Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.103455
Revised: January 11, 2025
Accepted: January 21, 2025
Published online: April 15, 2025
Processing time: 124 Days and 8.2 Hours
Adenocarcinoma of the esophagogastric junction (AEG) has distinct malignant features compared with other esophageal and gastric cancers. Its management is controversial and largely influenced by tumor location and esophageal involve
To evaluate the prognosis and clinicopathological features of patients with AEG, providing insights for management strategies.
This retrospective study analyzed cases with AEG admitted between January 2016 and December 2017. Patients meeting the inclusion criteria were categorized into three groups: Type E [tumors whose center was located within 5 cm above the esophagogastric junction (EGJ)]; Type Eg (tumors whose center was situated within 2 cm below the EGJ), with a 2-cm esophageal invasion; Type Ge (tumors whose center was situated within 2 cm below the EGJ, with an esophageal in
Totally, 153 patients with AEG were included (median follow up: 41.1 months; 22, 31, and 100 patients from type E, Eg, and Ge, respectively), with significant differences in maximum tumor length, esophageal involvement length, tumor type, pathology, differentiation, depth of invasion, and lymph node metastasis between the groups (P < 0.05). Lymph node metastasis rates at stations 1, 2, 3, and 7 were lower in type E than in Eg and Ge (P < 0.05). Survival rates for type E (45.5%) were significantly lower than those for Eg (48.4%) and Ge (73.0%) (P = 0.001). Type E tumors, vascular infiltration, T3-T4 invasion depth, and lymph node metastasis, were identified as independent prognostic factors (P < 0.05). The gastric cancer staging system outperformed the esophageal adenocarcinoma system for type Ge tumors.
Clinicopathological characteristics and prognoses varied between the AEG groups, with type E demonstrating distinct features. The gastric cancer staging system more accurately predicted type Ge AEG prognosis, guiding clinical decision-making.
Core Tip: This study introduces a novel subclassification for adenocarcinoma of the esophagogastric junction (AEG) based on the tumor center location and esophageal invasion. By distinguishing between types E, Eg, and Ge AEGs, the study identified significant differences in clinicopathological features and prognoses. Type E tumors were associated with poorer outcomes, and the American Joint Committee on Cancer/International Alliance against Cancer gastric cancer staging system more accurately predicted the prognosis of type Ge AEGs than did the esophageal adenocarcinoma system. This subclassification therefore improves prognosis prediction and may guide tailored clinical management for patients with AEG.
- Citation: Guo S, Liu FF, Yuan L, Ma WQ, Er LM, Zhao Q. Subclassification scheme for adenocarcinomas of the esophagogastric junction and prognostic analysis based on clinicopathological features. World J Gastrointest Oncol 2025; 17(4): 103455
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/103455.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.103455
Despite the annual declined in the global incidence of gastric adenocarcinoma in recent years, a notable increase has been observed in the global incidence of adenocarcinomas of the esophagogastric junction (AEGs)[1,2]. Hence, the deve
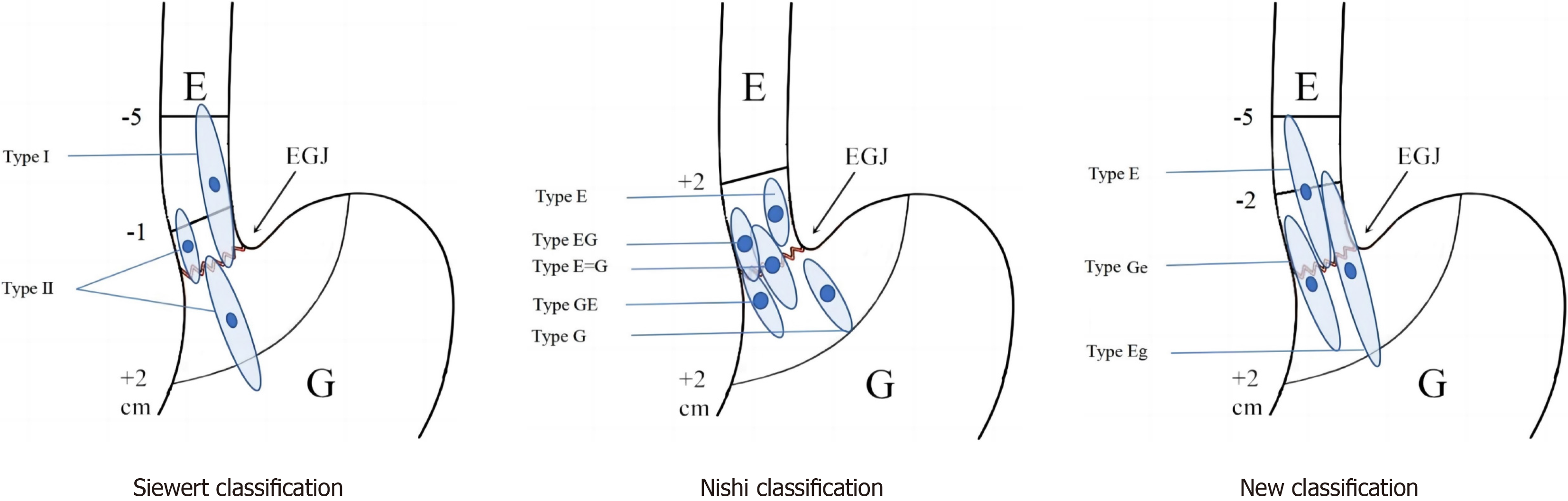
Currently, two primary classification methods have been utilized for tumors at the EGJ: The Siewert classification and the Nishi classification. The Siewert classification, initially introduced by Siewert et al[7] in 1987, categorizes adenocarcinomas into three types based on whether the tumor centers are located within a 5-cm range both proximal and distal to the EGJ, spanning or contacting the EGJ[7]. The Nishi classification, proposed by the Japanese scholar Nishi M in 1973, categorizes malignant tumors with a 4-cm diameter whose center is located around 2 cm from the EGJ into five types based on whether they involve or extend beyond the EGJ[8]. While the Nishi method is widely used in Japan, the Siewert classification remains common globally[9-11].
Another area of controversy is the staging system for AEGs. The 2016 International Alliance against Cancer (UICC)/American Joint Committee on Cancer (AJCC) has recommended the use of the gastric cancer staging system for Siewert type III AEGs for and the esophageal cancer staging system for type I and type II AEGs[12,13]. Recent studies suggest that the esophageal cancer staging system is universally applicable for tumors located above the EGJ. However, controversy persists among scholars from both Eastern and Western countries regarding the most appropriate staging system for AEG tumors located within 2 cm below the EGJ[14-17].
Considering the variations in the etiology and common classification of AEG among Chinese and Western populations, accurate assessment of the clinicopathological features of AEG, including tumor location, upper margin, and lymph node metastasis, is crucial for exploring the most suitable classification and staging systems for individualized treatment strategies. This study aimed to provide valuable insights for the clinical management of patients with AEG by retro
A retrospective study was conducted using case data from AEG patients admitted to the Fourth Hospital of Hebei Medical University from January 2016 to December 2017. The inclusion criteria were as follows: (1) AEG radical resection (R0, no microscopic tumor) and AEG confirmed by postoperative pathological examination, with the tumor center located within 2 cm above or below the EGJ; (2) Primary treatment; (3) No history of malignancy; and (4) Complete study data. The exclusion criteria were as follows: (1) Nonadenocarcinoma or microscopic residual lesions after R0 resection; (2) Patients with distant metastasis; and (3) Patients with combined organ resection. Ultimately, 153 patients, comprising 126 males and 27 females with a mean age of 62.4 ± 8.5 years and a median follow up time of 41.1 months, were included. All case data were approved for use by the Ethics Office of the Fourth Hospital of Hebei Medical University (No. 2024KY198).
This study defined the EGJ as the proximal part of the endoscopic gastric fold. The Nishi classification divides tumors into five types based on their relationship between the tumor center and the EGJ: E, EG, E = G, GE, and G. This classification is specifically applicable to regions within 2 cm of the EGJ and does not differentiate between pathological types (i.e., between adenocarcinoma or squamous carcinoma) in this area. Regarding the Siewert classification, type I tumors are those whose center is located within 1 cm to 5 cm of the EGJ, within the range of and involving the EGJ. Type II tumors are those whose center is positioned within 1 cm above the EGJ to 2 cm below the EGJ, also involving the EGJ. Type III tumors are those whose center is located 2 cm to 5 cm below the EGJ, still involving the EGJ. This study considered tumors whose center was located within or below the EGJ (as illustrated in Figure 1) and classified them into three types based on their distance from the EGJ: Type E (tumors whose center was located within 5 cm above the EGJ); Type Eg (tumors whose center was situated within 2 cm below the EGJ), with a 2-cm esophageal invasion; Type Ge (tumors whose center was situated within 2 cm below the EGJ, with an esophageal invasion of < 2 cm (Figure 1).
Owing to the absence of a serosal structure in the esophagus, discrepancies in the T stages have been observed between esophageal adenocarcinoma and gastric cancer. Consequently, stages T4a and T4b, as per the gastric cancer staging system, were reclassified as T3 and T4a in accordance with the esophageal adenocarcinoma staging system. The staging for esophageal cancer and gastric cancer was determined following the AJCC/UICC 8th edition tumor node metastasis (TNM) stage, adopting the TNM staging system for esophageal adenocarcinoma.
All patients underwent specific surgical treatment based on individual circumstances. This included preoperative or postoperative adjuvant chemotherapy and an adjuvant chemotherapy cycle. Postoperative follow up and telephone follow up were conducted, which concluded in August 2021. The endpoint for the follow up events was defined as either patient death or loss to follow up, and the total survival time (from the date of surgery to the date of death or last follow up) was recorded.
Based on clinical data, surgical records, and follow up data, the following parameters were assessed. First, the general clinical characteristics of the three types of AEG patients were determined, including gender, age, maximum tumor length, length of esophageal invasion, tumor pathology type, nerve invasion, vascular invasion, tumor differentiation, tumor T stage, tumor N stage, tumor TNM stage, and lymph node metastasis rate (calculated by dividing the number of pathologically confirmed lymph node metastases by the total number of dissected lymph nodes, expressed as a per
Statistical analysis was conducted using statistical product and service solutions version 22.0. Data consistent with a normal distribution were described as mean ± SD, whereas those that did not follow a normal distribution were presented as median (lower and upper quartiles). For measurement data exhibiting normal distribution and homogeneity of variance, one-way analysis of variance was employed. For nonhomogeneous data, a nonparametric rank-sum test was utilized. Count data were reported as percentages (%) and analyzed using the χ2 test or either the χ2 test or Fisher exact probability method for those requiring continuity correction. The Kruskal-Wallis test was applied for three-grade data. Survival rates were assessed via Kaplan-Meier survival analysis, and comparisons of survival rates were made using the log-rank test. Prognostic factors were identified using multivariate Cox regression. To evaluate the homogeneity and discriminative power of the staging system, likelihood ratios were calculated based on the evaluation index of the relevant staging system. Higher likelihood ratios suggested improved homogeneity, indicating a smaller difference in the prognosis among patients at the same stage. The linear trend χ2 test value was used to assess the discriminative capacity of the staging system, with higher values signifying greater discrimination and a more significant difference in prognosis among patients at different stages. The survival rate for patients at individual stages was calculated, and the log-likelihood estimate for each typing system was determined using the Cox proportional hazards model. Smaller log-likelihood estimates indicated that the model better predicted prognosis. In all analyses, a P value of < 0.05 indicated statistical significance.
This study included a total of 153 patients, comprising 126 males and 27 females, with a mean age of 62.4 ± 8.5 years and a mean maximum tumor of 44.2 mm ± 15.5 mm. Among the included patients, 86 (56.2%) and 67 (43.8%) had tumors measuring ≤ 40 mm and > 40 mm, respectively. The average length of esophageal invasion by the tumor was 13.4 mm ± 13.8 mm, with 101 (66.0%) and 52 patients (34.0%) having an invasion length of < 20 mm and ≥ 20 mm, respectively. According to the Siewert classification scheme, 11 and 142 patients were classified as Siewert type I and type II, re
| Characteristic | Variables | n | % |
| Age (year) | 62.4 ± 8.5 | ||
| ≤ 65 | 95 | 62.1 | |
| > 65 | 58 | 37.9 | |
| Sex | Male | 126 | 82.4 |
| Female | 27 | 17.6 | |
| Maximal tumor size (mm) | 44.2 ± 15.5 | ||
| ≤ 40 mm | 86 | 56.2 | |
| > 40 mm | 67 | 43.8 | |
| Esophageal invasion length (mm) | 13.4 ± 13.8 | ||
| < 20 mm | 101 | 66.0 | |
| ≥ 20 mm | 52 | 34.0 | |
| Siewert classification | I | 11 | 7.2 |
| II | 142 | 92.8 | |
| Nishi classification | E = G | 5 | 3.3 |
| GE | 74 | 48.4 | |
| EG | 7 | 4.6 | |
| Not applicable | 67 | 43.8 | |
| This classification | E | 22 | 14.4 |
| Eg | 31 | 20.3 | |
| Ge | 100 | 65.4 | |
| Surgical approaches | Transthoracic approach | 27 | 17.6 |
| Transabdominal hiatal approach | 126 | 82.4 | |
| Extent of surgical resection | Subtotal esophagectomy with partial gastrectomy | 27 | 17.6 |
| Proximal gastrectomy with partial esophagectomy | 34 | 22.2 | |
| Total gastrectomy with partial esophagectomy | 92 | 60.1 | |
| Histologic type | Adenocarcinoma | 145 | 94.8 |
| Mixed adenocarcinoma | 8 | 5.2 | |
| Histological grade | Well-moderately differentiated | 72 | 47.1 |
| Moderately-poorly differentiated | 80 | 52.3 | |
| Lauren type | Intestinal type | 56 | 36.6 |
| Diffuse type | 44 | 28.8 | |
| Mixed type | 51 | 33.3 | |
| Preoperative chemotherapy | No | 104 | 68.0 |
| Yes | 49 | 32.0 | |
| Vessel invasion | No | 128 | 83.7 |
| Yes | 25 | 16.3 | |
| Perineural invasion | No | 73 | 47.7 |
| Yes | 80 | 52.3 | |
| Pathological depth of tumor invasion | T1-2 | 48 | 31.4 |
| T3-4 | 105 | 68.6 | |
| Lymph node metastasis | N0 | 73 | 47.7 |
| N1-3 | 80 | 52.3 | |
| Distant metastasis | pM0 | 147 | 96.1 |
| pM1 | 6 | 3.9 | |
| TNM-GC staging system | IA | 24 | 15.7 |
| IB | 16 | 10.5 | |
| IIA | 8 | 5.2 | |
| IIB | 33 | 21.6 | |
| IIIA | 30 | 19.6 | |
| IIIB | 28 | 18.3 | |
| IIIC | 8 | 5.2 | |
| IV | 6 | 3.9 | |
| TNM-EC staging system | IA | 5 | 3.3 |
| IB | 17 | 11.1 | |
| IC | 16 | 10.5 | |
| IIA | 1 | 0.7 | |
| IIB | 4 | 2.6 | |
| IIIA | 5 | 3.3 | |
| IIIB | 49 | 32.0 | |
| IIIC/IVA | 50 | 32.7 | |
| IVB | 6 | 3.9 |
A comparison of the clinicopathological characteristics among patients with type E, type Eg, and type Ge AEGs is presented in Table 2. The average tumor length differed significantly between the three groups, with type Eg AEGs having the largest average tumor length (58.7 mm ± 12.6 mm), followed by type E (53.0 mm ± 18.2 mm), and type Ge (37.9 mm ± 11.2 mm) with the smallest average tumor length. The mean tumor esophageal infiltration length in type E AEG was 35.9 mm ± 13.0 mm, which was significantly greater than that type Eg (23.9 mm ± 6.0 mm) and type Ge (5.1 mm ± 5.4 mm) (P < 0.05). Significant differences in various aspects were observed between the three types of AEGs, including surgical approach, surgical resection range, tumor pathology type, tumor differentiation degree, depth of tumor invasion, lymph node metastasis, and tumor stage. Accordingly, 19 patients with type E AEGs underwent transthoracic surgery (86.4%), whereas 3 patients underwent proximal gastrectomy with partial esophagectomy through the transabdominal hiatal approach. Moreover, 8 patients with type Eg AEGs underwent transthoracic surgery (25.8%), whereas 23 patients underwent transabdominal hiatal surgery (74.2%), among whom 5 underwent proximal gastrectomy with partial esophagectomy and 18 underwent total gastrectomy with partial esophagectomy. All 100 patients with type Ge AEGs underwent transabdominal hiatal surgery, among whom 26 underwent proximal gastrectomy with partial esophagectomy and 74 underwent total gastrectomy with partial esophagectomy. Among our patients, 4 with type E (18.2%), 1 with type Eg (3.2%), and 3 with type Ge (3.0%) AEGs had mixed adenocarcinoma. Among type E, type Eg, and type Ge tumors, 12 (54.5%), 23 (74.2%), and 45 (45.0%) were moderately-poorly differentiated. In terms of tumor stage, a portion of type E (27.3%), type Eg (12.9%), and type Ge (38.0%) tumors were classified as T1-2. Lymph node metastases were observed in 59.1%, 24.0%, and 43.0% of patient with type E, of type Eg, and of type Ge AEGs, respectively. According to the gastric cancer staging system, 50.0%, 25.8%, and 62.0% of type E, type Eg, and type Ge tumors were classified as stage I-II, respectively. In contrast, according to the esophageal adenocarcinoma staging system, 9.1%, 12.9%, and 27.0% of type E, type Eg, and type Ge tumors were categorized as stage I–II, respectively. Notably, patients with type Ge AEGs tended to have earlier-stage tumors than did those with type E and type Eg AEGs (Table 2).
| Characteristic | Variables | Type E | Type Eg | Type Ge | P value |
| Age (year) | 61.5 ± 11.7 | 61.0 ± 9.7 | 63.0 ± 7.2 | 0.417 | |
| ≤ 65 | 13 | 20 | 62 | ||
| > 65 | 9 | 11 | 38 | 0.922 | |
| Sex | Male | 18 | 26 | 82 | 0.969 |
| Female | 4 | 5 | 18 | ||
| Tumor size (mm) | 53.0 ± 18.2 | 58.7 ± 12.6 | 37.9 ± 11.2 | 0.000a | |
| ≤ 40 mm | 7 | 4 | 75 | 0.000a | |
| > 40 mm | 15 | 27 | 25 | ||
| Esophageal invasion length (mm) | 35.9 ± 13.0 | 23.9 ± 6.0 | 5.1 ± 5.4 | 0.000a | |
| < 20 mm | 1 | 0 | 100 | 0.000a | |
| ≥ 20 mm | 21 | 31 | 0 | ||
| Surgical approaches | Transthoracic approach | 19 | 8 | 0 | 0.000a |
| Transabdominal hiatal approach | 3 | 23 | 100 | ||
| Extent of surgical resection | Subtotal esophagectomy with partial gastrectomy | 19 | 8 | 0 | 0.000a |
| Proximal gastrectomy with partial esophagectomy | 3 | 5 | 26 | ||
| Total gastrectomy with partial esophagectomy | 0 | 18 | 74 | ||
| Histologic type | Adenocarcinoma | 18 | 30 | 97 | 0.013a |
| Mixed adenocarcinoma | 4 | 1 | 3 | ||
| Histological grade | Well-moderately differentiated | 9 | 8 | 55 | 0.016a |
| Moderately-poorly differentiated | 12 | 23 | 45 | ||
| Lauren type | Intestinal type | 7 | 6 | 43 | 0.150 |
| Diffuse type | 7 | 13 | 24 | ||
| Mixed type | 7 | 12 | 32 | ||
| Preoperative chemotherapy | No | 20 | 19 | 65 | 0.042a |
| Yes | 2 | 12 | 35 | ||
| Vessel invasion | No | 18 | 24 | 86 | 0.512 |
| Yes | 4 | 7 | 14 | ||
| Perineural invasion | No | 10 | 14 | 49 | 0.908 |
| Yes | 12 | 17 | 51 | ||
| Pathological depth of tumor invasion | T1-2 | 6 | 4 | 38 | 0.028a |
| T3-4 | 16 | 27 | 62 | ||
| Lymph node metastasis | N0 | 9 | 7 | 57 | 0.003a |
| N1-3 | 13 | 24 | 43 | ||
| Distant metastasis | M0 | 21 | 29 | 97 | 0.679 |
| M1 | 1 | 2 | 3 | ||
| I-II | 11 | 8 | 62 | 0.002a | |
| III-IV | 11 | 23 | 38 | ||
| TNM-GC staging system | I-II | 11 | 8 | 62 | 0.002a |
| III-IV | 11 | 23 | 38 | ||
| TNM-EC staging system | I-II | 2 | 4 | 27 | 0.003a |
| III-IV | 20 | 27 | 63 |
Among the 153 patients, 80 (52.3%) exhibited lymph node metastasis classified as N1-3, with an average of 37.0 ± 21.9 lymph nodes involved. Among the 22 patients with type E AEGs, 13 showed lymph node metastasis, with 11 of them having more than 15% lymph node involvement (84.6%). Among the 31 patients with type Eg AEGs, 24 had lymph node metastases, with 13 having > 15% lymph node involvement (54.2%). Among the 100 patients with type Ge AEGs, 43 exhibited lymph node metastasis, with 16 having metastasis exceeding 15% (37.2%). Regarding lymph node metastasis distribution between the three groups, patients with type E, type Eg, and type Ge AEGs presented with lymph node involvement in various regional groups. Specifically, 6 (27.3%), 19 (61.3%), and 19 (19.0%) patients with type E, type Eg, and type Ge AEGs had group 1 lymph node metastasis (right of the cardia). Notably, no group 2 lymph node metastasis (left of the cardia) was observed in patients with type E AEGs. However, 9 (29.0%) and 18 (18.0%) patients with type Eg and type Ge AEGs experienced group 2 lymph node metastasis, respectively. Moreover, 2 (9.1%), 15 (48.4%), and 26 (26.0%) patients with type E, type Eg, and type Ge AEGs showed lymph node metastasis in group 4 (gastric curvature), with a significant difference between these groups. Differences in the pattern of lymph node metastasis for group 7 (left gastric artery) and group 20 (esophageal hiatus) were also observed among the different types of AEGs (Table 3).
| Variable | Variable | Type E | Type Eg | Type Ge | P value |
| Overall | 13/22 | 24/31 | 43/100 | 0.003a | |
| The rate of lymph node metastasis | ≤ 15% | 2 | 11 | 27 | 0.010a |
| > 15% | 11 | 13 | 16 | ||
| Pathological depth of tumor invasion | T1-2 | 4 | 3 | 4 | 0.141 |
| T3-4 | 9 | 21 | 39 | ||
| Pathological depth of tumor invasion | T1 | 0/1 | 0/1 | 1/23 | 0.956 |
| T2 | 4/5 | 1/3 | 3/15 | 0.051 | |
| T3 | 2/3 | 2/4 | 0.659 | ||
| T4 | 9/16 | 21/24 | 37/58 | 0.058 | |
| Location of lymph node | No. 1 | 6 | 19 | 19 | 0.017a |
| No. 2 | 0 | 9 | 18 | 0.018a | |
| No. 3 | 2 | 15 | 26 | 0.010a | |
| No. 4 | 1 | 6 | 9 | 0.443 | |
| No. 5 | 0 | 5 | 4 | 0.134 | |
| No. 6 | 0 | 1 | 2 | 0.735 | |
| No. 7 | 12 | 20 | 27 | 0.047a | |
| No. 8 | 0 | 2 | 4 | 0.527 | |
| No. 9 | 1 | 4 | 9 | 0.541 | |
| No. 10 | 0 | 0 | 1 | 0.647 | |
| No. 11 | 0 | 4 | 8 | 0.249 | |
| No. 20 | 2 | 1 | 0 | 0.038a | |
| No. 21 | 1 | 0 | 0 | 0.074 |
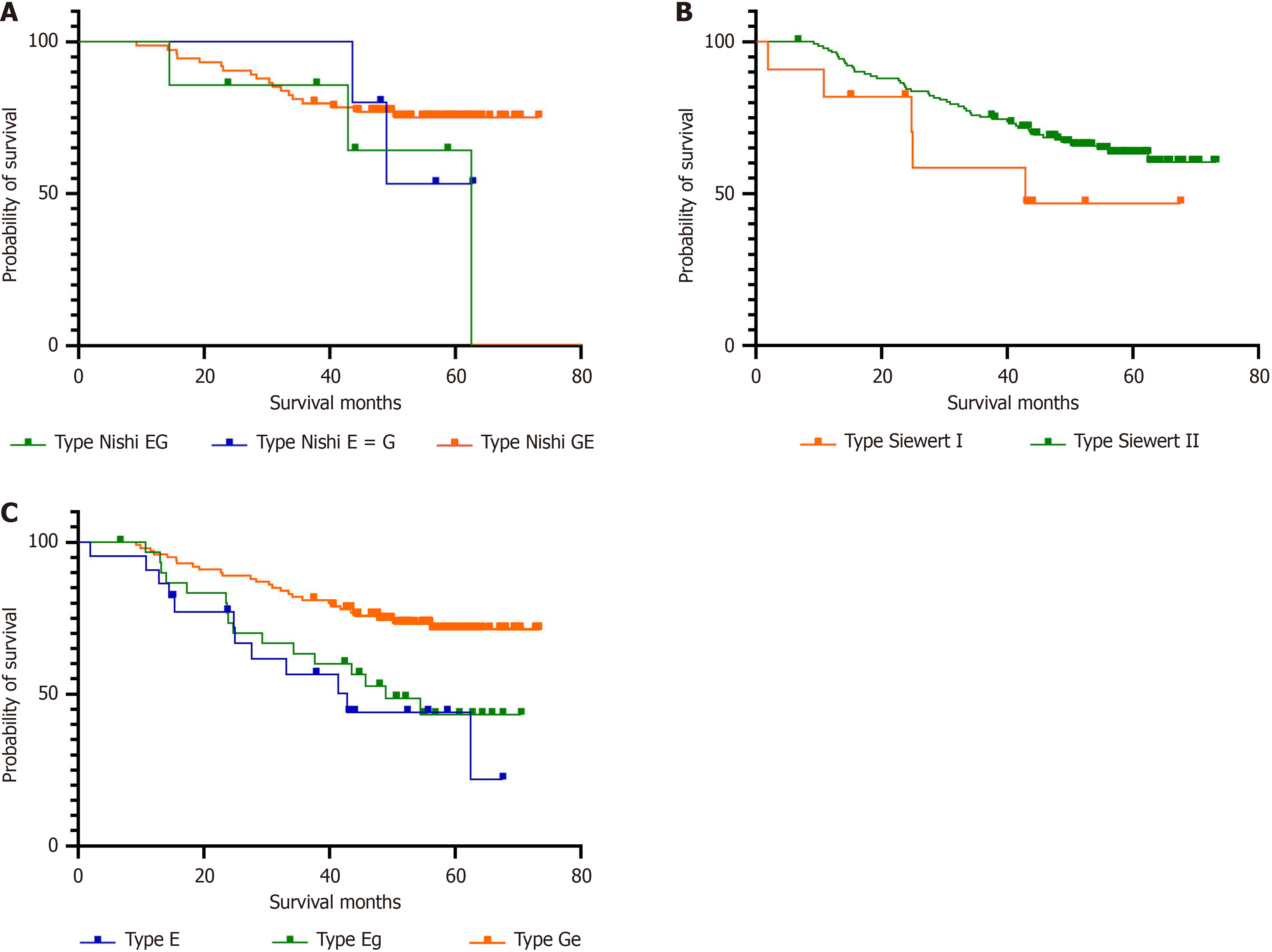
In this study, the overall survival rate of patients with AEG was 64.1%. Among the 55 patients who succumbed to their disease, 49 died due to their tumor, 1 patient died due to postoperative complications, and 5 patients experienced cardiovascular and other unexpected causes of death. The mean survival time for patients with type E, type Eg, and type Ge AEGs was 42.1, 47.6, and 61.0 months, respectively. The cumulative survival rates for these types were 45.5%, 48.4%, and 73.0%, respectively, with significant differences between them (P = 0.001). The survival rate of patients with type Siewert I tumors was slightly lower (54.6%) than that of patients with type II tumors (64.8%), though the difference was not significant (P = 0.156). According to the Nishi classification, those with type Eg AEGs had a survival rate of 57.1%, which was slightly lower than that for patients with type E = G (60.0%) and type GE (75.7%). However, the difference in survival rates was not significant (P = 0.402). Univariate Cox analysis revealed that type E tumors, esophageal invasion length ≥ 2 cm, tumor length > 4 cm, mixed carcinoma, vascular invasion, nerve invasion, invasion depth T3-4, and lymph node metastasis (N1-3) were prognostic factors for patients with AEGs. Multivariate Cox analysis demonstrated that type E tumors remained an important independent prognostic factor for patients with AEGs. Patients with type E tumors had a significantly higher survival risk than did patients with type Eg [hazard ratio (HR) = 0.451; 95% confidence interval (CI): 0.207-0.984; P = 0.004] and Type Ge (HR = 0.300; 95%CI: 0.147-0.609; P = 0.001) tumors. Vascular infiltration (HR = 2.545; 95%CI: 1.399-4.631; P = 0.002), depth of infiltration T3-4 (HR = 2.945; 95%CI: 1.095-7.922; P = 0.032), and lymph node metastasis (HR = 3.643; 95%CI: 1.728-7.678; P = 0.001) were also identified as independent factors influencing the outcomes of patients with AEGs (Figure 2, Table 4 and Table 5).
| Variable | Hazard ratio | 95% confidence interval | P value |
| This study classification | |||
| Type E | 1.0 | 0.002 | |
| Type Eg | 0.774 | 0.366-1.640 | 0.504 |
| Type Ge | 0.331 | 0.167-0.656 | 0.002 |
| Age | |||
| < 65 years | 1.0 | ||
| ≥ 65 years | 1.110 | 0.647-1.904 | 0.705 |
| Sex | |||
| Male | 1.0 | ||
| Female | 0.850 | 0.416-1.738 | 0.657 |
| Tumor size | |||
| ≤ 40 mm | 1.0 | ||
| > 40 mm | 2.235 | 1.307-3.823 | 0.003 |
| Esophageal invasion length | |||
| < 20 mm | 1.0 | ||
| ≥ 20 mm | 2.431 | 1.355-4.362 | 0.003 |
| Histologic type | |||
| Adenocarcinoma | 1.0 | ||
| Mixed adenocarcinoma | 2.775 | 1.102-6.988 | 0.030 |
| Histological grade | |||
| Well-moderately differentiated | 1.0 | ||
| Moderately-poorly differentiated | 1.707 | 0.993-2.936 | 0.053 |
| Lauren type | |||
| Intestinal type | 1.0 | 0.207 | |
| Diffuse type | 1.776 | 0.922-3.418 | 0.086 |
| Mixed type | 1.538 | 0.798-2.964 | 1.198 |
| Preoperative chemotherapy | |||
| No | 1.0 | ||
| Yes | 1.484 | 0.865-2.547 | 0.152 |
| Vessel invasion | |||
| No | 1.0 | ||
| Yes | 3.688 | 2.069-6.573 | 0.000 |
| Perineural invasion | |||
| No | 1.0 | ||
| Yes | 2.523 | 1.420-4.484 | 0.002 |
| Pathological depth of tumor invasion | |||
| T1-2 | 1.0 | ||
| T3-4 | 6.159 | 2.452-15.471 | 0.000 |
| Lymph node metastasis | |||
| L0 | 1.0 | ||
| L1 | 5.821 | 2.928-11.570 | 0.000 |
| Distant metastasis | |||
| M0 | 1.0 | ||
| M1 | 2.931 | 1.053-8.159 | 0.040 |
| Variable | Hazard ratio | 95% confidence interval | P value |
| This study classification | |||
| Type E | 1.0 | 0.004 | |
| Type Eg | 0.451 | 0.207-0.984 | 0.045 |
| Type Ge | 0.300 | 0.147-0.609 | 0.001 |
| Vessel invasion | |||
| V0 | 1.0 | ||
| V1 | 2.545 | 1.399-4.631 | 0.002 |
| Perineural invasion | |||
| P0 | 1.0 | ||
| P1 | 1.129 | 0.599-2.127 | 0.709 |
| Pathological depth of tumor invasion | |||
| T1-2 | 1.0 | ||
| T3-4 | 2.945 | 1.095-7.922 | 0.032 |
| Lymph node metastasis | |||
| L0 | 1.0 | ||
| L1 | 3.643 | 1.728-7.678 | 0.001 |
For patients with type E tumors, significant differences in survival curves were observed according to the staging system of gastric cancer and esophageal adenocarcinoma (P = 0.039 vs P = 0.011). Patients with type Eg AEGs also showed significant differences in survival curves according to the two staging systems (P = 0.000 vs P = 0.002). In contrast, patients with type Ge tumors showed significant differences according to stage when utilizing the gastric cancer staging system (P = 0.005) but not the esophageal adenocarcinoma staging system (P = 0.113) (Figure 3).
For patients with type E tumors, the survival curves exhibited significant differences between the staging system of gastric cancer and esophageal adenocarcinoma (P = 0.039 vs P = 0.011). Patients with type Eg AEG also showed significant differences in survival curves between the two staging systems (P = 0.000 vs P = 0.002). In contrast, for patients with type Ge tumors, the survival curves of different stages were significantly different when utilizing the gastric cancer staging system (P = 0.005), but in the esophageal adenocarcinoma staging system, there was no significant difference in survival curves (P = 0.113) (Figure 3).
Several statistical values were assessed to comprehensively compare the staging systems for gastric cancer and eso
| Project | Likelihood ratio χ2 price | Linear trend χ2 price | The log-likelihood estimate | |||
| Gastric cancer TNM staging system | Esophageal cancer TNM staging system | Gastric cancer TNM staging system | Esophageal cancer TNM staging system | Gastric cancer TNM staging system | Esophageal cancer TNM staging system | |
| Type E | 10.896 | 6.615 | 3.327 | 5.178 | 37.018 | 37.300 |
| Type Eg | 17.032 | 18.760 | 11.923 | 9.899 | 55.624 | 62.748 |
| Type Ge | 13.514 | 7.575 | 2.873 | 1.196 | 181.106 | 186.322 |
Surgical resection has been the treatment of choice for patients with resectable AEGs. The primary goal of surgery is the complete removal of the primary tumor and its lymphatic drainage. An individualized therapeutic strategy, tailored to the tumor’s location, is essential for improving overall survival[18]. Therefore, accurate preoperative evaluation is a prerequisite for selecting the optimal therapeutic strategy.
The EGJ is the virtual anatomical junction where the tubular esophagus connects with the distal stomach. However, the definition of this region varies based on whether endoscopy, imaging, and histopathology is used. During endoscopy, the EGJ is identified based on the presence of the distal longitudinal palisade vessels in the lower esophagus or the proximal margin of the gastric mucosa. On imaging, the EGJ is defined as the narrowest part of the lower esophageal segment, whereas during histopathology, it is determined as the transition from squamous to gastric epithelium. Given that the location of the distal end of the palisade vessels and the lower esophagus can be influenced by the presence of tumors at the junction and that the anatomical junction does not precisely coincide with these definitions, we adopted the definition based on the location of the proximal gastric fold, which is consistent with the widely accepted World Health Or
The Nishi classification, also referred to as the Japanese classification, presents a significant limitation in that it uniformly categorizes malignant tumors near the EGJ without differentiating between squamous cell carcinoma and adenocarcinoma. Moreover, its applicability is confined to tumors located within 2 cm above and below the EGJ. In Japan, where the early detection rates for gastric cancer has been relatively high, EGJ tumors are often identified at earlier stages. Outside of Japan, however, particularly in China and Western countries, AEG tumors are frequently diagnosed at more advanced stages, with patients often presenting with tumor diameters exceeding 4 cm. Consequently, the Nishi classification exhibits certain limitations and has been predominantly utilized within Japan, with limited international applicability. However, the widely used Siewert classification also has certain limitations, such as its inability to account for the extent of proximal and distal tumor spread and limited guidance for determining the appropriate scope of surgical resection. For example, a large-diameter Siewert type II tumor located at or below the EGJ may exhibit more esophageal infiltration than would a small-diameter Siewert type I or II AEG tumor. Apart from tumor staging and Siewert classification, the distance between the proximal tumor and the EGJ is of particular significance in clinical practice[9]. This distance is crucial considering the close association between the length of tumor invasion and the rate of mediastinal lymph node metastasis and plays a pivotal role in determining the surgical approach, resection method, and recons
The current study defined AEG cases as those in which the tumor was located within 5 cm above and 2 cm below the EGJ and categorized them into the following three groups based on the location of the tumor’s center and 2 cm of esophageal invasion: Type E (esophageal type), type Eg (esophagogastric type with predominant esophageal involve
The 8th edition of the AJCC/UICC TNM staging system for esophageal cancer and gastric cancer was used in the staging of AEGs. This suggests that for Siewert I AEGs, the esophageal cancer TNM staging system (TNM-EC) and corresponding treatment guidelines should be applied, whereas for Siewert III AEGs, the gastric cancer TNM staging system (TNM-GC) and related guidelines are more appropriate. Nevertheless, the classification and treatment of Siewert II AEGs remains controversial[29]. In fact, a retrospective study found that the 8th TNM-GC scheme is superior to TNM-EC in predicting the prognosis of Siewert II AEGs[14,30]. In the current study, the prognosis of different AEG types was evaluated using both the TNM-EC and TNM-GC systems. Our findings revealed that for type E AEGs, the TNM-EC better predicted the survival risk of patients than did the TNM-GC, indicating greater homogeneity and discriminative power. This could be due to the similarities in esophageal invasion length, lymph node metastasis range, and histological type between type E AEGs and esophageal cancer. For type Ge AEG, the TNM-GC was more effective at distinguishing survival differences among patients with different tumor stages than did the TNM-EC, showing an increasing trend in the risk ratio and better separation of survival curves. This might be related to the similarities in gastric involvement, lymph node metastasis range, and histological type between type Ge AEGs and gastric cancer. In the case of type Eg AEGs, both systems effectively differentiated survival differences among stages, but the TNM-GC demonstrated better risk ratio trends, survival curve separation, homogeneity, and discriminative power than did the TNM-EC. Given the patients with type Eg AEGs exhibit mixed biological behavior of esophageal and gastric cancer, comprehensive pro
The present study retrospectively analyzed 153 patients with AEGs and proposed a novel and practical classification method for AEG by comparing the applicability of two commonly used staging systems for different AEG types. Al
| 1. | Li Z, Dong J, Huang Q, Zhang W, Tao K. Comparison of three digestive tract reconstruction methods for the treatment of Siewert II and III adenocarcinoma of esophagogastric junction: a prospective, randomized controlled study. World J Surg Oncol. 2019;17:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Kusano C, Gotoda T, Khor CJ, Katai H, Kato H, Taniguchi H, Shimoda T. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23:1662-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Lu YK, Li YM, Gu YZ. Cancer of esophagus and esophagogastric junction: analysis of results of 1,025 resections after 5 to 20 years. Ann Thorac Surg. 1987;43:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Ito H, Inoue H, Odaka N, Satodate H, Suzuki M, Mukai S, Takehara Y, Kida H, Kudo SE. Clinicopathological characteristics and optimal management for esophagogastric junctional cancer; a single center retrospective cohort study. J Exp Clin Cancer Res. 2013;32:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Kauppila JH, Lagergren J. The surgical management of esophago-gastric junctional cancer. Surg Oncol. 2016;25:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Shoji Y, Koyanagi K, Kanamori K, Tajima K, Ogimi M, Yatabe K, Yamamoto M, Kazuno A, Nabeshima K, Nakamura K, Nishi T, Mori M. Current status and future perspectives for the treatment of resectable locally advanced esophagogastric junction cancer: A narrative review. World J Gastroenterol. 2023;29:3758-3769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (0)] |
| 7. | Siewert JR, Hölscher AH, Becker K, Gössner W. [Cardia cancer: attempt at a therapeutically relevant classification]. Chirurg. 1987;58:25-32. [PubMed] |
| 8. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1913] [Article Influence: 239.1] [Reference Citation Analysis (1)] |
| 9. | Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 914] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 10. | Siewert JR, Feith M, Stein HJ. Biologic and clinical variations of adenocarcinoma at the esophago-gastric junction: relevance of a topographic-anatomic subclassification. J Surg Oncol. 2005;90:139-46; discussion 146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Ajani JA, Barthel JS, Bentrem DJ, D'Amico TA, Das P, Denlinger CS, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, Hofstetter WL, Ilson DH, Keswani RN, Kleinberg LR, Korn WM, Lockhart AC, Mulcahy MF, Orringer MB, Osarogiagbon RU, Posey JA, Sasson AR, Scott WJ, Shibata S, Strong VE, Varghese TK Jr, Warren G, Washington MK, Willett C, Wright CD; National Comprehensive Cancer Network. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 12. | Rice TW, Ishwaran H, Hofstetter WL, Kelsen DP, Apperson-Hansen C, Blackstone EH; Worldwide Esophageal Cancer Collaboration Investigators. Recommendations for pathologic staging (pTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus. 2016;29:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 13. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4396] [Article Influence: 549.5] [Reference Citation Analysis (4)] |
| 14. | Zhao B, Zhang Z, Mo D, Lu Y, Hu Y, Yu J, Liu H, Li G. Optimal Extent of Transhiatal Gastrectomy and Lymphadenectomy for the Stomach-Predominant Adenocarcinoma of Esophagogastric Junction: Retrospective Single-Institution Study in China. Front Oncol. 2018;8:639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Rice TW, Gress DM, Patil DT, Hofstetter WL, Kelsen DP, Blackstone EH. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 16. | Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T, Nashimoto A, Hiratsuka M; Japan Clinical Oncology Group (JCOG9502). Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol. 2006;7:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 303] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Wang W, Xiong W, Xue Y, Li J, Huang H, Zheng Y, Luo L, Wan J. Totally laparoscopic plus one transthoracic port esophagogastrectomy for Siewert-type II adenocarcinoma of esophagogastric junction. J Clin Oncol. 2020;38:e16568-e16568. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Grotenhuis BA, Wijnhoven BP, Poley JW, Hermans JJ, Biermann K, Spaander MC, Bruno MJ, Tilanus HW, van Lanschot JJ. Preoperative assessment of tumor location and station-specific lymph node status in patients with adenocarcinoma of the gastroesophageal junction. World J Surg. 2013;37:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Ichihara S, Uedo N, Gotoda T. Considering the esophagogastric junction as a 'zone'. Dig Endosc. 2017;29 Suppl 2:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Ectors N, Driessen A, De Hertog G, Lerut T, Geboes K. Is adenocarcinoma of the esophagogastric junction or cardia different from Barrett adenocarcinoma? Arch Pathol Lab Med. 2005;129:183-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Sharma P, McQuaid K, Dent J, Fennerty MB, Sampliner R, Spechler S, Cameron A, Corley D, Falk G, Goldblum J, Hunter J, Jankowski J, Lundell L, Reid B, Shaheen NJ, Sonnenberg A, Wang K, Weinstein W; AGA Chicago Workshop. A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 351] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 22. | Barbour AP, Rizk NP, Gonen M, Tang L, Bains MS, Rusch VW, Coit DG, Brennan MF. Lymphadenectomy for adenocarcinoma of the gastroesophageal junction (GEJ): impact of adequate staging on outcome. Ann Surg Oncol. 2007;14:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Mine S, Sano T, Hiki N, Yamada K, Kosuga T, Nunobe S, Yamaguchi T. Proximal margin length with transhiatal gastrectomy for Siewert type II and III adenocarcinomas of the oesophagogastric junction. Br J Surg. 2013;100:1050-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Kurokawa Y, Hiki N, Yoshikawa T, Kishi K, Ito Y, Ohi M, Wada N, Takiguchi S, Mine S, Hasegawa S, Matsuda T, Takeuchi H. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery. 2015;157:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Hosoda K, Yamashita K, Moriya H, Mieno H, Watanabe M. Optimal treatment for Siewert type II and III adenocarcinoma of the esophagogastric junction: A retrospective cohort study with long-term follow-up. World J Gastroenterol. 2017;23:2723-2730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Zheng Z, Shang Y, Xu R, Zhang H, Yin J, Zhang J, Zhang Z. Risk factors and prognosis in patients with adenocarcinoma of esophagogastric junction with lymph node metastasis of Siewert II/III. Int J Clin Exp Pathol. 2020;13:1262-1269. [PubMed] |
| 27. | Kulig P, Sierzega M, Pach R, Kolodziejczyk P, Kulig J; Polish Gastric Cancer Study Group. Differences in prognosis of Siewert II and III oesophagogastric junction cancers are determined by the baseline tumour staging but not its anatomical location. Eur J Surg Oncol. 2016;42:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Pang T, Nie M, Yin K. The correlation between the margin of resection and prognosis in esophagogastric junction adenocarcinoma. World J Surg Oncol. 2023;21:316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Ajani JA, D'Amico TA, Bentrem DJ, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Farjah F, Gerdes H, Gibson M, Grierson P, Hofstetter WL, Ilson DH, Jalal S, Keswani RN, Kim S, Kleinberg LR, Klempner S, Lacy J, Licciardi F, Ly QP, Matkowskyj KA, McNamara M, Miller A, Mukherjee S, Mulcahy MF, Outlaw D, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:393-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 218] [Reference Citation Analysis (0)] |
| 30. | Liu K, Feng F, Chen XZ, Zhou XY, Zhang JY, Chen XL, Zhang WH, Yang K, Zhang B, Zhang HW, Zhou ZG, Hu JK. Comparison between gastric and esophageal classification system among adenocarcinomas of esophagogastric junction according to AJCC 8th edition: a retrospective observational study from two high-volume institutions in China. Gastric Cancer. 2019;22:506-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |