oncol. 2013;39:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Vogl TJ, Naguib NN, Nour-Eldin NE, Bechstein WO, Zeuzem S, Trojan J, Gruber-Rouh T. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int J Cancer. 2012;131:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Kim JH, Yoon HK, Sung KB, Ko GY, Gwon DI, Shin JH, Song HY. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer. 2008;113:1614-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Liang B, Zhao D, Liu Y, Guo X, Zhang H, Zhang L, Zheng C. Chemoembolization of liver cancer with doxorubicin-loaded CalliSpheres microspheres: plasma pharmacokinetics, intratumoral drug concentration, and tumor necrosis in a rabbit model. Drug Deliv Transl Res. 2020;10:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5061] [Cited by in RCA: 5591] [Article Influence: 372.7] [Reference Citation Analysis (1)] |
| 16. | He SW, Zhang Y, Chen L, Luo WJ, Li XM, Chen Y, Huang SY, He QM, Yang XJ, Li YQ, Liu N, Zhao Y, Ma J. Gemcitabine synergizes with cisplatin to inhibit nasopharyngeal carcinoma cell proliferation and tumor growth. FASEB J. 2021;35:e21885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Lu T, Lokerse WJM, Seynhaeve ALB, Koning GA, Ten Hagen TLM. Formulation and optimization of idarubicin thermosensitive liposomes provides ultrafast triggered release at mild hyperthermia and improves tumor response. J Control Release. 2015;220:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Roth GS, Teyssier Y, Abousalihac M, Seigneurin A, Ghelfi J, Sengel C, Decaens T. Idarubicin vs doxorubicin in transarterial chemoembolization of intermediate stage hepatocellular carcinoma. World J Gastroenterol. 2020;26:324-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 19. | Guiu B, Chevallier P, Assenat E, Barbier E, Merle P, Bouvier A, Dumortier J, Nguyen-Khac E, Gugenheim J, Rode A, Oberti F, Valette PJ, Yzet T, Chevallier O, Barbare JC, Latournerie M, Boulin M. Idarubicin-loaded Beads for Chemoembolization of Hepatocellular Carcinoma: The IDASPHERE II Single-Arm Phase II Trial. Radiology. 2019;291:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Boulin M, Schmitt A, Delhom E, Cercueil JP, Wendremaire M, Imbs DC, Fohlen A, Panaro F, Herrero A, Denys A, Guiu B. Improved stability of lipiodol-drug emulsion for transarterial chemoembolisation of hepatocellular carcinoma results in improved pharmacokinetic profile: Proof of concept using idarubicin. Eur Radiol. 2016;26:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Berman E, Heller G, Santorsa J, McKenzie S, Gee T, Kempin S, Gulati S, Andreeff M, Kolitz J, Gabrilove J. Results of a randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood. 1991;77:1666-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 244] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, Primrose JN, Rimassa L, Stenzinger A, Valle JW, Ducreux M; ESMO Guidelines Committee. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:127-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 288] [Article Influence: 144.0] [Reference Citation Analysis (0)] |