Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.102831
Revised: January 17, 2025
Accepted: February 5, 2025
Published online: April 15, 2025
Processing time: 140 Days and 13.9 Hours
In this editorial we comment on the article published in the recent issue of World Journal of Gastrointestinal Oncology. This study aims to explore the relationship be
Core Tip: The importance of predicting prognosis in the management of gastrointestinal stromal tumors (GIST) is well known, with a particular focus on mitotic activity, tumor size, anatomical location, and KIT and platelet-derived growth factor receptor alpha mutation status. It underscores the emerging significance of inflammatory markers such as the neutrophil-to-lymphocyte ratio, systemic immune-to-inflammation index, platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) in predicting GIST prognosis and recurrence risk. This study identified the MLR and PLR as independent risk factors, thereby providing a predictive model for recurrence-free survival. However, it is important to note that the study has limitations, including its retrospective design, small sample size, and lack of external validation.
- Citation: Sun YF, Cao XK, Wei Q, Gao YH. Potential biomarkers for the prognosis of gastrointestinal stromal tumors. World J Gastrointest Oncol 2025; 17(4): 102831
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/102831.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.102831
Gastrointestinal stromal tumors (GIST) are mesenchymal tumor with variable behavior, with an incidence ranging from 1.1 cases per 100000 person/years to 1.5 cases per 100000 person/years[1]. In contrast, microGIST are relatively common. Prognostic parameters play a crucial role in the management of GIST, helping to estimate the risk of recurrence after surgery, which is essential for establishing adjuvant therapy schemes. The best-documented prognostic parameters for GIST are mitotic activity, tumor size and anatomical site. Besides, mutation status of KIT and platelet-derived growth factor receptor alpha serves as a prognostic and predictive parameter[2,3]. These elements are incorporated into the National Institutes of Health risk classification system, which categorizes GIST cases into low-risk, intermediate-risk, and high-risk groups for recurrence[4,5]. This classification facilitates establish the strategy of adjuvant therapy, highlighting the importance of prognostic indicators. It is recommended that patients with high-risk GSIT may get privileged form a standard three-years adjuvant treatment. Many clinical trials have recommended imatinib as the first-line treatment for GIST, with sunitinib, regorafenib, and ripretinib identified as subsequent therapeutic options for advanced cases[3]. Additionally, various tyrosine kinase inhibitors are utilized in different treatment settings[6].
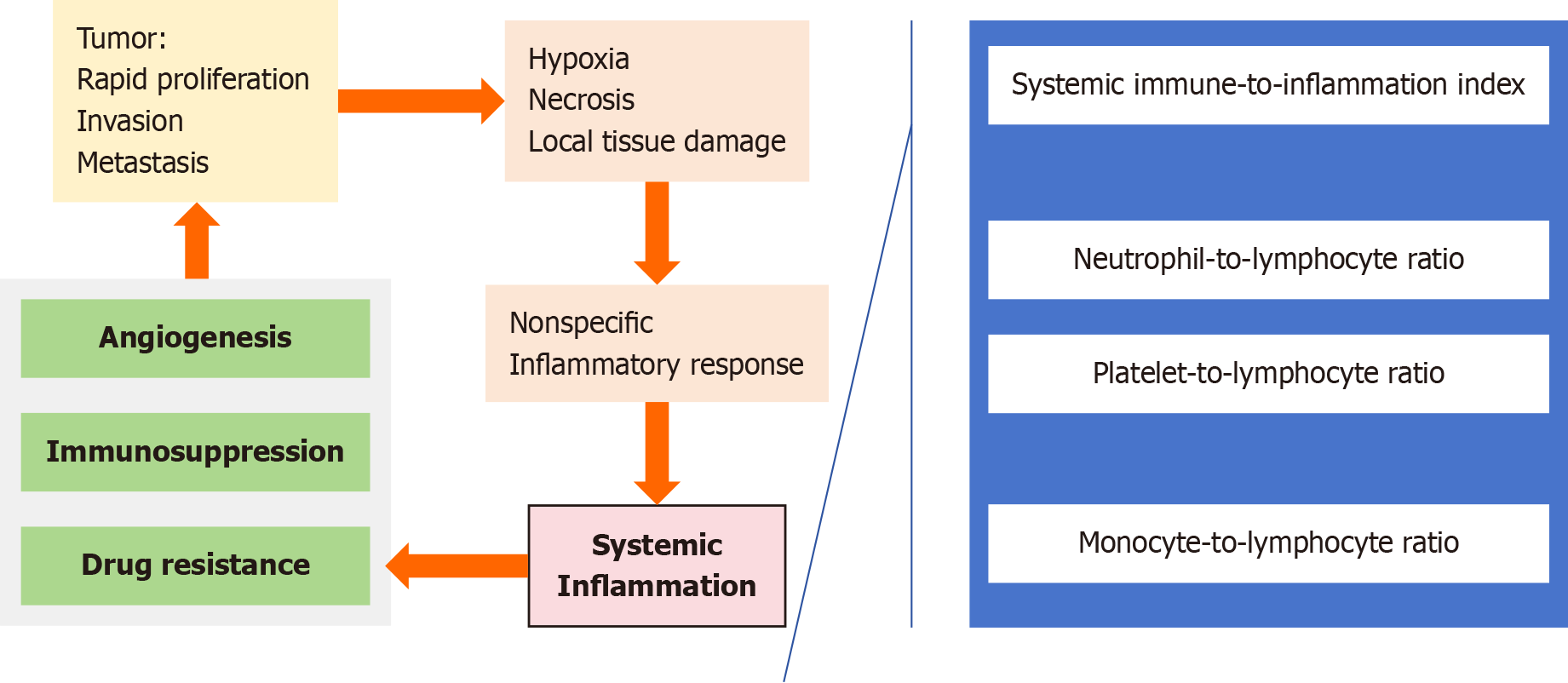
In recent years, inflammatory markers, such as the neutrophil-to-lymphocyte ratio, systemic immune-to-inflammation index, platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR), have demonstrated potential value for the prognosis of various cancers, including GIST[7-10]. Compared with classical prognostic parameters, inflammatory markers are readily accessible and cost effective to examine, and they provide additional prognostic information that allows clinicians to conduct more comprehensive assessments[8,9]. In patients with GIST, inflammatory markers are associated with the risk of disease recurrence. These markers not only reflect the systemic antitumour inflammatory response but are also closely associated with tumour progression, invasion, and prognosis. Elevated levels of certain markers may indicate a more aggressive disease phenotype and poorer prognosis in patients with GIST. Additionally, inflammatory markers have also been explored for their ability to predict neoadjuvant and immunotherapy efficacy in advanced tumour patients[11,12]. Additionally, in GIST, inflammatory markers are related to angiogenesis and the immune response within the tumour microenvironment. These markers provide insights into the host immune status, which can be useful for making personalized treatment decisions. Furthermore, certain inflammatory markers may serve as predictors of adjuvant treatment efficacy, indicating promising prognostic value for recurrence-free survival (RFS) in patients (Table 1)[3,13-21].
| Inflammatory markers | Impact | Mechanism | Threshold value | Ref. |
| NLR | High preoperative NLR is associated with decreased DFS and is an independent prognostic factor | Higher NLR may reflect the inflammatory state and immunosuppressive state of the body, which may be related to tumor progression and response to treatment | 1.92-6 | Kumarasamy et al[14], Bigot et al[20], Malietzis et al[21] |
| SII | A promising predictor for RFS and effect of neoadjuvant therapy | A high level of SII indicates an enhanced systemic inflammatory state and a weakened immune response, while a high level of SII may indicate that the inflammatory microenvironment promotes tumor invasion and metastasis | 544.6-820.0 | Goh et al[16], Lu et al[17] |
| PLR | High PLR is an independent prognostic factor for RFS in patients with GIST | High PLR may indicate the immune surveillance ability is decreased, which may be related to tumor progression | 275 | |
| PENK | High expression of PENK was associated with superior OS and RFS in patients with GIST | PENK is a neuropeptide precursor, which may affect the proliferation and apoptosis of tumor cells through its interaction with opioid receptors. The high expression of PENK may be related to the tumor inhibition pathway | IHC score ≥ 4: PENK positive. IHC score < 4: PENK negative | |
| GNRI | A promising predictor for RFS | GNRI is an objective nutritional assessment method based on serum albumin levels and body weight ratios, reflecting the nutritional status of patients to influence prognosis | 98.3 | Lu et al[17] |
| PNI | A promising predictor for effect of neoadjuvant therapy | PNI reflects the nutritional status and immune function of patients. Low PNI may indicate decreased immune function and poor nutritional status, further promoting the formation of an inflammatory microenvironment | 47.2 | |
| MLR | As a prognostic indicator for the recurrence of GIST, with elevated MLR correlating with an increased risk of postoperative recurrence | Monocytes and lymphocytes play crucial roles in the immune modulation within the tumor microenvironment. A high MLR may signify underlying chronic inflammation, which could facilitate tumor progression and recurrence. Furthermore, an elevated MLR might reflect compromised immune surveillance capabilities | / | |
| SLITRK3 | SLITRK3 expression is closely associated with OS and DFS in GIST patients | Elevated levels of SLITRK3 may be linked to the aggressiveness and unfavorable prognosis of GIST; however, the precise mechanisms underlying its action and the critical pathways involved in GIST remain to be elucidated | IHC score ≥ 4: SLITRK3 positive. IHC score < 4: SLITRK3 negative |
However, these results may be influenced by various factors, including infection status, nutritional status, and other conditions unrelated to cancer. This could result in false-positive outcomes during prognostic assessments. Therefore, ensuring the consistency and reproducibility of inflammatory markers is essential for their clinical application. Further validation of their prognostic value calls for larger, multicenter studies. Standardized thresholds should be established for these markers[3,14]. It is highly important to establish standardized thresholds and interpretations for these markers to increase their reliability across different studies and clinical settings.
In this study, which identified the MLR and PLR as independent risk factors among four inflammatory biomarkers derived from neutrophils, platelets, monocytes, and lymphocytes (Figure 1), the authors established a line chart pre
Nevertheless, as a relatively small-sample retrospective study, there was inevitable bias in the results. Second, the study only conducted internal validation without external or multicenter data verification. Third, the impact of postope
Research into novel prognostic parameters for GIST is thriving. New markers such as cytokines and chemokines are being explored for their potential role in GIST prognosis. Recent studies identified SLITRK3 as a significant predictor for the recurrence and metastasis of GIST, with higher expression levels correlating with poorer patient outcomes[18]. On the other hand, another study found that high proenkephalin (PENK) expression in GIST is associated with better overall survival and RFS[19]. On the molecular front, next-generation sequencing of liquid biopsy detecting circulating tumor DNA offers a non-invasive approach to detect mutation status and monitor progression and response to treatment in real-time[22].
In conclusion, integration both classical and novel prognostic parameters-including inflammatory markers-into the clinical management of GIST, is essential for personalized treatment strategies. These markers hold promising value for enhancing risk stratification, guiding adjuvant therapy decisions, and improving our ability to predict and manage disease recurrence among GIST patients. Further research is warranted to fully elucidate the mechanisms underlying the association between inflammation and GIST progression and to translate these findings into clinical practice.
| 1. | Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 875] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 2. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 3. | Zhao JL, Wang MY, Lv YZ, Zhou YJ. Prognostic value of inflammatory markers in predicting recurrence-free survival in gastrointestinal stromal tumor patients: A nomogram-based approach. World J Gastrointest Oncol. 2024;17:94956. |
| 4. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 5. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 6. | van de Wal D, Elie M, Le Cesne A, Fumagalli E, den Hollander D, Jones RL, Marquina G, Steeghs N, van der Graaf WTA, Husson O. Health-Related Quality of Life and Side Effects in Gastrointestinal Stromal Tumor (GIST) Patients Treated with Tyrosine Kinase Inhibitors: A Systematic Review of the Literature. Cancers (Basel). 2022;14:1832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, Liu L. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci Rep. 2019;9:3284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 8. | Yilmaz A, Mirili C, Bilici M, Tekin SB. A novel predictor in patients with gastrointestinal stromal tumors: Systemic immune-inflammation index (SII). J BUON. 2019;24:2127-2135. [PubMed] |
| 9. | Yang J, Gu Y, Huang X, Xu J, Zhang Y, Yang X, Tian H, Zhan W. Prognostic impact of preoperative neutrophil-lymphocyte ratio for surgically resected gastrointestinal stromal tumors. Medicine (Baltimore). 2019;98:e15319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Wei ZW, Huang WB, Yang DJ, Yuan YJ, He YL, Zhang CH. The prognostic roles of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in gastrointestinal stromal tumours: a meta-analysis. Transl Cancer Res. 2020;9:5128-5138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Zheng F, Meng Q, Zhang L, Chen J, Zhao L, Zhou Z, Liu Y. Prognostic roles of hematological indicators for the efficacy and prognosis of immune checkpoint inhibitors in patients with advanced tumors: a retrospective cohort study. World J Surg Oncol. 2023;21:198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 12. | Ding P, Wu J, Wu H, Sun C, Guo H, Lowe S, Yang P, Tian Y, Liu Y, Meng L, Zhao Q. Inflammation and nutritional status indicators as prognostic indicators for patients with locally advanced gastrointestinal stromal tumors treated with neoadjuvant imatinib. BMC Gastroenterol. 2023;23:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Cananzi FCM, Minerva EM, Samà L, Ruspi L, Sicoli F, Conti L, Fumagalli Romario U, Quagliuolo VL. Preoperative monocyte-to-lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J Surg Oncol. 2019;119:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Kumarasamy C, Tiwary V, Sunil K, Suresh D, Shetty S, Muthukaliannan GK, Baxi S, Jayaraj R. Prognostic Utility of Platelet-Lymphocyte Ratio, Neutrophil-Lymphocyte Ratio and Monocyte-Lymphocyte Ratio in Head and Neck Cancers: A Detailed PRISMA Compliant Systematic Review and Meta-Analysis. Cancers (Basel). 2021;13:4166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 15. | Luo XF, Zhou LH. Prognostic significance of neutrophil to lymphocyte ratio in patients with gastrointestinal stromal tumors: A meta-analysis. Clin Chim Acta. 2018;477:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Goh BK, Chok AY, Allen JC Jr, Quek R, Teo MC, Chow PK, Chung AY, Ong HS, Wong WK. Blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are independent prognostic factors for surgically resected gastrointestinal stromal tumors. Surgery. 2016;159:1146-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Lu Z, Li R, Cao X, Liu C, Sun Z, Shi X, Shao W, Zheng Y, Song J. Assessment of Systemic Inflammation and Nutritional Indicators in Predicting Recurrence-Free Survival After Surgical Resection of Gastrointestinal Stromal Tumors. Front Oncol. 2021;11:710191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Wang CJ, Zhang ZZ, Xu J, Wang M, Zhao WY, Tu L, Zhuang C, Liu Q, Shen YY, Cao H, Zhang ZG. SLITRK3 expression correlation to gastrointestinal stromal tumor risk rating and prognosis. World J Gastroenterol. 2015;21:8398-8407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Tang D, Lin T, Wang Y, Cao H. High expression of proenkephalin is associated with favorable outcomes in patients with gastrointestinal stromal tumors. Cancer Manag Res. 2019;11:6681-6690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S, Angevin E, Armand JP, Ribrag V, Aspeslagh S, Varga A, Bahleda R, Menis J, Gazzah A, Michot JM, Marabelle A, Soria JC, Massard C. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: The Gustave Roussy Immune Score (GRIm-Score). Eur J Cancer. 2017;84:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 21. | Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, Aziz O, Jenkins JT. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2014;260:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Calderillo-Ruíz G, Pérez-Yepez EA, García-Gámez MA, Millan-Catalan O, Díaz-Romero C, Ugalde-Silva P, Salas-Benavides R, Pérez-Plasencia C, Carbajal-López B. Genomic profiling in GIST: Implications in clinical outcome and future challenges. Neoplasia. 2024;48:100959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |